Familial Aggregation of Age-Related Hearing Loss in an
Epidemiological Study of Older Adults
Laura A. Raynor, University of Minnesota James S. Pankow, University of Minnesota Michael B. Miller, University of Minnesota Guan-Hua Huang,
National Chiao Tung University Dayna Dalton,
University of Wisconsin-Madison Ronald Klein,
University of Wisconsin-Madison Barbara E. K. Klein, and University of Wisconsin-Madison Karen J. Cruickshanks
University of Wisconsin-Madison
Laura A. Raynor: Rayno007@umn.edu
Abstract
Purpose—To estimate the genetic contributions to presbycusis
Method—Presbycusis was assessed by audiometric measurements at three waves of the population-based Epidemiology of Hearing Loss Study (EHLS). Measurements from the most recent hearing examination were used, at which time subjects were between 48 and 100 years of age. Heritability of presbycusis was estimated using maximum likelihood methods in 973 biological relative pairs from 376 families. Familial aggregation was also evaluated by tetrachoric correlations, odds ratios, and lambda statistics in 594 sibling pairs from 373 sibships.
Subjects—3,510 participants from the EHLS study
Results—The prevalence of presbycusis conformed to previous research, increasing with age and male sex. Heritability estimates for presbycusis adjusted for age, sex, education level, and exposure to work noise exceeded 50%, and siblings of an affected relative were at 30% higher risk. When stratified by sex, estimates of familial aggregation were higher in women than men.
Conclusions—There is evidence that genetic factors contribute to age-related hearing loss in this population-based sample. The familial aggregation is stronger in women than in men.
Age-related hearing loss, also known as presbycusis, is the most common form of hearing impairment in humans and is characterized by decreased hearing sensitivity, decreased ability to understand speech in a noisy environment, slowed central processing of acoustic stimuli, and impaired ability to detect the location of a sound. It is typically described as a downward
NIH Public Access
Author Manuscript
Am J Audiol. Author manuscript; available in PMC 2010 December 1.
Published in final edited form as:
Am J Audiol. 2009 December ; 18(2): 114–118. doi:10.1044/1059-0889(2009/08-0035).
NIH-PA Author Manuscript
NIH-PA Author Manuscript
sloping high-frequency loss, meaning that hearing is better in the low and middle frequencies than in the high frequencies.
Presbycusis is the third most prevalent chronic condition in older Americans, ranging from 25 to 40% in individuals ≥ 65 years of age (Cruickshanks et al., 1998; Gates et al., 1999; Reuben, Walsh, Moore, Damesyn, & Greendale, 1998; U.S. Department of Commerce, 1997). Prevalence has been shown to increase with age, ranging from 40–66% in individuals ≥ 75 years of age. By the year 2050, 20.7% of the United States, over 86 million adults, will be aged 65 and older (U.S. Census, 2008).
Epidemiological and biological studies in human and mouse models suggest that both genetic and environmental risk factors play roles in the development and progression of presbycusis (Christensen, Frederiksen, & Hoffman, 2001; DeStefano, Gates, Heard-Costa, Myers, & Baldwin, 2003; Gates, Couropmitree, & Myers, 1999; Johnson, Erway, Cook, Willot, & Zheng, 1997; Parving, Sakihara, & Christensen, 2000). Previous studies have found that age-related hearing loss aggregates in families and heritability analyses have found that 25–75% of the variance in presbycusis is genetic (Christensen et al. 2001; Gates et al. 1999; Karlsson, Harris, & Svarengren, 1997; Viljanen et al., 2007). Age-related hearing loss is likely a polygenic disorder and thus far, genetic contributions to this phenotype include mitochondrial deletions (Van Eyken, Van Camp, & Van Laer, 2007). A recent genomewide linkage analysis using participants of the Framingham Heart Study has identified a number of new candidate regions for further study (DeStefano et al., 2003). In the present study, we used data from one of the largest population-based studies with auditory assessment to estimate familial aggregation of age-related hearing loss.
Methods
ParticipantsDuring 1987–1988, a private census was conducted to identify residents of the city or township of Beaver Dam, Wisconsin who were ages 43–84 years. These residents were subsequently invited to participate in the Beaver Dam Eye Study, a study of age-related ocular disorders. Of the 5,924 eligible individuals, 4,926 participated in the eye examination phase (1988–1990). Some of the participants were later determined to be part of the nuclear or extended families, and pedigree structures were collected. Details regarding the original cohort and baseline examination have been described in earlier publications (Klein, Klein, Linton, & De Mets, 1991; Linton, Klein, & Klein, 1991).
Participants, who were alive as of March 1, 1993 (n=4,541), were eligible to participate in the first Epidemiology of Hearing Loss Study (EHLS) examination that occurred at the time of the 5-year follow-up visit for the eye study, conducted from 1993–95. Of those eligible, 3,753 participated in the first EHLS examination. A 5-year follow-up EHLS examination was conducted from 1998–2000 with 2,851 participants. Finally, a ten year-follow up examination, that had 2,411 participants, was conducted from 2003–2005. The EHLS was approved by the Human Subjects Committee of the University of Wisconsin-Madison and informed consent was obtained from each participant at the beginning of the examination.
Procedures
A standardized questionnaire that included relevant questions about potential covariates was administered by interview. The hearing examination included an otoscopic evaluation and pure-tone air- and bone-conduction audiometry. Audiometric testing was conducted according to the guidelines of the American-Speech-Language Hearing Association in sound treated booths (The American Speech-Hearing-Language Association, 1978). Virtual 320 clinical
NIH-PA Author Manuscript
NIH-PA Author Manuscript
audiometers (Virtual Corp, Seattle, Washington) were used during the first examination phase. Grason Stadler GSI 61 audiometers (Grason Stadler Inc, Madison, Wisconsin) were used in the 5 and 10 year follow-up examinations. Audiometers were equipped with TDH 50 headphones (Telephonics Corp, Farmingdale, NY). Insert earphones (ERA 3A, Cabot Safety Corporation, Indianapolis, IN) and masking were used as necessary. Participants unable to travel to clinic sites were tested at their residences using a Beltone portable audiometer (Beltone Electronic Corp, Chicago, Illinois). Testing was performed by trained examiners.
Pure tone air-conduction thresholds were obtained for each ear at 250, 500, 1000, 2000, 3000, 4000, 6000, and 8000 Hz at each examination period. Bone-conduction thresholds were obtained at 500 Hz and 4000 Hz at the baseline examination and at 500 Hz, 2000 Hz, and 4000 Hz at the 5 and 10 year follow-up examinations. Examination time constraints limited the bone conduction testing conducted at baseline and follow-up examination; thus, bone conduction testing was not done at 1000 and 2000 Hz at the baseline examination and not done at 1000 Hz at subsequent follow-up examinations. The presence of a hearing loss was defined as a pure tone average of thresholds at 500, 1000, 2000 and 4000 Hz greater than 25 dB HL in either ear.
All audiometers were initially calibrated according to the American National Standards Institute (ANSI) and were recalibrated every six months during the study period (American National Standards Institute, 1989). Ambient noise levels were measured daily and at home visits. Thresholds were set to missing values if the appropriate ANSI standards were exceeded (varied by transducer). Noise levels with the sound-treated booth did not exceed ANSI standards.
Statistical Analyses
Individuals with patterns of hearing loss not consistent with age-related hearing loss were excluded from all analyses (N=243, 187, 179 at baseline, 5-year, and 10-year examinations, respectively). Participants were considered to have non age-related hearing loss if they reported a history of significant ear surgery (tympanoplasty, mastoidectomy or stapedectomy), onset of hearing loss before age 30, unilateral hearing loss (hearing loss in only one ear, and a difference in pure tone average > 20 dB HL between ears), or a conductive loss (air conduction threshold ≥ 15 dB HL worse than bone conduction threshold) that if resolved would result in normal hearing. Hearing loss was modeled as a dichotomous trait, using the last recorded (most recent) measurement of hearing loss for each individual to best capture the age-related expression of the phenotype. Of the 3,510 subjects included in the analysis, 57% provided data from the 10-year examination, 19% provided data from the 5-10-year examination, and 24% provided data from the baseline examination.
Heritability of hearing loss in pedigrees was estimated by a liability threshold model, as implemented in the Sequential Oligogenic Linkage Analysis Routines (SOLAR) software package (http://www.sfbr.org/solar/index.html; Version 2.1.5) (Almasy & Blangero, 1998). The maximum-likelihood estimation is applied to a polygenic model, whereby the phenotypic variance is divided between additive genetic heritability and the non-additive, non-familial effects. The heritability is a proportion of additive genetic variance divided by the total phenotypic variance and ranges from 0 to 100%, with 0% signifying no genetic influence and 100% signifying total genetic influence. Heritability estimates for hearing loss were calculated as follows: (a) unadjusted (b) adjusted for age and sex and (c) adjusted for age, sex, level of education, smoking status, and exposure to work noise.
In addition, familial aggregation was estimated in sibships by tetrachoric correlations, odds ratios, and relative risks (λ). The tetrachoric correlation estimates likeness between relatives (Neale & Cardon, 1992). The calculation of the tetrachoric correlation is defined under the
NIH-PA Author Manuscript
NIH-PA Author Manuscript
liability threshold model, where it is assumed that the influence of many genes and
environmental factors result in an underlying, normally distributed liability to the disease and there exists a threshold that divides the population into affected and unaffected subjects. This threshold is inferred from the disease prevalence in the sample and the tetrachoric correlation is the correlation between siblings for the underlying liability (Sham, 1998). The pairwise odds ratio for any two siblings is the ratio of the odds that, given that any sibling selected at random has the disorder, a second sibling selected at random will also have the disorder over the odds that, given that any sibling selected at random does not have the disorder, a second sibling picked at random will have the disorder (Rende & Weissman, 1999). Odds ratios that are greater than one indicate sibling aggregation. Finally, the λ is defined as the risk to sibling of an affected individual divided by the population prevalence (Risch, 2001).
Results
Subjects with patterns of hearing loss not consistent with age related hearing loss, which included the reported onset of hearing impairment before 30 years of age, hearing loss with a history of ear surgery, a conductive loss without evidence of decreased sensitivity if the conductive loss was resolved and an asymmetric hearing loss (≥20 dB difference in the PTA between ears), were excluded from analysis. The prevalence of hearing loss was as follows: 42% at the baseline examination of EHLS, 48% at 5-year up, 53% at 10-year follow-up, and 57% using the most recent measurement for each individual. Prevalence of hearing loss is highest at the most recent measurement due to differential participation in the examinations. Individuals without hearing loss were more likely to participate in ongoing examinations as opposed to individuals with hearing loss. Subjects with hearing loss were more likely to be older, male, have less education, and have been exposed to work noise (p<.0001) (Table 1).
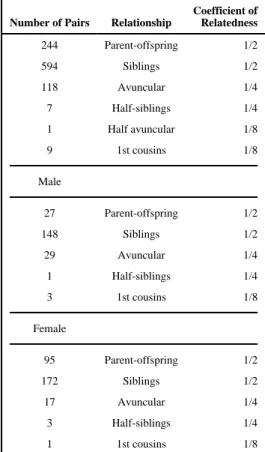
Table 2 shows the relationships of all relative pairs available for family-based analysis. Auditory measurements were available for 973 biological relative pairs from 376 families, including 594 sibling pairs from 373 sibships. Within these families there were more female than male relative pairs. Estimates of familial aggregation for presbycusis at last measurement are presented in Table 3. Heritability estimates were significant (P<.0001), and ranged from 0.47 (unadjusted) to 0.68 adjusting for age, sex, education level, and exposure to work noise. Additional adjustment for exposure to hobby noise, body mass index, diabetes status, a history of head injury, physical activity levels, exposure to gunfire, and use of statins had little effect on the heritability estimates (data not shown). The relative high concordance of hearing loss between siblings resulted in a tetrachoric correlation of 0.54 (95% CI: 0.49–0.59) and a pairwise odds ratio of 4.69. Thus, siblings of individuals with hearing loss had 4.69 times higher odds of having hearing loss as siblings of participants without hearing loss. Siblings of participants with hearing loss were 30% more likely themselves to have hearing loss than the average member of the population (λ=1.30). Modeling of individual frequency ranges yielded similar heritability estimates across strata; thus, neither low or high frequencies were more heritable than the other.
When the subjects were stratified by sex, there were differences in estimates of familial aggregation between men and women (Table 3). Sibling tetrachoric correlations, sibling pairwise odds ratios, and sibling risk ratios were greater in women than men.
Conclusions
We conducted a study of the hereditary basis of presbycusis in a population-based sample using audiometric and questionnaire data for important covariates. We found clear evidence of familial aggregation of presbycusis within the EHLS cohort using multiple methodologies.
NIH-PA Author Manuscript
NIH-PA Author Manuscript
These results suggest that genetic factors play a large role in age-related hearing loss in this population, explaining at least half the variation in audiometrically measured hearing loss after controlling for major risk factors. Tetrachoric correlations, pairwise odds ratios and sibling risk ratios indicate that one’s risk increases if a sibling is affected.
As aforementioned, we live in an aging population where the prevalence of presbycusis is likely to increase dramatically in forthcoming decades affecting the overall quality of life for a large segment of the population. Further insights into the etiology of this condition, including genetic determinants, may provide the basis for new approaches to prevention and treatment. For example, a genetic predisposition may warrant greater protection from environmental exposures that contribute to hearing loss, earlier and more frequent screening for hearing loss, and earlier fittings of hearing aids (Viljanen et al., 2007)
Results from our study and others suggest that presbycusis is a multi-factorial disorder that involves both genetic and environmental factors. While we controlled for age, sex, education level, and work noise exposure in our analyses, it is possible that heritability estimates were overestimated because they did not account for unmeasured or unknown environmental factors for hearing loss that are shared within families. Furthermore, we do not know how many environmental factors actively contribute to the etiology of hearing loss. Additional research into environmental causes of hearing loss is warranted to understand how genes, environmental factors, and their interactions contribute to the development of presbycusis.
Previous age-related hearing loss research has shown that men were more likely to be affected by hearing loss than women in this cohort (Cruickshanks et al., 1998). Additionally, there are sex-specific differences in possible effects of risk factors including age, education level, and exposure to work noise. We found that despite the greater prevalence of hearing loss at last measurement in men, the familial aggregation of hearing loss is stronger in women, which has been documented in prior research studies.
Viljanen et al. (2007) found that female twins had greater estimates of heritability of
audiometrically measured hearing loss when compared to a study of audiometrically measured hearing loss in male twins. Likewise, the Framingham study found stronger heritability estimates in women compared to men (Gates et al, 1999; DeStefano et al. 2003). Gates et al. (1999) proposed that the weaker correlations in men results from exposure to disparate environmental factors, such as noise exposure, between men and women. Sex-specific differences in heritability could also indicate that mitochondrial inheritance (maternal inheritance) of mutations plays a role in presbycusis (Hutchin & Cortopassi, 2000; Sakihara, Christensen, & Parving, 1999).
The strengths of this study include: the availability of a large, stable, population-based sample for the estimation of heritability of presbycusis at last visit, the use of standardized
examinations and questionnaires to measure outcomes and potential confounders, and the high response rate at both baseline and follow-up examinations. There are several important limitations to the study. We did not exclude individuals with ototoxicity; however, we believe that this condition would have a very low prevalence in this population. The generalizability of these results is restricted, as 98% of the participants are Caucasian (Klein et al., 1991). The results when stratified by sex are based upon a small number of affected individuals with women contributing more data than men, which could lead to imprecision in the estimation of familial aggregation by sex. Finally, the inability to control for unmeasured confounders may affect the estimates.
Previous studies have shown that genetic factors play a moderate role in the development of presbycusis in humans (Christensen et al., 2001; Gates et al., 1999). The results of this study are consistent with these earlier studies, supporting the need for further studies to identify the
NIH-PA Author Manuscript
NIH-PA Author Manuscript
patterns of familial transmission and the genes responsible for presbycusis. Future studies of age-related hearing loss should include information on a family history of hearing loss in addition, to biological samples for future candidate gene studies (Christensen et al., 2002). Recent linkage analyses have identified genetic regions on chromosomes 3, 11, and 14 linked to age-related hearing losses; thus, forthcoming association analyses should begin with candidate genes in these regions (DeStefano et al., 2003; Garringer et al., 2006). As the U.S. population continues to age, the prevalence of this disorder will increase and impact the quality of life for older Americans, making presbycusis an important area of future public health research.
Acknowledgments
This research is supported by the National Institutes of Health grants AG021917 (Cruickshanks) and AG11099 (Cruickshanks).
References
Almasy L, Blangero J. Multipoint quantitative trait linkage analysis in general pedigrees. American Journal of Human Genetics 1998;62(5):1198–1211. [PubMed: 9545414]
American National Standards Institute. American National Standard Specification for audiometers. New York: American National Standards Institute, Inc.; 1989.
The American Speech-Hearing-Language Association. Guidelines for manual pure-tone audiometry. ASHA 1978;20:297–301. [PubMed: 656172]
Christensen K, Frederiksen H, Hoffman HJ. Genetic and environmental influences on self-reported hearing in the old and oldest old. Journal of the American Geriatric Society 2001;49(11):1512–1517. Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, Klein R, Mares-Perlman JA, et al. Prevalence of
hearing loss in older adults in Beaver Dam, Wisconsin: the Epidemiology of Hearing Loss Study. American Journal of Epidemiology 1998;148(9):879–886. [PubMed: 9801018]
DeStefano AL, Gates GA, Heard-Costa N, Myers RH, Baldwin CT. Genomewide linkage analysis to presbycusis in the Framingham Heart Study. Archives of Otolaryngology, Head & Neck Surgery 2003;129(3):285–289. [PubMed: 12622536]
Gates GA, Couropmitree NN, Myers RH. Genetic associations in age-related hearing thresholds. Archives of Otolaryngology, Head & Neck Surgery 1999;125(11):654–659. [PubMed: 10367922] Hutchin TP, Cortopassi GA. Mitochondrial defects and hearing loss. Cellular and molecular life sciences
2000;57(13–14):127–137.
Johnson KR, Erway LC, Cook SA, Willot JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hearing Research 1997;114(1–2):83–92. [PubMed: 9447922]
Karlsson KK, Harris JR, Svarengren M. Description and primary results from an audiometric study of male twins. Ear and Hearing 1997;18(2):114–120. [PubMed: 9099560]
Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology 1991;98(8):1310–1315. [PubMed: 1923372]
Linton KL, Klein BE, Klein R. The validity of self-reported and surrogate-reported cataract and age-related macular degeneration in the Beaver Dam Eye Study. American Journal of Epidemiology 1991;134(12):1438–1446. [PubMed: 1776618]
Neale, MC.; Cardon, LR. Methodology for genetic studies of twins and families. Boston: Kluwer Academic Publishers; 1992.
Parving A, Sakihara Y, Christensen B. Inherited sensorineural low-frequency hearing impairment: some aspects of phenotype and epidemiology. Audiology 2000;39(1):50–60. [PubMed: 10749071] Rende R, Weissman MM. Sibling aggregation for psychopathology in offspring of opiate addicts: effects
of parental comorbidity. Journal of Clinical Child Psychology 1999;28(3):342–348. [PubMed: 10446683]
Reuben DB, Walsh K, Moore AA, Damesyn M, Greendale GA. Hearing loss in community-dwelling older persons: national prevalence data and identification using simple questions. Journal of the American Geriatric Society 1998;46(8):1108–1111.
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Risch N. The genetic epidemiology of cancer. Interpreting family and twin studies and their implications for molecular genetic approaches. Cancer Epidemiology, Biomarkers & Prevention 2001;10(7):733– 741.
Sakihara Y, Christensen B, Parving A. Prevalence of hereditary hearing impairment in adults. Scandinavian Audiology 1999;28(1):39–46. [PubMed: 10207955]
19. Sham, P. Statistics in human genetics. London: Hodder Arnold Publication; 1998.
U.S. Census (nd) Table 2a. Projected population of the United States, by age and sex: 2000 to 2050. [Retrieved September 3, 2008]. from
http://www.census.gov/population www/projections/usinterimproj/natprojtab02a.pdf
U.S. Department of Commerce. Statistical Abstract of the United States. 117th ed.Washington, DC: U.S. Census Bureau;
Van Eyken E, Van Camp G, Van Laer L. The complexity of age-related hearing impairment: contributing environmental and genetic factors. Audiology & Neurotology 2007;12(6):345–358. [PubMed: 17664866]
Viljanen A, Era P, Kaprio J, Pyykko I, Koskenvuo M, Rantanen T. Genetic and environmental influences on hearing in older women. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2007;62(4):447–452.
NIH-PA Author Manuscript
NIH-PA Author Manuscript
NIH-PA Author Manuscript
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Table 1
Prevalence of hearing loss at most recent EHLS examination by selected characteristics
Total (N) Hearing Loss* (%)
Sex Male 1497 68.1 Female 2013 49.1 Age (years) 43–54 73 20.6 55–64 845 25.4 65–74 1171 50.7 ≥ 75 1421 83.3 Years of Education < High school 843 77.2 High School 1612 54.2 Some college 538 49.6 College graduate 515 41.8 Work Noise Yes 1934 61.0 No 1576 52.5 *
NIH-PA Author Manuscript
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Table 2 Pairwise relationships among individuals and by sex
Number of Pairs Relationship
Coefficient of Relatedness 244 Parent-offspring 1/2 594 Siblings 1/2 118 Avuncular 1/4 7 Half-siblings 1/4 1 Half avuncular 1/8 9 1st cousins 1/8 Male 27 Parent-offspring 1/2 148 Siblings 1/2 29 Avuncular 1/4 1 Half-siblings 1/4 3 1st cousins 1/8 Female 95 Parent-offspring 1/2 172 Siblings 1/2 17 Avuncular 1/4 3 Half-siblings 1/4 1 1st cousins 1/8
NIH-PA Author Manuscript
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Table 3
Familial aggregation of presbycusis for all participants and stratified by sex
Model a h 2 (SE) p value Odds Ratio b Tetrachoric Correlation (SE) b Lambda b ,c 0.47 All subjects 1 (0.28) <.0001 4.69 0.54 (0.05) 1.30 0.67 2 (0.14) <.0001 0.68 3 (0.14) <.0001 Males only 2.51 0.31 (0.15) 1.16 Females only 5.46 0.59 (0.09) 1.35