Original Paper
Int Arch Allergy Immunol 318861 DOI: 10.1159/000318861
The Efficacy and Safety of a Chinese Herbal
Product (Xiao-Feng-San) for the Treatment of
Refractory Atopic Dermatitis: A Randomized,
Double-Blind, Placebo-Controlled Trial
Hui-Man Cheng
a, b
Leih-Chin Chiang
a
Ya-Min Jan
a
Guang-Wei Chen
b
Tsai-Chung Li
b
a Department of Integration of Traditional Chinese and Western Medicine, China Medical University Hospital, and b School of Chinese Medicine, College of Chinese Medicine, China Medical University, Taichung , Taiwan, ROC
scores. The difference between the 2 groups was still sig-nificant for all outcome measures except the erythema score at the 12-week follow-up, 4 weeks after the 8-week treat-ment had ended. Patients reported no side effects from treatment, although some commented on the unpalatabili-ty of the medication. Conclusion: Our study results suggest that the traditional Chinese herbal medicine XFS may be an alternative choice of therapy for severe, refractory, extensive and nonexudative atopic dermatitis.
Copyright © 2010 S. Karger AG, Basel
Introduction
Atopic dermatitis is a chronic, relapsing,
inflamma-tory skin disease that affects 10–20% of children and
1–3% of adults in industrialized countries [1, 2] . Topical
emollients, corticosteroid creams and oral
antihista-mines are effective in controlling mild to moderate
dis-ease with minimal side effects. However, current
treat-ments for severe and widespread disease (e.g. systemic
steroids, azathioprine, cyclosporine, PUVA), although
beneficial, all have undesirable adverse effects. As a
Key Words
Atopic dermatitis ⴢ Traditional Chinese medicine ⴢ Randomized controlled trial
Abstract
Background: Severe and widespread atopic dermatitis of-ten fails to respond adequately to topical steroids and oral antihistamines and requires immunomodulatory drugs which, although effective, have undesirable toxic effects. Methods: In this prospective, randomized, double-blind, placebo-controlled trial, 71 patients with severe intractable atopic dermatitis were given an 8-week treatment with oral Xiao-Feng-San (XFS; 47 patients) or placebo (24 patients). To-tal lesion score, erythema score, surface damage score, pru-ritus score and sleep score were measured at 4-week inter-vals. Results: Fifty-six patients completed both the treat-ment and follow-up periods. The decrease in the total lesion score in the treatment group at 8 weeks was significantly greater than that of the placebo group (79.7 8 5.8% vs. 13.5 8 7.64%; p ! 0.001). There was also a statistically significant difference between the treatment and placebo groups with regard to erythema, surface damage, pruritus and sleep
Received: January 20, 2010 Accepted after revision: June 28, 2010 Published online: $ $ $
Cheng /Chiang /Jan /Chen /Li Int Arch Allergy Immunol 318861
2
sult, there have been intensive efforts to develop better
and safer treatment. One such treatment is the use of
tra-ditional Chinese medicine.
Although traditional Chinese medicine is widely used
in many Asian countries, its beneficial effects in patients
with atopic dermatitis have not been consistently
demon-strated, and undesirable side effects have been noted [3, 4] .
A recent Cochrane Review of Chinese herbal medicine for
atopic dermatitis [5] found only 4 randomized controlled
trials that met the Cochrane inclusion criteria, and these
trials were all for a herbal mixture that is no longer being
manufactured. Xiao-Feng-San (XFS) is a common
Chi-nese herbal preparation, composed of 12 herbs, which is
used to treat patients with atopic dermatitis in Asian
clin-ical practice. The purpose of this study was to evaluate the
efficacy and safety of XFS in atopic dermatitis, using a
ran-domized, double-blind, placebo-controlled study design.
Methods
Patient Selection
Seventy-one Chinese patients with refractory atopic dermati-tis, diagnosed by recognized clinical criteria [6] , were recruited from the department of Integration of Traditional Chinese and Western Medicine, China Medical University Hospital, Taiwan. The study received approval from the Institutional Review Board of the China Medical University Hospital, and all patients gave written informed consent.
Inclusion criteria were the following: extensive (not limited to the skin folds and covering 1 20% of the body surface area) lichen-ified or erythematous papules or plaques of atopic dermatitis, no active exudation or infection and poor response to conventional treatment (topical steroids and oral antihistamines).
Patients were excluded if they had secondary bacterial infec-tions or had received oral or intravenous steroid treatment, anti-biotics, phototherapy or other immunosuppressive therapies (such as cyclosporine or azathioprine) in the previous 2 months. Other exclusion criteria were abnormal liver enzymes or kidney function tests (1.5 times higher than the upper normal limit), abnormal blood chemistry, concurrent systemic illness (except asthma or al-lergic rhinitis), current breastfeeding and pregnancy or the inten-tion of becoming pregnant. In addiinten-tion, all women of childbearing age agreed to take appropriate contraceptive precautions.
Patients were required to have normal full blood counts and renal and hepatic function tests before starting the study. They also answered questionnaires with regard to their age, sex, height, weight, disease progress, personal allergy, family allergy, past treatments and exacerbating factors, among others. Patients were asked to maintain their current diet and dermatological treat-ments (in particular, not to increase the potency or frequency of topical corticosteroid use) throughout the trial. Topical steroids were used with the same frequency and strength during the study and prior to the study in both groups.
Randomization and Blinding
Eligible patients were randomized at a ratio of 2: 1 to receive XFS or placebo for an 8-week treatment period. The computer-generated randomization list was drawn up by an independent statistician and placed in an envelope until the study was com-pleted. Eligible patients were assigned consecutive randomized numbers as they entered the study. Patients and the evaluating physicians were unaware during the study of whether the medica-tion taken by the patients was placebo or the treatment drug.
XFS and Placebo Preparation and Dosage
XFS, the active treatment, consisted of a standardized formu-lation of plant materials in widespread use in China ( table 1 ). The powder was manufactured, packaged and labeled by the Sheng Chang Pharmaceutical Company (Taiwan), using good manufac-turing practice standards. The optimal composition of each herb included was standardized prior to manufacturing. The powder
Table 1. Composition of XFS herbal medicine
Medicinal plants Weight
ratio, mg
Family Species Actions
Saposhnikovia divaricata 2.5 Umbelliferae Saposhnikovia divaricata (Turcz.) Schischk relieves itching, relieves pain
Schizonepeta tenuifolia 2.5 Labiatae Schizonepeta tenuifolia (Benth.) Briq. relieves pain, relieves itching,
anti-inflammatory
Angelica sinensis 2.5 Umbelliferae Angelica sinensis (Oliv.) Diels promotes blood circulation
Rehmannia glutinosa 2.5 Scrophulariaceae Rehmannia glutinosa Libosch. antipyretic, anti-inflammatory
Sophora flavescens 2.5 Leguminosae Sophora flavescens Ait. anti-inflammatory, relieves itching
Atractylodes lancea 2.5 Compositae Atractylodes lancea (Thunb.) DC. harmonizes water metabolism
Cryptotympana pustulata 2.5 Cicadidae Cryptotympana pustulata Fabricius sedative, relieves itching
Linum usitatissimum 2.5 Pedaliaceae Sesamum indicum L. moistens
Anemarrhena asphodeloides 2.5 Liliaceae Anemarrhena asphodeloides Bunge sedative, anti-inflammatory
Gypsum fibrosum 2.5 Gypsum Gypsum fibrosum: CaSO4, 2H2O anti-inflammatory
Clematis armandii 1.25 Ranunculaceae Clematis armandii Franch. harmonizes water metabolism
Glycyrrhiza uralensis 1.25 Leguminosae Glycyrrhiza uralensis Fisch. antitoxic, sedative, protects digestive system
Arctium lappa 2.5 Compositae Arctium lappa L. antibacterial, relieves itching
IAA318861.indd 2
was formulated into uniform dose packets under the supervision of the Clinical Trials Section, China Medical University Hospital, according to established procedures. All materials were checked before use for heavy metal content and for possible microbial con-taminants. Thin-layer chromatography was used to ‘fingerprint’ each batch of every constituent, and batches were rejected (about 10%) if they differed substantially from the reference material.
Patients took the medicine 3 times a day, and the number of packs taken differed according to the enrollee’s age. Those 3–7 years of age took 1 pack, those 8–12 years of age took 2 packs and those aged 13 and over took 3 packs at each dosing point. There were 3 g of XFS concentrated particles or placebo in each pack. Placebo was made of caramel, lactose and starch at a ratio of 2: 1:1 and put into identical-appearing 3-gram packs. The placebo mix-ture has no known benefit in atopic dermatitis but has a similar appearance and taste to the active treatment.
Patients were instructed to take the medicine by mixing it in a cup with 120 ml of warm drinking water and then drinking the mixture.
Assessment
Patients were randomly assigned to the treatment or control group. They took the herbal treatment or placebo daily for 8 weeks and were assessed at the beginning of the study and after 4, 8 and 12 weeks. The following investigations were performed during
each assessment: full blood count, serum bilirubin, aspartate aminotransferase, alkaline phosphatase, albumin, urea and elec-trolytes, creatinine, calcium, phosphate, glucose, creatine phos-phokinase and immunologic markers (IgE, eosinophil count, eo-sinophil cationic protein, IL-5, IL-13). Blood pressure and weight were also measured and side effects were monitored.
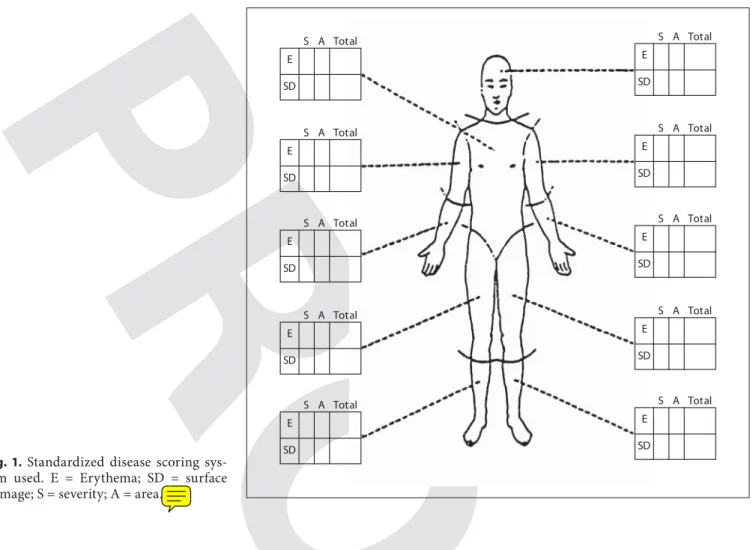
The extent and severity of the dermatitis was assessed by quan-titative measurement of erythema and surface damage (i.e. papu-lation, vesicupapu-lation, scaling, excoriation and lichenification) us-ing a standardized scorus-ing system [7, 8]. The body surface was divided into 20 roughly equal areas, and within each area, a score of 0 (none) to 3 (severe) for the degree of erythema and surface damage was given ( fig. 1 ). For each of these clinical features, an estimate of the percentage of the area within each zone affected by that particular feature was measured; a score of 1 was given where the area affected was ! 33%, 2 where the area was between 34 and 66%, and 3 where the area was 1 67%. The sum of the se-verity scores multiplied by the area scores provided a total body score for each feature, the maximum score being 180.
Patients were asked to keep a daily diary during the study to record their compliance with treatment and any side effects. At each monthly visit, patients were asked to record the severity of itching (0 = no itching at all; 1 = slight itching; 2 = moderate itch-ing; 3 = severe itchitch-ing; 4 = very severe itching) and sleep distur-bance (0 = no sleep interruptions; 1 = sleep interrupted 1 or 2
E SD S A Total E SD S A Total E SD S A Total E SD S A Total E SD S A Total E SD S A Total E SD S A Total E SD S A Total E SD S A Total E SD S A Total
Fig. 1. Standardized disease scoring sys-tem used. E = Erythema; SD = surface damage; S = severity; A = area.
Cheng /Chiang /Jan /Chen /Li Int Arch Allergy Immunol 318861
4
times; 2 = sleep interrupted 3 or 4 times; 3 = sleep interrupted more than 5 times; 4 = unable to sleep) and whether they had fewer episodes or less severe asthma than usual during that phase of the trial.
The protocol specified that patients who showed a persistent abnormality in blood chemistry, including increases in serum to-tal bilirubin or liver enzymes 1 1.5 times the normal range, dia-stolic blood pressure persistently above 95 mm Hg or other com-plications believed to be due to treatment should be withdrawn from the study. Furthermore, patients who failed to comply with the protocol (a failure to take the treatment on more than 5 days in any 4-week period) or who were given systemic antibiotics or corticosteroids for any reason during the study were withdrawn.
Sample Size Determination
For the primary outcome, the clinical lesion score, a sample size of 67 subjects (45 for the XFS group and 22 for the placebo group) would provide a statistical power of 80% to detect a mean difference of 40.
Statistical Analysis
Continuous data are presented as means 8 SD, and the two-sample t test was used for comparisons. When the hypothesis of normal distribution was violated, the Mann-Whitney U test was adopted. Categorical data are presented as numbers of patients (percentages) and were compared with Fisher’s exact test.
The primary outcome, clinical lesion score, was the total of the erythema score plus the surface damage score. There were 4 sec-ondary outcomes: erythema score, surface damage score, pruritus score and sleep score. All efficacy endpoints were defined as the mean improvement from baseline. Because the sleep score at
base-line showed a borderbase-line nonsignificant difference between the 2 treatment groups, analysis of covariance was used to adjust the baseline sleep score for efficacy analysis. The mean score im-provement is presented as the least-squares mean 8 SE. Statistical assessments were two-sided, and the 0.05 level was considered statistically significant. Statistical analyses were performed using SPSS 15.0 statistics software (SPSS Inc., Chicago, Ill., USA).
Results
Patients
Seventy-one patients were enrolled in this study, and
47 and 24 patients were randomly divided into the XFS
group and placebo group, respectively. Two patients (1 in
the XFS group, 1 in the control group) who were not
treat-ed with any investigational drugs were dropptreat-ed from the
study at baseline (week 0), so that a total of 69 patients (46
in the XFS group, 23 in the placebo group) were included
in the intention-to-treat population.
During the 8-week treatment period, 10 patients (9 in
the XFS group and 1 in the placebo group) were excluded
from the per-protocol population due to poor compliance
(5 in the XFS group and 1 in the placebo group), refusal
to continue treatment (in the XFS group, 2 were too busy,
1 was afraid to have blood drawn) and use of a prohibited
drug (1 in the XFS group).
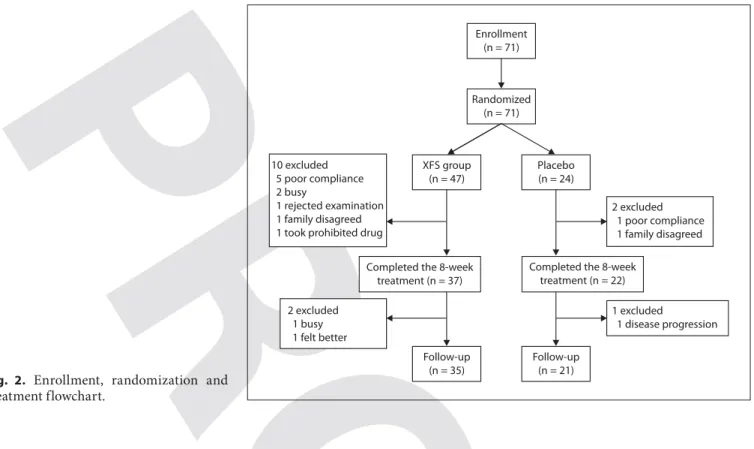
Enrollment (n = 71) Randomized (n = 71) Placebo (n = 24) 2 excluded 1 poor compliance 1 family disagreed
Completed the 8-week treatment (n = 22) 1 excluded 1 disease progression Follow-up (n = 21) XFS group (n = 47)
Completed the 8-week treatment (n = 37) Follow-up (n = 35) 2 excluded 1 busy 1 felt better 10 excluded 5 poor compliance 2 busy 1 rejected examination 1 family disagreed 1 took prohibited drug
Fig. 2. Enrollment, randomization and treatment flowchart.
IAA318861.indd 4
After the treatment period (week 8), 1 patient in the
XFS group felt better and refused to continue the trial and
a second patient in the XFS group was too busy to
con-tinue the trial. One patient in the placebo group was
dis-continued due to disease progression. Fifty-nine of the
patients (83%) completed the treatment period, and 56
(79%) completed the entire study period. This
informa-tion is summarized in the flowchart shown in figure 2 .
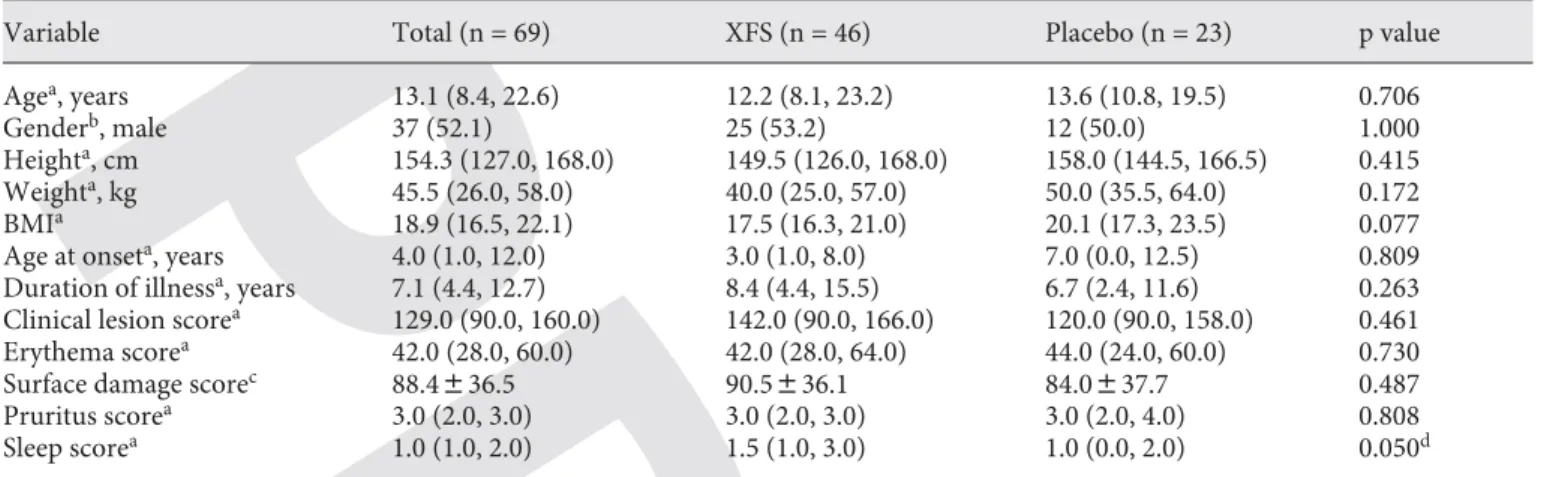
Table 2 shows the baseline characteristics for the
in-tention-to-treat population of the 2 treatment groups.
There was no significant difference between the 2
treat-ment groups with regard to baseline characteristics.
However, there was a borderline nonsignificant
differ-ence in the sleep scores [median (interquartile range): 1.5
(1.0, 3.0) for XFS vs. 1.0 (0.0, 2.0) for placebo].
Efficacy
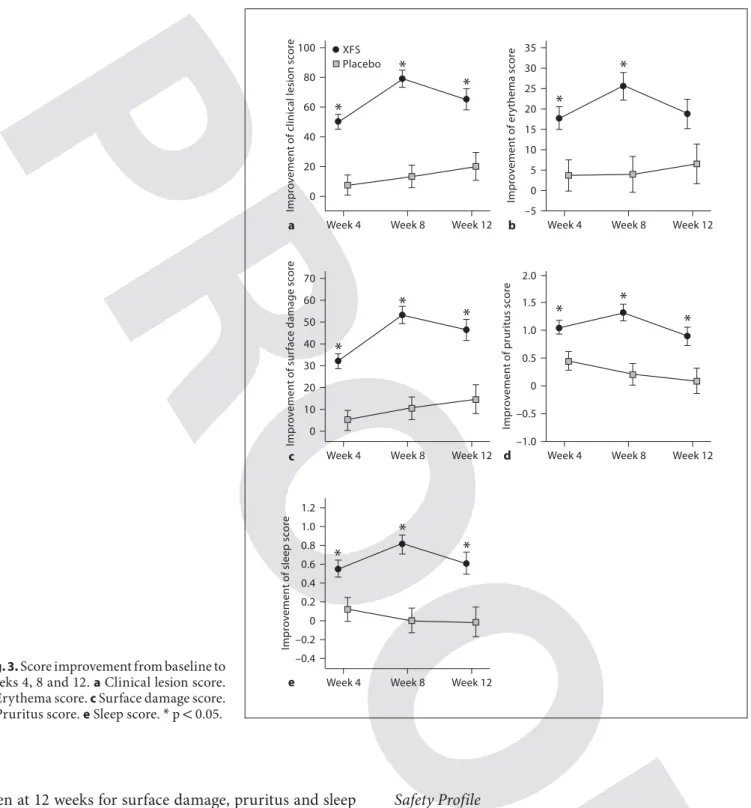
Efficacy outcomes are shown in table 3 and figure 3 .
At the end of the 8-week treatment period, the primary
endpoint, mean improvement in the total clinical lesion
score, was significantly higher in the XFS group than in
the placebo group (least-squares mean 8 SE with
adjust-ment for baseline sleep scores: 79.10 8 5.70 vs. 13.50 8
7.56; p ! 0.001). The 4 secondary endpoints (erythema
score, surface damage score, pruritus score and sleep
score) all also showed significantly greater improvement
in the XFS group than the placebo group (p ! 0.001 for
all comparisons; table 3 ). In addition, 4 weeks after
ter-mination of the treatment, the mean improvement in the
clinical lesion score in the XFS group was still
signifi-cantly higher than that of the placebo group (p ! 0.05).
Significantly better scores in the XFS group were also
Table 2. Demographic and baseline characteristics for the intention-to-treat populationVariable Total (n = 69) XFS (n = 46) Placebo (n = 23) p value
Agea, years 13.1 (8.4, 22.6) 12.2 (8.1, 23.2) 13.6 (10.8, 19.5) 0.706
Genderb, male 37 (52.1) 25 (53.2) 12 (50.0) 1.000
Heighta, cm 154.3 (127.0, 168.0) 149.5 (126.0, 168.0) 158.0 (144.5, 166.5) 0.415
Weighta, kg 45.5 (26.0, 58.0) 40.0 (25.0, 57.0) 50.0 (35.5, 64.0) 0.172
BMIa 18.9 (16.5, 22.1) 17.5 (16.3, 21.0) 20.1 (17.3, 23.5) 0.077
Age at onseta, years 4.0 (1.0, 12.0) 3.0 (1.0, 8.0) 7.0 (0.0, 12.5) 0.809
Duration of illnessa, years 7.1 (4.4, 12.7) 8.4 (4.4, 15.5) 6.7 (2.4, 11.6) 0.263
Clinical lesion scorea 129.0 (90.0, 160.0) 142.0 (90.0, 166.0) 120.0 (90.0, 158.0) 0.461
Erythema scorea 42.0 (28.0, 60.0) 42.0 (28.0, 64.0) 44.0 (24.0, 60.0) 0.730
Surface damage scorec 88.4836.5 90.5836.1 84.0837.7 0.487
Pruritus scorea 3.0 (2.0, 3.0) 3.0 (2.0, 3.0) 3.0 (2.0, 4.0) 0.808
Sleep scorea 1.0 (1.0, 2.0) 1.5 (1.0, 3.0) 1.0 (0.0, 2.0) 0.050d
a Data presented as median (interquartile range); the Mann-Whitney U test was used to compare the difference between the 2 treat-ment groups.
b Data presented as number of patients (percentage); Pearson’s 2 test was used to compare the difference between the 2 treatment groups.
c Data presented as mean 8 SD; the two-sample t test was used to compare the difference between the 2 treatment groups. d There was a borderline nonsignificant difference in sleep scores between the XFS and placebo groups.
Table 3. Improvement in scores between baseline and week 8
Index XFS (n = 46) Placebo (n = 23) p value Improvement in clinical lesion score 79.1085.70 13.5087.56 <0.001* Improvement in erythema score 25.7083.33 4.1084.41 <0.001* Improvement in surface damage score 53.4083.96 10.3085.25 <0.001* Improvement in pruritus score
Improvement in sleep score
1.3080.15 0.2080.20 <0.001* 0.8080.10 0.0080.13 <0.001* D ata are presented as least-squares means 8 SE. Analysis of covariance was used to compare the difference between the 2 treatment groups, with baseline sleep score adjustment. There were 5 missing values in the XFS group and 1 missing value in the placebo group. * p < 0.05: significant difference between the XFS and placebo groups.
Cheng /Chiang /Jan /Chen /Li Int Arch Allergy Immunol 318861
6
seen at 12 weeks for surface damage, pruritus and sleep
scores ( fig. 3 ).
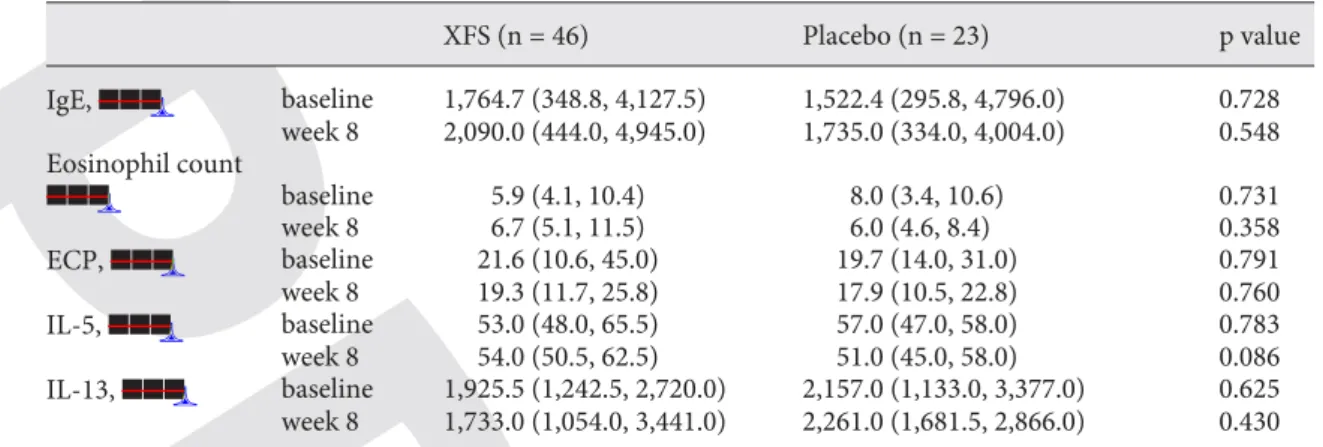
The immunologic markers, IgE, eosinophil count,
eo-sinophil cationic protein, IL-5 and IL-13, were above the
normal range at baseline. Eleven patients had intrinsic
atopic dermatitis (serum IgE ! 165 IU/ml). However, there
were no statistical differences in immunologic markers
between the active treatment and placebo groups during
the 8-week treatment period ( table 4 ).
Safety Profile
No abnormalities were detected in the patients’ blood
chemistry or renal function tests at any time. Transient
elevation of aspartate aminotransferase was noted in 1
patient, but this was reversed within 8 weeks of stopping
treatment. Two patients in the active group complained
of gastrointestinal upsets, including abdominal colic and
dyspepsia, in the first week. This side effect was transient.
There was no change in blood pressure or weight.
100 0 Week 4 Impr o v ement o f clinical lesion sc or e 20 40 60 80
*
XFS Placebo Week 8 Week 12*
*
35 Week 4 Impr o v ement o f e rythema sc or e*
Week 8 Week 12*
30 25 20 15 10 5 0 –5 a b 70 0 Week 4 Impr o v ement o f sur fac e damage sc or e*
Week 8 Week 12*
*
2.0 Week 4 Impr o v ement o f pruritus sc or e*
Week 8 Week 12*
–1.0 c d 10 20 30 40 50 60 –0.5 0 0.5 1.0 1.5*
Week 4 Impr o v ement o f sleep sc or e*
Week 8 Week 12*
–0.4*
–0.2 0 0.2 0.4 0.6 0.8 1.0 1.2 eFig. 3. Score improvement from baseline to weeks 4, 8 and 12. a Clinical lesion score.
b Erythema score. c Surface damage score.
d Pruritus score. e Sleep score. * p ! 0.05.
IAA318861.indd 6
Concomitant Medications
There were 21 patients (45.7%) in the XFS group and 7
(30.4%) in the placebo group who took concomitant
med-ications during the study period (p = 0.301); 12 patients
(26.1%) in the XFS group and 4 (17.4%) in the placebo
group took antihistamines (p = 0.550), and 12 patients
(26.1%) in the XFS group and 5 (21.7%) in the placebo
group had taken medications for colds (p = 0.774). No
significant difference was found in concomitant
medica-tion usage between the 2 treatment groups.
Discussion
In this prospective, randomized, double-blind,
place-bo-controlled trial, XFS formula significantly lessened
disease severity in patients with atopic dermatitis as well
as the distressing symptoms of itch and sleep loss. These
therapeutic effects persisted for 4 weeks after treatment
was stopped.
Chronic atopic dermatitis, such as was seen in our
pa-tients, is thought to be a disorder involving cell-mediated
immunity [9] . Although it is unclear by what mechanisms
Chinese herbal preparations, which contain mixtures of
herbal ingredients, act, a number of reports have been
published showing the anti-inflammatory actions of
sin-gle species. Some reports have demonstrated that the
spe-cies Schizonepeta tenuifolia Briq. and Saposhnikovia
di-varicata (Turcz.) Schischk have significant
anti-inflam-matory effects [10, 11] . An aqueous extract of the steamed
root of Rehmannia glutinosa dose-dependently inhibited
the skin allergic reaction activated by anti-DNP IgE [12] .
Polysaccharide isolated from Angelica sinensis has
im-munomodulatory activity by regulating the expression of
Th1- and Th2-related cytokines [13] . Another study
re-ports that polysaccharides isolated from Glycyrrhiza
ura-lensis Fisch. have macrophage immunomodulatory
activ-ity [14] .
Modulation of some part of the immune system is a
likely explanation of the action of XFS, but it must be
noted that in our study, serum levels of inflammatory
markers were not changed by XFS administration. The
exact mechanism of action of XFS formula is not known.
Further investigations are needed to delineate the exact
biological mechanisms of XFS formula.
It has been reported that the use of some Chinese
herb-al preparations has resulted in serious adverse systemic
effects such as liver toxicity and dilated cardiomyopathy
[15–17] . However, XFS, as used in this study, was found
to be quite safe. Transient elevation of aspartate
amino-transferase was noted in 1 patient, but this was reversed
within 8 weeks of stopping treatment. We did not detect
any hematologic or biochemical abnormalities in our
patients. Complete blood chemistry and renal function
were all normal throughout the entire treatment period.
The lack of pharmacokinetic and pharmacodynamic
data for the XFS formula is a limitation in understanding
the mechanism of action of the drug.
Although the mechanism of action of XFS formula in
the treatment of atopic dermatitis still needs to be
ex-plored, our studies showed that XFS formula was able to
effectively improve lesion scores, pruritus symptoms and
Table 4. I mmunologic markers before and after treatmentXFS (n = 46) Placebo (n = 23) p value IgE, $$$ baseline 1,764.7 (348.8, 4,127.5) 1,522.4 (295.8, 4,796.0) 0.728 week 8 2,090.0 (444.0, 4,945.0) 1,735.0 (334.0, 4,004.0) 0.548 Eosinophil count $$$ baseline 5.9 (4.1, 10.4) 8.0 (3.4, 10.6) 0.731 week 8 6.7 (5.1, 11.5) 6.0 (4.6, 8.4) 0.358 ECP, $$$ baseline 21.6 (10.6, 45.0) 19.7 (14.0, 31.0) 0.791 week 8 19.3 (11.7, 25.8) 17.9 (10.5, 22.8) 0.760 IL-5, $$$ baseline 53.0 (48.0, 65.5) 57.0 (47.0, 58.0) 0.783 week 8 54.0 (50.5, 62.5) 51.0 (45.0, 58.0) 0.086 IL-13, $$$ baseline 1,925.5 (1,242.5, 2,720.0) 2,157.0 (1,133.0, 3,377.0) 0.625 week 8 1,733.0 (1,054.0, 3,441.0) 2,261.0 (1,681.5, 2,866.0) 0.430
D ata are presented as medians (interquartile range); the Mann-Whiney U test was used to compare the dif-ference between the 2 treatment groups. ECP = Eosinophil cationic protein.
Cheng /Chiang /Jan /Chen /Li Int Arch Allergy Immunol 318861
8
References
1 Maintz L, Novak N: Getting more and more complex: the pathophysiology of atopic
ec-zema. Eur J Dermatol 2007; 17: 267–283.
2 Boguniewicz M, Leung DY: Atopic
dermati-tis. J Allergy Clin Immunol 2006; 117:S475–
S480.
3 Fung AY, Look PC, Chong LY, But PP, Wong E: A controlled trial of traditional Chinese herbal medicine in Chinese patients with re-calcitrant atopic dermatitis. Int J Dermatol
1999; 38: 387–392.
4 Hon KL, Leung TF, Ng PC, Lam MC, Kam WY, Wong KY, Lee KC, Sung YT, Cheng KF, Fok TF, Fung KP, Leung PC: Efficacy and tol-erability of a Chinese herbal medicine con-coction for treatment of atopic dermatitis: a randomized, double-blind,
placebo-con-trolled study. Br J Dermatol 2007; 157: 357–
363.
5 Zhang W, Leonard T, Bath-Hextall F, Cham-bers CA, Lee C, Humphreys R, Williams HC: Chinese herbal medicine for atopic eczema. Cochrane Database Syst Rev 2004;(4): CD002291.
6 Hanifin JM, Rajka RG: Diagnostic features of atopic dermatitis. Acta Derm Venereol
(Stockh) 1980; 92(suppl 144):44–47.
7 Sheehan MP, Rustin MH, Atherton DJ, Buckley C, Harris DW, Brostoff J, Ostlere L, Dawson A: Efficacy of traditional Chinese herbal therapy in adult atopic dermatitis.
Lancet 1992; 340: 13–17.
8 Sheehan MP, Atherton DJ: A controlled trial of traditional Chinese medicinal plants in widespread non-exudative atopic eczema. Br
J Dermatol 1992; 126: 179–184.
9 Leung DY: Atopic dermatitis: new insights and opportunities for therapeutic
interven-tion. J Allergy Clin Immunol 2000; 106: 860–
876.
10 Ge WH, Guo JY, Shen YJ, Chen ML, Shi SL, Han YH, Lin J: Effects of volatile oil of Schizonepeta tenuifolia Briq herb and
Sa-poshnikovia divaricata Schischke root on
proinflammatory cytokine expression and regulation (in Chinese). Zhongguo Zhong
Yao Za Zhi 2007; 17: 1777–1779.
11 Wang CC, Chen LG, Yang LL: Inducible ni-tric oxide synthase inhibitor of the Chinese
herb I. Saposhnikovia divaricata (Turcz.)
Schischk. Cancer Lett 1999; 145: 151–157.
12 Kim H, Lee E, Lee S, Shin T, Kim Y, Kim J: Effect of Rehmannia glutinosa on immediate type allergic reaction. Int J
Immunopharma-col 1998; 20: 231–241.
13 Yang T, Jia M, Meng J, Wu H, Mei Q: Immu-nomodulatory activity of polysaccharide isolated from Angelica sinenesis. Int J Biol
Macromol 2006; 39: 179–184.
14 Cheng A, Wan F, Wang J, Jin Z, Xu X: Mac-rophage immunomodulatory activity of
polysaccharides isolated from Glycyrrhiza
uralensis Fish. Int Immunopharmacol 2008;
8: 43–50.
15 Perharic L, Shaw D, Leon C, De Smet PA, Murray VS: Possible association of liver damage with the use of Chinese herbal med-icine for skin disease. Vet Hum Toxicol 1995;
37: 562–566.
16 Perharic-Walton L, Murray V: Toxicity of
Chinese herbal remedies. Lancet 1992; 340:
674.
17 Graham-Brown R: Toxicity of Chinese
herb-al remedies. Lancet 1992; 340: 673–674.
sleep conditions. There were no obvious adverse events
noted during the intervention period. The results of this
study suggest that XFS formula can be a useful treatment
for patients with recalcitrant atopic dermatitis. However,
additional randomized, controlled trials with adequate
sample sizes need to be conducted to corroborate our
findings.
Acknowledgements
This clinical trial was supported by the Department of Health, Committee on Chinese Medicine and Pharmacy. We also thank Sheng Chang Pharmaceutical Co. Ltd. for the preparation of pla-cebo.
IAA318861.indd 8