© 2002 Wiley-Liss, Inc.
Superoxide Dismutase Activities of Spermatozoa and Seminal
Plasma Are Not Correlated With Male Infertility
Yao-Yuan Hsieh,
1Yu-Ling Sun,
2Chi-Chen Chang,
1Yi-San Lee,
2Horng-Der Tsai,
1and Chich-Sheng Lin
2*
1Department of Obstetrics and Gynecology, China Medical College Hospital, Taichung, Taiwan 2Department of Biological Science and Technology, National Chiao Tung University, Hsinchu, Taiwan
Abnormal reactive oxygen species (ROS) production is associated with defective sperm function. Superoxide dismutase (SOD) is related with the scavenging of seminal ROS. We aimed to determine the effect of SOD activities of spermatozoa and seminal plasma on sperm quality. Semen samples from infertile couples who con-sented to the analyses were divided into two groups: 1) normospermia (n = 20); and 2) oligoasthenozoospermia (n = 31). The SOD activities of the spermatozoa and seminal plasma were measured by determining the inhibition of pyrogallol autoxidation. The SOD activities of spermatozoa and semi-nal plasma in both groups were compared. The relationships between the SOD activi-ties and the sperm qualiactivi-ties were deter-mined. We noted that SOD activities of
sperm/seminal plasma in both groups were nonsignificantly different (group 1 vs. 2 = 0.77 ± 0.33/0.84 ± 0.40 U/mg protein for sperm, and 0.66 ± and 0.36/0.83 ± 0.47 U/ ml for seminal plasma). SOD activities of sperm/seminal plasma were positively but nonsignificantly correlated with the sperm motility (SOD of sperm = 0.0008 × motility + 0.67; SOD of seminal plasma = 0.0006 × motility + 0.81) and concentration (SOD of sperm = 0.0006 × concentration + 0.67; SOD of seminal plasma = 0.0021 × con-centration + 0.73). We concluded that SOD activities of sperm and seminal plasma were nonsignificantly correlated with the seminal quality. It appears that the SOD survey is not a useful tool for determining sperm fer-tilization potential. J. Clin. Lab. Anal. 16:127– 131, 2002. © 2002 Wiley-Liss, Inc.
Key words: antioxidant; male infertility; oligoasthenozoospermia; reactive oxygen species; sperm; superoxide dismutase
INTRODUCTION
Reactive oxygen species (ROS) play an important role in human reproduction. Free radicals have beneficial or detri-mental effects upon sperm functions, depending on their na-ture and concentration. The generations of ROS, such as the superoxide anion, hydrogen peroxide, and hydroxyl radical, can result in damage to cell membranes. Spermatozoa are highly sensitive to injuries caused by high ROS concentra-tion (1). Excessive generaconcentra-tion of ROS in semen, mainly by neutrophils but also by abnormal spermatozoa, could be a cause of infertility.
The major ROS generated by human spermatozoa is the superoxide anion. Superoxide dismutase (SOD), an ROS scav-enger, catalyzes the further transformation of superoxide an-ion into hydrogen peroxide (2). The disorder in the regulatan-ion of the SOD may be related with the pathology of certain types of male infertility. Decreased seminal plasma antioxidant ac-tivity and increased ROS production can be responsible for idiopathic male infertility (3). Treatment with oxygen radical scavengers provides significant rescue of testicular function
(4). There are few reports in the literature concerning the ef-fects of SOD of spermatozoa and seminal plasma upon sperm concentration and motility. This study is among the first such reports.
MATERIALS AND METHODS
This study included 51 infertile couples who consented to the semen analyses. Semen samples were obtained by mas-turbation after at least 72 hr of sexual abstinence, less than 30 min after ejaculation. Samples were collected into sterile con-tainers for immediate transportation to the laboratory. This series was approved by the Ethics Committee and
Institu-*Correspondence to: Chich-Sheng Lin, Ph.D., Department of Biological Science and Technology, National Chiao Tung University, 1001 Ta Hsueh Road, Hsinchu, Taiwan. E-mail: lincs@cc.nctu.edu.tw
Received 15 May 2001; Accepted 31 October 2001 DOI: 10.1002/jcla.10029
tional Review Board of the China Medical College Hospital. Informed consents were signed by all couples who donated their semen.
After liquefaction, samples were analyzed for volume, leu-kocyte count, and sperm morphology according to WHO guidelines (5). Then a 6-µl aliquot of each specimen was loaded into a 30-mm microcell slide and subjected to com-puter-assisted semen analysis (CASA) with the use of an HTM-S motility analyzer (Hamilton-Thorne Research, Beverly, MA). All semen samples were divided into two groups: 1) idiopathic infertility (normospermia, n = 20); and 2) male infertility (oligo- or asthenozoospermia, n = 31, count <20 × 106/ml, motility <50%). Then the semen was centri-fuged to separate sperm from seminal plasma. Seminal plasma and homogenate sperm were loaded into an Eppendorf tube and stored in a liquid nitrogen tank before SOD analyses.
SOD activity was measured by determining the inhibition of pyrogallol autoxidation (6). A 0.1-ml sample was added to 2.7 ml of 50 mM Tris-cacodylic acid buffer with 1 mM EDTA (pH 8.2). Then 0.2 ml of 0.2 mM pyrogallol was added to immediately determine changes in absorbance at 420 nm for 5 min. The rate of pyrogallol autoxidation was taken from the decrease in A420 per min. An inhibition of 50% by sample was defined as 1 unit SOD. Results were reported as U/ml for seminal plasma or U/mg of protein for sperm.
The SOD activities in both seminal plasma and sperm in the two groups were determined and compared. The relationships between the SOD activities and the sperm motility or concen-trations were determined. The SAS system with t-test and a linear regression model were utilized for statistical analyses. A P-value of <0.05 was considered statistically significant.
RESULTS
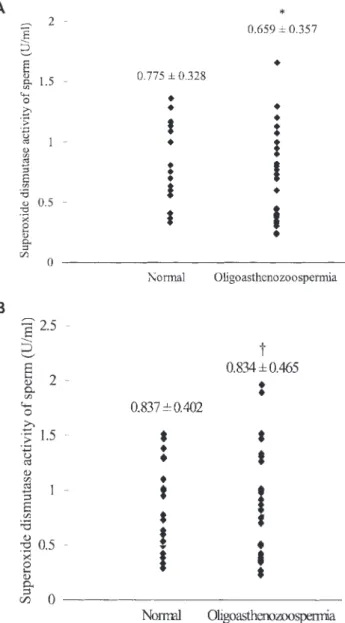
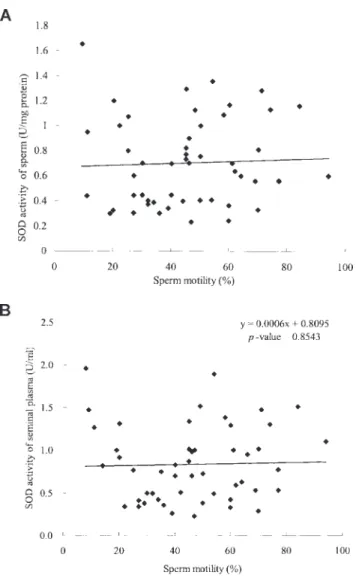
SOD activities of sperm/seminal plasma in both groups were nonsignificantly different (P-value = 0.25/0.99). SOD activities of sperm/seminal plasma in groups 1 and 2 were 0.77 ± 0.33/0.84 ± 0.40 U/mg protein vs. 0.66 ± and 0.36/ 0.83 ± 0.47 U/ml, respectively (Fig. 1A and B). We observed a positive but nonsignificant correlation between the sperm motility and SOD activities of sperm/seminal plasma. We established the formula of their relationship as: SOD = 0.0008
× motility + 0.67/SOD = 0.0006 × motility + 0.81 (P-value = 0.54/0.57) (Fig. 2A and B).
We also noted the positive but nonsignificant correlation between the sperm concentrations and the SOD activity of sperm/seminal plasma. The formula for their relationship was: SOD = 0.0006 × concentration + 0.67/SOD = 0.0021 × con-centration + 0.73 (P-value = 0.43/0.26) (Fig. 3A and B).
DISCUSSION
Excessive generation of ROS in semen, mainly by neutro-phils but also by abnormal spermatozoa, could be a cause for infertility. Abnormal ROS production is associated with
de-fective sperm function (2,7,8). The incidence of spontaneous pregnancy was negatively correlated with ROS production (9). Low concentrations of ROS do not affect sperm viability but do cause sperm immobilization, mostly via depletion of intra-cellular ATP and the subsequent decrease in the phosphoryla-tion of axonemal proteins. Polyunsaturated fatty acids in the phospholipids of human spermatozoa are highly susceptible to peroxidation generated by ROS (10). In fact, ROS have beneficial or detrimental effects on sperm functions depend-ing on the nature and concentration of the ROS involved, as well as the moment and the location of exposure (11).
ROS are related to spermatozoa hyperactivation, capacita-tion, and acrosome reaction (12,13). ROS production is nega-tively related with the sperm-oocyte fusion capacity of human
Fig. 1. Comparisons of superoxide dismutase (SOD) activities of (A) sper-matozoa and (B) seminal plasma in two groups of semen samples (*P-value = 0.25; †P-value = 0.99).
spermatozoa (2). ROS at low concentrations may inhibit sperm-egg fusion via oxidation of the sulfhydryl (SH) pro-teins in the sperm membrane (14). High lipid peroxidation may reduce the capacity of the sperm to undergo acrosomal reaction and fertilization (2). The capability of the spermato-zoa for oocyte fusion was impaired with the addition of hy-drogen peroxide (15).
Significantly higher levels of superoxide anion have been observed in infertile males (8). The positive correlation be-tween superoxide anion level and sperm morphology abnor-malities has been demonstrated (7,8). Lipid hyperoxidation produced by ROS is strongly related with the morphological abnormalities of spermatozoa, primarily in tail defects (16). This could be due to loss of membrane fluidity (17) or to selective inactivation of some of the biochemical pathways leading to acrosomal reaction, such as second messenger
sys-tems (18). Lipid peroxidation triggers the loss of membrane integrity, causing increased cell permeability, enzyme inacti-vation, structural damage to DNA, and cell death (19).
There is controversy about the role of SOD activity in sperm viability. Different studies have reported that: 1) SOD could prevent oxidative damage in sperm (20); 2) diminished SOD activity in seminal plasma was associated with male infertil-ity (21); 3) decreased SOD was responsible for decreased sperm motion and viability (22); 4) high concentrations of SOD prevented the loss of motility in mouse sperm (23); and 5) the addition of SOD to the sperm suspension significantly improved sperm motility (24).
In contrast, some investigators demonstrated that ROS ac-tivity was not influenced by the presence of SOD (15). In fact, in one study sperm SOD activities varied widely be-tween individuals (25). The SOD activity in seminiferous
Fig. 2. The lack of correlation between sperm motility and SOD activity is shown: (A) sperm motility vs. SOD activities of spermatozoa (y = 0.0008x + 0.6679, P-value = 0.7471); and (B) sperm motility vs. SOD activities of seminal plasma (y = 0.0006x + 0.8095, P-value = 0.8543).
Fig. 3. The positive but nonsignificant correlation between sperm con-centration and SOD activities is shown: (A) sperm concon-centration vs. SOD activities of spermatozoa (y = 0.0006x + 0.6714, P-value = 0.4339); and (B) sperm concentration vs. SOD activities of seminal plasma (y = 0.0021x + 0.7263, P-value = 0.2555).
epithelium is regulated over a wide range during spermato-genesis. Another study reported no difference in the SOD activities for spermatozoa or seminal plasma in samples that either did or did not produce ROS (26). Nonsignificant dif-ferences in SOD levels were detected in seminal plasma of fertile and infertile men (27). Higher ROS production in in-fertile patients may be due to increased ROS production rather than defective ROS scavenging activity (28). High SOD ac-tivities reflect errors in spermatogenesis associated with germ cell exfoliation and the retention of excess residual cytoplasm by the spermatozoa (29).
Hydrogen peroxide is the primary toxic ROS for human spermatozoa (30). High concentrations of hydrogen peroxide induce lipid peroxidation and result in cell death. Therefore, the balance of the SOD in semen and sperm is important for maintaining sperm motility. Some agents used for improving sperm quality can influence the SOD activity. Brezezinska-Slebodzinska et al. (31) observed increased SOD and de-creased ROS levels after vitamin E treatment. This suggests that vitamin E may improve the sperm motility and fertiliza-tion rates by reducing the ROS.
In this study, we observed that the SOD level was positive but nonsignificantly correlated with sperm mobility and con-centration. Higher SOD may scavenge the generation of ROS, which may lower the cytotoxicity to human spermatozoa. Higher concentrations of spermatozoa may produce higher levels of SOD. This could interpret their correlation. Although their correlation in this study was nonstatistically different, it may be better defined after larger series are studied. In com-paring the SOD activities between both groups, we observed that the difference in spermatozoa was more significant than that in seminal plasma. This may be due to fact that the SOD and ROS were produced mainly by abnormal spermatozoa and then released into the seminal plasma (32).
In conclusion, the SOD activities in sperm and semen were not significantly correlated with seminal quality. A seminal SOD survey may not be a useful tool for determining sperm fertilization potential. However, the results of this study could provide a database for further research into the effects of SOD on sperm. SOD dysfunction is not the single determiner for sperm regulation, which is a complex process involving many factors. The real roles played by SOD and other antioxidants in sperm quality merit further investigation.
REFERENCES
1. Aiken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil 1987;81:459–469.
2. Aiken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen spe-cies, lipid peroxidation and human sperm function. Biol Reprod 1989;41:183–187.
3. Alkan I, Simsek F, Haklar G, Kervancioglu E, Ozveri H, Yalcin S, Akdas A. Reactive oxygen species production by the spermatozoa of patients with idiopathic infertility: relationship to seminal plasma antioxidants. J Urol 1997;157:140–143.
4. Prillaman HM, Turner TT. Rescue of testicular function after acute ex-perimental torsion. J Urol 1997;157:340–345.
5. World Health Organization. Laboratory manual for the examination of human semen and semen-cervical mucus interaction. 3rd ed. Cambridge: Cambridge University Press; 1992. p. 10–15.
6. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974;47:469–474.
7. Iwasaki A, Gagnon C. Formation of reactive oxygen species in sperma-tozoa of infertile patients. Fertil Steril 1992;57:409–416.
8. Mazzilli F, Rossi T, Marchesini M, Ronconi C, Dondero F. Superoxide anion in human semen related to seminal parameters and clinical as-pects. Fertil Steril 1994;62:862–868.
9. Aitken RJ, Irvine DS, Wu FC. Prospective analysis of sperm-oocyte fusion and reactive oxygen species generation as criteria for the diag-nosis of infertility. Am J Obstet Gynecol 1991;164:542–551. 10. Selly MU, Lacey MJ, Bartlett MR, Copeland CM, Ardlie NG. Content
of significant amounts of a cytotoxic end-product of lipid peroxidation in human semen. J Reprod Fertil 1991;92:291–298.
11. de Lamirande E, Gagnon C. Impact of reactive oxygen species on sper-matozoa: a balancing act between beneficial and detrimental effects. Hum Reprod 1995;10:15–21.
12. Griveau JF, Renard P, Le Lannou D. Superoxide anion production by human spermatozoa as a part of the ionophore-induced acrosome reac-tion process. Int J Androl 1995;18:67–74.
13. de Lamirande E, Gagnon C. A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int J Androl 1993;16:21–25.
14. Mammoto A, Masumoto N, Tahara M, Ikebuchi Y, Ohmichi M, Tasaka K, Miyake A. Reactive oxygen species block sperm-egg fusion via oxidation of sperm sulfhydryl proteins in mice. Biol Reprod 1996;55:1063–1068.
15. Aitken RJ, Buckingham D, Harkiss D. Use of a xanthine oxidase free radical generating system to investigate the cytotoxic effects of reac-tive oxygen species on human spermatozoa. J Reprod Fertil 1993;97: 441–450.
16. Rao B, Souflir JC, Martin M, David G. Lipid peroxidation in human spermatozoa as related to midpiece abnormalities and motility. Gamete Res 1989;24:127–134.
17. Ohyashiki T, Ohtsuka T, Mohri T. Increase of the molecular rigidity of the protein conformation in the intestinal brush-border membranes by lipid peroxidation. Biochim Biophys Acta 1988;939:383–392. 18. De Jonge CJ, Han HL, Lawrie HL, Mack SR, Zaneveld LJD.
Modula-tion of the human sperm acrosome reacton by effectors of adehylate cyclase/cyclic AMP second-messenger system. J Exp Zool 1991; 258:113–125.
19. Halliwell B. Free radicals, antioxidants, and human diseases: curiosity, cause, or consequence? Lancet 1994;344:721–724.
20. Griveau JF, Le Lannou D. Effects of antioxidants on human sperm prepa-ration techniques. Int J Androl 1994;17:225–231.
21. Sanocka D, Miesel R, Jedrzejczak P, Kurpisz MK. Oxidative stress and male infertility. J Androl 1996;17:449–454.
22. Rajasekaran M, Hellstrom WJ, Sparks RL, Sikka SC. Sperm-damaging effects of electric current: possible role of free radicals. Reprod Toxicol 1994;8:427–432.
23. Nonogaki T, Noda Y, Narimoto K, Umaoka Y, Mori T. Effects of super-oxide dismutase on mouse in vitro fertilization and embryo culture sys-tem. J Assist Reprod Genet 1992;9:274–280.
24. Kobayashi T, Miyazaki T, Natori M, Nozawa S. Protective role of su-peroxide dismutase in human sperm motility: susu-peroxide dismutase ac-tivity and lipid peroxide in human seminal plasma and spermatozoa. Hum Reprod 1991;6:987–991.
25. Alvarez JG, Storey BT. Evidence for increased lipid peroxidative dam-age and loss of superoxide dismutase activity as a mode of sublethal
cryodamage to human sperm during cryopreservation. J Androl 1992;13:232–241.
26. Peltola V, Parvinen M, Ahotupa M. Superoxide dismutase activity along rat seminiferous epithelial wave: effects of ethane dimethanesulphonate and 3.0 Gy of X-irradiation. Andrologia 1994;26:79–85.
27. Miesel R, Jedrzejczak P, Sanocka D, Kurpisz MK. Severe antioxidase deficiency in human semen samples with pathological spermiogram parameters. Andrologia 1997;29:77–83.
28. Zini A, de Lamirande E, Gagnon C. Reactive oxygen species in se-men of infertile patients: levels of superoxide dismutase- and cata-lase-like activities in seminal plasma and spermatozoa. Int J Androl 1993;16:183–188.
29. Aitken RJ, Buckingham DW, Carreras A, Irvine DS. Superoxide
dismutase in human sperm suspensions: relationship with cellular com-position, oxidative stress, and sperm function. Free Radic Biol Med 1996;21:495–504.
30. Hernandez Alvarado SR, Guzman Grenfell AM, Hicks Gomez JJ. Oxy-gen reactive species in spermatozoa. Ginecol Obstet Mex 1995;63: 50–54.
31. Brezezinska-Slebodzinska E, Slebodzinski AB, Pietras B, Wieczorek G. Antioxidant effect of vitamin E and glutathione on lipid peroxidation in boar semen plasma. Biol Trace Elem Res 1995;47: 69–74.
32. Griveau JF, Dumont E, Renard P, Callegari JP, Le Lannou D. Reactive oxygen species, lipid peroxidation and enzymatic defence systems in human spermatozoa. J Reprod Fertil 1995;103:17–26.