Original Article
Dyspnea and Its Correlates in Taiwanese
Patients with Terminal Cancer
Tai-Yuan Chiu, MD, MHSci, Wen-Yu Hu, RN, PhD, Bee-Horng Lue, MD, Chien-An Yao, MD, MPH, Ching-Yu Chen, MD, and Susumu Wakai, MD, PhD Hospice and Palliative Care Unit, Departments of Family Medicine (T.-Y.C., C.-A.Y, C.-Y.C.), School of Nursing Science (W.-Y.H.), and Department of Social Medicine (B.-H.L.), College of Medicine and Hospital, National Taiwan University, Taipei, Taiwan, and Department of International Community Health (T.-Y.C., S.W.), Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
Abstract
This study prospectively assessed dyspnea and related bio-psycho-social-spiritual factors— including severity, cause, psychological distress, and fear of death—that were possibly related to dyspnea in 125 terminal cancer patients at admission and two days before their death. At admission, 74 patients had dyspnea, which improved but later worsened. Causes included cachexia, anemia, pleural effusion, and lymphangitis. Quality of life, anxiety, depression, and fear of death improved after admission; anxiety was correlated with dyspnea before death (r⫽ 0.211, P ⬍ 0.05, univariate analysis). Lung infection (odds ratio⫽ 2.29, 95% confidence interval ⫽ 0.68–3.90; multiple regression), airway obstruction (2.27, 1.41–3.13), acidemia (1.82, 0.72-–2.98), and pericardial effusion (1.38, 0.44–2.32) were independent correlates of dyspnea severity at admission (42.8% of explained variance). Before death, airway obstruction, esophageal cancer, pericardial effusion, lung infection, and mediastinal mass were independent correlates of severity (42.7% of explained variance). Comprehensive care, including improved psychospiritual status, can help in controlling dyspnea and enhancing patients’ quality of life. J Pain Symptom Manage 2004;28:123–132.
쑖
2004 U.S. Cancer Pain Relief Committee. Published by Elsevier Inc. All rights reserved.Key Words
Dyspnea, terminal cancer, severity, correlated factors
Introduction
Dyspnea is a common and distressing symp-tom in patients with terminal cancer. It may be difficult to control. Previous studies have shown that as many as 50%–70% of terminal cancer
Address reprint requests to: Tai-Yuan Chiu, MD, MHSci,
No. 7 Chung-Shan South Road, Taipei, Taiwan.
Accepted for publication: November 22, 2003.
쑖2004 U.S. Cancer Pain Relief Committee 0885-3924/04/$–see front matter Published by Elsevier Inc. All rights reserved. doi:10.1016/j.jpainsymman.2003.11.009
patients experience dyspnea during the last 6 weeks of life and that the symptom is aggravated with the progression of disease. Moreover, dys-pnea is often accompanied by anxiety and fear, which severely diminish the patient’s quality of life.1–8Thus, dyspnea usually persists, it can be uncontrollable, and it may be aggravated. This problem greatly challenges the goal of easing the patient’s death and also deeply affects family members and medical professionals. Therefore, the comprehensive management of dyspnea,
especially in the late terminal stage, has become one of the most important issues in palliative care.
To improve therapeutic success, the corre-lates of dyspnea in patients with terminal cancer must be understood. Bruera et al.6 found that anxiety, maximal inspiratory pressure, and the presence of cancer in the lungs are correlates of the intensity of dyspnea in patients with ad-vanced cancer. Dudgeon et al.9 conducted a study in a general cancer population and found that some baseline data, such as a history of smoking, asthma, and chronic obstructive pul-monary disease, are significantly related to the presence of dyspnea. They also found in an-other study that only anxiety remained signifi-cant in a multivariate model based on data from 75 outpatients of a general oncology clinic.10 In a third study, patients had a median of five different abnormalities that could have contrib-uted to their shortness of breath, but only anxi-ety, a history of smoking, and pCO2 levels were statistically significantly correlated with short-ness of breath in 100 terminally ill cancer pa-tients.11Reuben and Mor3 found that 75% of patients with lung involvement reported dys-pnea at some point, but patients with lung or pleural involvement constituted only 39% of these terminally ill patients reporting shortness of breath. Heyse-Moore et al.12found in a study of 150 advanced cancer patients that correla-tion between dyspnea scores and spirometry was low.
The issue of dyspnea and its correlated fac-tors in cancer patients also has drawn attention in Japan. Tanaka et al.13reported that psycho-logical distress and the presence of organic causes, coughing, and pain are significantly cor-related with dyspnea in patients with advanced lung cancer. Their results confirmed that dys-pnea is multifactorial and that a beneficial ther-apeutic strategy might include intervention for psychological distress and pain.
No formal studies of dyspnea and its corre-lated factors have been conducted in patients with terminal cancer in Taiwan, though dyspnea occurs in 56.6% of such patients.8The aim of our study was to investigate factors correlated with dyspnea—including a comprehensive range of medico-psycho-social-spiritual factors—in pa-tients with terminal cancer. The findings may lead to the development of a model for manag-ing dyspnea in terminal cancer patients in Taiwan.
Methods
Patients
All consecutive patients admitted to the hos-pice and palliative care unit of the National Taiwan University Hospital between February 2002 and January 2003 were enrolled in the study. Patients whose cancers were not respon-sive to curative treatment were identified in an initial assessment performed by members of the admissions committee. Patients who met the following inclusion criteria were considered eligible: 1) The patient was conscious and able to communicate, both at admission and in the 48 hours before his or her death, 2) the patient could provide informed consent or verbally agreed to participate, and 3) the patient was not so weak that answering the questions was a major burden. The patients’ physicians and pri-mary nurses determined their eligibility. The selection of patients and the design of this study were approved by both the Department of Health, Executive Yuan, Taiwan, and the ethical committee at the hospital. By the end of the study period, 125 of the 470 consecutive pa-tients met the inclusion criteria and had com-pleted the study.
Instruments
An assessment form was designed after the investigators carefully scrutinized the litera-ture in this area and was used daily. On this form, we recorded the patients’ demographic data, dyspnea scores (on a 0–10 scale), or-ganic causes of dyspnea, psychological status (including anxious and depressed moods), family function, extent of any fear of death, and quality of life. A panel comprising 2 physicians, 2 nurses, 1 psychologist, and 1 chaplain tested the entire instrument for content validity. All members of the panel were experienced in the care of the terminally ill. Each item in the ques-tionnaire was appraised on a scale of 1, “very inappropriate and not relevant,” to 5, “very ap-propriate and relevant.” A content validity index was used to determine the validity of the structured questionnaire; this instrument yielded an index of 0.96. In addition, a pilot study was conducted for 1 month in the same unit. The results of this pilot study further con-firmed the instrument’s content validity and ease of application.
Demographic and Medical Data. Demographic characteristics assessed included sex, age, edu-cation, primary tumor sites, metastasis sites, and survival. The organic causes of dyspnea were classified as follows: cancer (including pleural effusion, airway obstruction, pulmonary mass, mediastinal mass, pericardial effusion, lym-phangitis, ascites, and cachexia); treatment-related causes (including lobectomy, irradia-tion, and chemotherapy); cancer-related causes (including anemia, pulmonary embolism, and infection); and co-morbidities (including chronic obstructive pulmonary disease and heart failure).
Modified Borg Scale of Dyspnea. The instrument for measuring dyspnea was modified from the Borg scale, which was introduced in 1982 as a category scale with ratio properties. Burdon et al.14 adapted this scale to measure the intensity of the sensation of dyspnea. The self-reported scale consisted of a vertical scale labeled 0–10, with corresponding verbal expres-sions of progressively increasing sensation in-tensity, such as “nothing at all” to “maximal.” This is the format most commonly used.15–17 The modified Borg scale has been found to have good relation with visual analogue scale (VAS)18 and currently is used frequently in Taiwan because it is easier for patients to under-stand and familiar to the local medical staff.
Psychological Distress Scale. The psychological distress scale consisted of the subscales for anx-ious mood and depressed mood. The reliability and validity of these subscales have been estab-lished in Taiwanese palliative care units.19The scale of anxious mood had 4 items: easily wor-rying too much, anticipating the most severe condition, fearing that something bad will happen, and easily displaying outbursts of temper. The scale of depressed mood com-prised 5 items: feeling depressed, feeling sad, crying, having an attack of anger, and experi-encing negative emotions in the morning. For each item, patients were asked to rate the extent of their anxious or depressed mood on a 0–3 Likert scale (0⫽ none, 1 ⫽ mild, 2 ⫽ moder-ate, 3⫽ severe). An exploratory factor analysis was used to analyze the measure for construct validity. This process extracted 2 factors: anxiety (easily worrying too much, anticipating the most severe condition, and fearing that
something bad will happen) and depression (easily displaying outbursts of temper, feeling depressed, feeling sad, crying, having an attack of anger, and experiencing negative emotions in the morning). The factor loading for all items was above 0.5. Cronbach alpha values for these 2 factors were 0.93 and 0.88, respectively. The alpha value for all 9 items was 0.92 for this study sample.
Family Function. Family function was assessed by using a family adaptability, partnership, growth, affection, and resolve (APGAR) index, which contained 5 structured questions about family interactions. The scores were as follows: 0⫽ seldom, 1 ⫽ sometimes, and 2 ⫽ always, where a higher score indicated higher quality of family support.20The alpha value for all 5 items was 0.912 for this study sample.
Extent of the Fear of Death. We evaluated the extent of the patients’ fear of death with one item scored on a 1–5 Likert scale (1⫽ not at all, 2⫽ a little bit, 3 ⫽ moderately, 4 ⫽ quite a bit, 5⫽ extremely). A spiritual care team de-signed this measure for use in Taiwanese pa-tients with terminal disease and used it to assess spirituality in Taiwanese patients in palliative care units.21 The chaplains in the study unit used this measure to evaluate each patient.
Single-Item Scale for Quality of Life. Cohen et al. originally designed the single-item scale (SIS) in 1997.22It is a self-reported scale related to the patient’s overall quality of life from his or her perspective: The SIS “considers all parts of my life: physical, emotional, social, spiritual, and financial—my quality of life in the past two days was…very bad (0)/excellent (10).” The SIS score is assumed to be the best available in-dicator of quality of life as perceived by the patient and its Taiwanese version has been ini-tially validated.23
Procedure
Staff members asked patients about the sever-ity of dyspnea and recorded the results on a daily basis in the normal process of caring for them. Otherwise, the staff members evaluated and discussed the causes of dyspnea according to the patient’s history of disease, physical ex-amination findings, imaging studies (chest radiography, computed tomography, magnetic
resonance imaging), and hemoglobin level. The collected data were analyzed at the time of admission, at 1 week after admission, and in the 48 hours prior to the patient’s death (usu-ally retrospectively) in weekly team meetings.
Statistical Analysis
Data management and statistical analysis was performed by using SPSS 11.0 statistical software (SPSS, Chicago, IL). A frequency dis-tribution was used to describe the demographic data and the distribution of each variable. Means and standard deviations (SD) were used to analyze the intensity of dyspnea and psycho-social-spiritual scores. A paired t test was used to compare the differences in the scores for these items at different times. Univariate analy-sis, including the Chi-square test, Fisher’s exact method, independent t test, and Pearson cor-relation coefficient analysis, was performed between the possible correlates (demographic data, anxious and depressed mood, family function, extent of the fear of death, quality of life) and the intensity of dyspnea to identify sig-nificant differences. Afterward, backward step-wise multiple regression analysis was used to investigate significant predictors of dyspnea. A probability value of less than 0.05 was con-sidered to indicate a statistically significant difference.
Results
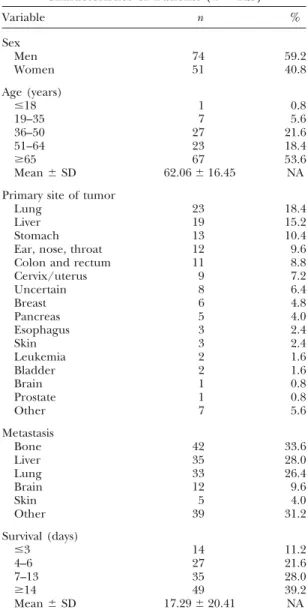
Of 470 consecutive patients with terminal cancer, 125 were conscious and able to commu-nicate 2 days before their death. Characteristics of these 125 evaluable patients are summarized in Table 1. Of them, 74 (59.2%) were men, and 51 (40.8%) were women. A total of 53.6% of the patients were older than 65 years, and only 1 patient was younger than 18 years. The primary sites of cancer were the lung (18.4%), liver (15.3%), stomach (10.4%), and head/ neck (9.6%). Thirty-three patients (26.4%) whose primary sites of cancer were not the lung were found to have lung metastasis, as noted on imaging studies. The mean survival of these patients after admission was 17.29⫾ 20.41 days.
Table 2shows the prevalence and severity of dyspnea. At admission, 74 patients (59.2%) had dyspnea, which was rated mild in 25 patients
Table 1 Characteristics of Patients (n⫽ 125) Variable n % Sex Men 74 59.2 Women 51 40.8 Age (years) ⱕ18 1 0.8 19–35 7 5.6 36–50 27 21.6 51–64 23 18.4 ⱖ65 67 53.6 Mean⫾ SD 62.06⫾ 16.45 NA Primary site of tumor
Lung 23 18.4
Liver 19 15.2
Stomach 13 10.4
Ear, nose, throat 12 9.6 Colon and rectum 11 8.8
Cervix/uterus 9 7.2 Uncertain 8 6.4 Breast 6 4.8 Pancreas 5 4.0 Esophagus 3 2.4 Skin 3 2.4 Leukemia 2 1.6 Bladder 2 1.6 Brain 1 0.8 Prostate 1 0.8 Other 7 5.6 Metastasis Bone 42 33.6 Liver 35 28.0 Lung 33 26.4 Brain 12 9.6 Skin 5 4.0 Other 39 31.2 Survival (days) ⱕ3 14 11.2 4–6 27 21.6 7–13 35 28.0 ⱖ14 49 39.2 Mean⫾ SD 17.29⫾ 20.41 NA NA indicates not applicable.
(20.0%) and moderate or severe in 49 (39.2%). One week after admission, their dyspnea im-proved; 38 (52.8%) of 72 patients who survived longer than 1 week still had this symptom. The severity of dyspnea, compared with the severity at admission, improved; however, the change was not statistically significant (t⫽ 0.065,
P⫽ 0.948). Seventy-five (60.0%) patients had
dyspnea 2 days prior to their death; this symp-tom was moderate or severe in 41.6%. The mean dyspnea score again increased, but the score was still lower than that at admission, and the change was not statistically significant (t⫽ 0.886,
Table 2
Frequency and Severity of Dyspnea (n⫽ 125)
At admission At 1 week 2 days before Dyspneaa (%) (%)b death (%)c None 51 (40.8) 34 (47.2) 50 (40.0) Mild (1–2) 25 (20.0) 15 (20.8) 23 (18.4) Moderate 39 (31.2) 20 (27.8) 46 (36.8) (3–7) Severe 10 (8.0) 3 (4.2) 6 (4.8) (8–10) Total 125 (100.0) 72 (100.0) 125 (100.0) Mean⫾ SD 2.31⫾ 2.67 1.76⫾ 2.24 2.16⫾ 2.39 aNumbers in parentheses are scores.
bAdmission vs. 1 week: t⫽ 0.065; P ⫽ 0.948.
cAdmission vs. 2 days before death: t⫽ 0.886; P ⫽ 0.377.
The organic causes of dyspnea in the pa-tients’ last 48 hours of life are shown inTable 3. Causes of moderate or severe dyspnea included cachexia (86.5%), anemia (80.8%), pleural ef-fusion (73.1%), lung mass (67.3%), airway ob-struction (61.5%), and lymphangitis (59.6%). About 76% of patients without dyspnea also had anemia, 48.0% had ascites, 36.0% had lym-phangitis, and 30.0% had a lung mass.
Table 4shows possible psycho-social-spiritual factors related to the intensity of dyspnea. These included psychological status, family
Table 3
Dyspnea and Organic Causes in the Last 48 Hours of Life (n⫽ 125)
Dyspnea score Cause ⱖ3 (%) ⬍3 (%) 0 (%) Total (%) Cancer Pleural effusion 38 (73.1) 11 (47.8) 13 (26.0) 62 (49.6) Airway obstruction 32 (61.5) 10 (43.5) 9 (18.0) 51 (40.8) Pulmonary mass 35 (67.3) 13 (56.5) 15 (30.0) 63 (50.4) Mediastinal mass 28 (53.8) 14 (60.9) 14 (28.0) 56 (44.8) Pericardial effusion 9 (17.3) 2 (8.7) 1 (2.0) 12 (9.6) Lymphangitis 31 (59.6) 15 (65.2) 18 (36.0) 64 (51.2) Ascites 29 (55.8) 11 (47.8) 24 (48.0) 64 (51.2) Cachexia 45 (86.5) 21 (91.3) 41 (82.0) 107 (85.6) Treatment-related Lobectomy 1 (1.9) 0 (0.0) 0 (0.0) 1 (0.8) Irradiation 9 (17.3) 6 (26.1) 5 (10.0) 20 (16.8) Chemotherapy 14 (26.9) 10 (43.5) 12 (24.0) 36 (28.8) Cancer-related Anemia 42 (80.8) 17 (73.9) 38 (76.0) 97 (77.6) Pulmonary embolism 2 (3.8) 1 (4.3) 1 (2.0) 4 (3.2) Infection 27 (51.9) 6 (26.1) 10 (20.0) 43 (34.4) Co-morbidities COPD 15 (28.8) 5 (21.7) 5 (10.0) 25 (20.0) Asthma 3 (5.8) 2 (8.7) 1 (2.0) 6 (4.8) Heart failure 17 (32.7) 2 (8.7) 10 (20.0) 29 (23.2) Acidemia 16 (30.8) 5 (21.7) 4 (8.0) 25 (20.0) Total 52 (100.0) 23 (100.0) 50 (100.0) 125 (100.0)
COPD⫽ Chronic obstructive pulmonary disease.
function, extent of the fear of death, and qual-ity of life. The mean scores of the 2 subscales for anxiety and depressed mood at admission were 3.68 (range 0–12) and 5.58 (range 0–24), respectively. These scores significantly im-proved in the 48 hours before death (mean⫽ 2.92, P⬍ 0.001; mean ⫽ 4.52, P ⬍ 0.01, respec-tively). The total mean score for family function was 8.13⫾ 2.43 (SD), range 0–10), indicating good family support for the study patients.
Regarding fear of death, the mean score de-clined from 2.45 at admission to 2.06 before death (P⬍ 0.001), indicating the effects of spiri-tual care. As for the quality of life, the mean SIS score increased from 3.16 at admission to 3.74 before death (P⬍ 0.001).
Tables 5 and 6summarize the univariate cor-relations between the severity of dyspnea and significantly different variables at admission and in the 48 hours before death. At the time of admission, only organic causes (such as lung cancer, cervical cancer, lung metastases, pleural effusion, lung mass, and lung infection) were significantly correlated with the severity of dyspnea. Anxiety and fear of death did not show a significant correlation (r⫽ 0.122 and
Table 4
Variables Potentially Related to the Severity of Dyspnea (n⫽ 125)
Variable At admission 2 days before death Range t Value
Psychological status 9.26⫾ 6.938 7.43⫾ 5.901 0–36 3.809c Anxiety mood 3.68⫾ 2.570 2.92⫾ 2.171 0–12 4.419c Easily worrying too much 1.20⫾ 0.897 1.00⫾ 0.791 0–4 2.884b Anticipating the most severe condition 1.22⫾ 0.953 0.97⫾ 0.807 0–4 3.684c Fear something bad will happen 1.25⫾ 0.883 0.95⫾ 0.767 0–4 4.310c Depression mood 5.58⫾ 4.790 4.52⫾ 3.873 0–24 3.019b Temper outbursts 0.78⫾ 0.983 0.60⫾ 0.799 0–4 2.405a Feeling depressed 1.37⫾ 1.044 1.09⫾ 0.906 0–4 3.436c Feeling sad 1.12⫾ 1.031 0.90⫾ 0.894 0–4 2.968b
Crying 0.73⫾ 0.927 0.47⫾ 0.700 0–4 3.295c
Negative emotion in morning 0.71⫾ 0.862 0.59⫾ 0.723 0–4 1.687
Anger attack 1.00⫾ 1.034 0.84⫾ 0.851 0–4 1.942 Family function 8.13⫾ 2.426 NA 0–10 NA Adaptability 1.69⫾ 0.545 NA 0–2 NA Partnership 1.54⫾ 0.644 NA 0–2 NA Growth 1.61⫾ 0.582 NA 0–2 NA Affection 1.57⫾ 0.587 NA 0–2 NA Resolve 1.72⫾ 0.504 NA 0–2 NA Fear of death 2.45⫾ 0.687 2.06⫾ 0.658 1–5 5.910c Quality of life in the last 2 days 3.16⫾ 0.820 3.74⫾ 0.730 1–5 ⫺8.295c NA indicates not applicable.
aP⬍ 0.05. bP⬍ 0.01. cP⬍ 0.001.
Among psychosocial demographic factors, only anxiety was significantly correlated with the severity of dyspnea (r⫽ 0.211, P ⬍ 0.05) in the 48 hours before the patients’ death. Organic causes, such as pleural effusion, airway obstruction, lung mass, pleural effusion, and
Table 5
Univariate Analysis Between Dyspnea Scores
and Potential Factors at Admission (n⫽ 125)
Mean dyspnea
Potential factor score (no/yes) t Value
Primary site of tumor
Lung 2.07/3.39 ⫺2.177a Cervix/uterus 2.17/4.11 ⫺2.127a Lung metastasis 1.92/3.39 ⫺2.785a Cancer Pleural effusion 1.34/3.47 ⫺4.837c Airway obstruction 1.19/4.10 ⫺6.270c Pulmonary mass 1.27/3.33 ⫺4.666c Mediastinal mass 1.75/3.21 ⫺2.870b Pericardial effusion 2.05/6.13 ⫺4.483b Lymphangitis 1.63/3.03 ⫺3.042b Lobectomy 2.77/8.00 ⫺2.170a Cancer-related infection 1.77/3.88 ⫺3.574c Complications COPD 1.94/3.80 ⫺3.230b Asthma 2.18/4.57 ⫺2.345a Heart failure 2.04/3.46 ⫺2.382a Acidemia 1.99/4.22 –3.417b COPD⫽ Chronic obstructive pulmonary disease.
aP⬍ 0.05 bP⬍ 0.01 cP⬍ 0.001.
lung infection, had strongly significant differ-ences (all P ⬍ 0.001) (Table 6). In the study, patients had a median of four different abnor-malities that could have contributed to the in-tensity of dyspnea at admission, and a median of three in the 48 hours before death.
Table 6
Univariate Analysis Between Dyspnea Score and Potential Factors at 2 Days Before Death
(n⫽ 125)
Mean dyspnea
Potential factor score (no/yes) t Value
Primary site of tumor
Stomach 2.31/0.85 3.144a Esophagus 2.02/8.00 ⫺4.628b Lung metastasis 1.80/3.15 ⫺2.860a Cancer Pleural effusion 1.34/3.14 ⫺4.521b Airway obstruction 1.34/3.48 ⫺4.975b Pulmonary mass 1.32/2.98 ⫺4.144b Mediastinal mass 1.70/2.90 ⫺2.647a Pericardial effusion 1.95/5.25 ⫺4.007b Cancer-related infection 1.75/3.34 ⫺3.386b COPD 1.82/3.52 ⫺2.796a Level of consciousness Clear 1.33 ⫺2.566c Drowsy 1.82
Anxious mood (0–9) had a correlation coefficient of 0.211. COPD⫽ Chronic obstructive pulmonary disease. aP⬍ 0.01.
bP⬍ 0.001. cP⬍ 0.05.
Results of multiple regression analysis of the factors correlated with the dyspnea score are shown in Tables 7 and 8. Airway obstruction, pleural effusion, acidemia, and lung infection were independently correlated with the dyspnea score at admission (odds ratio [OR]⫽ 2.27, 95% confidence interval [CI]⫽ 1.41–3.13; OR⫽ 1.38, 95% CI ⫽ 0.44–3.32; OR ⫽ 1.83, 95% CI⫽ 0.72–2.98; OR ⫽ 2.29, 95% CI ⫽ 0.68–3.90, respectively). This model accounted for 42.8% (multiple R2) of the variance in the dyspnea score (Table 7). Multivariate analysis in the patients with dyspnea in the 48 hours before death revealed that airway obstruction, esopha-geal cancer, pericardial effusion, lung infec-tion, and mediastinal mass were independent correlates of the dyspnea score. The model of this multivariate analysis accounted for 42.7% of the variance in the dyspnea score. Psychoso-cial and demographic factors were not in the models of multivariate analysis, either at admis-sion or at 2 days prior to death. This finding indicated that the organic causes were strong correlates of the dyspnea score in these termi-nal cancer patients.
Some studies found that the influence in quality of life due to a specific symptom appears to be detected within intensity values ofⱖ 30% of maximum.6,24,25 Thus the study also per-formed the multiple regression analysis for the moderate and severe intensity (ⱖ 3/10) of dys-pnea. The results showed airway obstruction and lung infection were independent correlates of the dyspnea score ⱖ 3/10 at admission (OR⫽ 1.61, 95% CI ⫽ 0.40–2.90; OR ⫽ 1.38, 95% CI⫽ 0.15–2.61). On the other hand, airway obstruction, sex and esophageal cancer
Table 7
Multiple Regression Analysis of Factors Independently Correlated With Dyspnea
at Admission (n⫽ 125)
Variable Coefficient Beta t Value 95% CI
Airway 2.27 0.40 5.243a 1.41–3.13 obstruction Pericardial 1.38 0.22 2.901b 0.44–2.32 effusion Acidemia 1.82 0.24 3.248b 0.72–2.99 Lung infection 2.29 0.22 2.814b 0.68–3.90 Constant 0.76 NA 2.796b 0.22–1.29 Multiple R value⫽ 0.670; multiple R2⫽ 0.428.
CI indicates confidence interval; NA, not applicable. aP⬍ 0.001.
bP⬍ 0.01.
Table 8
Multiple Regression Analysis of Factors Independently Correlated with Dyspnea at 2 Days
Prior to Death (n⫽ 125)
Variable Coefficient Beta t Value 95% CI Airway 2.23 0.44 4.831a 1.32–3.14 obstruction Esophageal 4.97 0.32 4.420a 2.74–7.20 cancer Pericardial 1.96 0.25 3.220b 0.75–3.16 effusion Lung 1.11 0.22 2.903b 0.35–1.87 infection Mediastinal ⫺0.95 ⫺0.19 ⫺2.036c ⫺1.87–0.02 mass Constant 1.01 NA 3.950a 0.50–1.52 Multiple R value⫽ 0.673; Multiple R2⫽ 0.427.
CI indicates confidence interval; NA, not applicable. aP⬍ 0.001.
bP⬍ 0.01. cP⬍ 0.05.
were independent correlates of the dyspnea score ⱖ 3/10 in the 48 hours before death (OR⫽ 1.76, 95% CI ⫽ 0.92–2.61; OR ⫽ ⫺1.12, 95% CI⫽ ⫺1.95–0.29; OR ⫽ 2.30, 95% CI ⫽ 0.57–4.02, respectively).
Discussion
To our knowledge, this study is one of the first to prospectively reveal the correlates of dys-pnea in terminal cancer patients, particularly in patients with a Confucian culture. Previous studies showed that organic causes are signifi-cantly associated with the presence and inten-sity of dyspnea.6,9,13 However, Bruera et al.6 found that lung involvement was not an inde-pendent correlate for the intensity of dyspnea in patients with moderate-to-severe dyspnea. They suggested that better characterization of the severity of lung involvement may be re-quired. On the other hand, Tanaka et al.13 reported that, despite the significant correla-tion of organic causes with dyspnea, these causes accounted for only a relatively small part of the total score in the cancer-related dyspnea scale (multiple R2⫽ 0.098). They indicated the same need for better characterization of or-ganic causes. Therefore, in our study, we characterized the organic causes related to the severity of dyspnea and grouped them into several categories, including cancer, treat-ment-related, cancer-related, and co-morbidi-ties. This characterization can be helpful for the
evaluation and management of possible organic factors to improve the severity of dyspnea. Meanwhile, this characterization contributed to the multivariate model that accounted for 42.8% (at admission) and 42.7% (2 days before death) of the variation scores, which were higher than in previous studies.
In previous studies, psychological distress has been correlated with dyspnea.6,9–11,13However, we believed that the intensity of psychological distress in terminal cancer patients should be related to their cultural background. Hence, we used the psychological distress measure, the reliability and validity of which have been established in a Taiwanese palliative care unit.19 This measure also demonstrated a good level of reliability and validity in this study sample. To evaluate the patient’s spiritual status, we tried to use one item to measure the extent to which patients had a fear of death, which is believed to be an important index of spiritual status. Our spiritual care team developed this measure, which is commonly used to evaluate the spiri-tual status of terminal cancer patients in Taiwan.21 Although the psychospiritual and sociodemographic factors were not in the final model in the study, a higher percentage was achieved in accounting for the variation in dyspnea scores. Future studies to investigate the comprehensive factors related to the sensation of dyspnea are still a worthwhile effort.
Dyspnea is one of the most common symp-toms in patients with terminal cancer. After admission, the frequency of dyspnea decreased and the severity of dyspnea improved in our patients. Nevertheless, the prevalence of dys-pnea increased (60%) again in the late terminal stage. These findings, which were compatible with those of a previous study,8 revealed the difficulties in managing dyspnea in the late terminal stage.
The organic causes that were significantly correlated with the intensity of dyspnea in the multivariate analysis included airway obstruc-tion, pleural effusion, and lung infection. In the hospice, the medical staff carefully calculates all of the medical benefits and risks/burdens for possible treatments (such as palliative radio-therapy, pleurocentesis, and antimicrobial ther-apy) to relieve dyspnea. Any medical procedure can be offered with the patient’s consent (if possible). Medications, including broncho-dilators, corticosteroids, and anxiolytics, and
supportive care, such as nebulizer therapy, breathing exercises, and emotional support, are also provided. In our hospice, invasive treat-ments (such as pleurocentesis and palliative ra-diotherapy) are usually withheld because of the deteriorating condition of patients with termi-nal cancer and because of their short survival. Some patients without dyspnea had lung le-sions or other possible organic causes of dys-pnea. The results in this study also showed a significant correlation between anxiety and the severity of dyspnea in the 2 days prior to death, as demonstrated in the univariate analysis. These findings might be partially explained by the compression of tumors at different loca-tions, but they also allow for the possibility of a nonpharmacologic approach to control dys-pnea. Clinically, we try to provide a warm, caring environment and give full support to terminally ill patients in our hospice. Instead of using benzodiazepines to control dyspnea,26 we try to communicate with our patients about their concerns and ensure their continuous care. In addition, we explain that our medical team is able to give comprehensive care for any distress, including dyspnea.
In Asian countries, some breathing methods are believed to be useful for the management of dyspnea. The Queisee method is a kind of religious breathing exercise practiced in Taoism. This technique helps people to breathe calmly and to use the least amount of oxygen to maintain survival. Most Taiwanese people are familiar with this breathing method, which can help patients relieve their anxiety and effec-tively control their breathing. In addition to introducing the Queisee breathing method, we also explain to our patients that the residual volume in their lungs should be adequate to maintain their body’s real need for oxygen. We usually measure the hemoglobin oxygen satu-ration (which had no significant correlation with dyspnea in previous studies), and we show normal data from the pulse oximeter to patients and their families to decrease their worries. These interventions seem to be useful for con-trolling dyspnea in the study patients. It is worthwhile to further investigate the effects of these methods.
Dyspnea near the late terminal stage often becomes severe and difficult to control.1–11In addition to medical treatment, we often teach patients in the active dying process to
strengthen their religious faith by means of prayer with Buddhist Sanskrit (A-Mi-Tou-Fo). Since more than 80% of people in Taiwan have their faith in the Buddhist philosophy, they believe that prayer with Buddhist words in the stage of imminent death is helpful in their death and afterlife. Prayer involving patients and their families may help distract them from the dyspnea and is useful for releasing anxiety in patients, their families, and staff.
After patients are provided with active total care for dyspnea, the severity could be im-proved after their admission and controlled to the extent possible during the dying process. In this study, several organic causes were inde-pendently correlated with the dyspnea score, both at admission and prior to death. Although reversing these causes is difficult in patients with terminal illness, comprehensive care could still decrease the severity of their dyspnea.
Lung infection was strongly correlated with the severity of dyspnea, both at admission and prior to death. Although the use of anti-microbial agents in terminal patients is contro-versial because the benefits and burdens are unclear,27,28our findings suggest that control-ling lung infections may have an important role in relieving dyspnea.
Certain caveats should be mentioned in rela-tion to our study. First, we attempted to research the correlates of dyspnea in patients with ter-minal cancer, but respiratory function tests were not performed. Results of these test have previously been found to be predictors of the in-tensity of dyspnea. However, these tests may not be a practical tool in patients with terminal cancer because almost all of them are unable to complete these tests. Second, the participants were in a unit for only palliative care, and with a 3-week mean survival, the generalizability of the findings is a concern.
In conclusion, the severity of dyspnea in pa-tients with terminal cancer could be improved with active total care, despite the fact that or-ganic causes may irreversibly progress and de-spite their strong correlation with the intensity of dyspnea. More studies are required to iden-tify other psychosocial-spiritual factors and the sensitivity of these measures.
Acknowledgments
This research was supported by the Depart-ment of Health, Executive Yuan, Taiwan. The
authors are indebted to the faculties of the Department of Family Medicine, National Taiwan University Hospital, particularly the psy-chologist Ms. Y.R. Cheng, and also Ms. K.H. Chao and Ms. Y.P. Pan for their assistance in preparing the manuscript.
References
1. Ventafridda V, Ripamonti C, De Conno F, et al. Symptom prevalence and control during cancer pa-tients’ last days of life. J Palliat Care 1990;6:7–11.
2. Heyse-Moore LH, Ross V, Mullee MA, et al. How much of a problem is dyspnea in advanced cancer? Palliat Med 1991;5:20–26.
3. Reuben DB, Mor V. Dyspnea in terminally ill cancer patients. Chest 1986;89:234–236.
4. Fainsinger R, MacEachern T, Hanson J, et al. Symptom control during the last week of life on a palliative care unit. J Palliat Care 1991;7:5–11.
5. Muers MF, Round CE. Palliation of symptoms in non-small cell lung cancer: a study by the Yorkshire Regional Cancer Organization Thoracic Group. Thorax 1993;48:339–343.
6. Bruera E, Schmitz B, Pither J, et al. The fre-quency and correlates of dyspnea in patients with advanced cancer. J Pain Symptom Manage 2000;19: 357–362.
7. Ripamonti C. Management of dyspnea in ad-vanced cancer patients. Support Care Cancer 1999; 7:233–243.
8. Chiu TY, Hu WY, Chen CY. Prevalence and sever-ity of symptoms in terminal cancer patients: a study in Taiwan. Support Care Cancer 2000;8:311–313.
9. Dudgeon DJ, Kristjanson L, Sloan JA, et al. Dys-pnea in cancer patients: prevalence and associated factors. J Pain Symptom Manage 2001;21:95–102. 10. Dudgeon DJ, Lertzman M, Askew GR. Physiologi-cal changes and cliniPhysiologi-cal correlations of dyspnea in cancer outpatients. J Pain Symptom Manage 2001; 21:373–379.
11. Dudgeon DJ, Lertzman M. Dyspnea in the ad-vanced cancer patient. J Pain Symptom Manage 1998; 16:212–219.
12. Heyse-Moore L, Beynon T, Ross V. Does spirome-try predict dyspnea in advanced cancer? Palliat Med 2000;14:189–195.
13. Tanaka K, Akechi T, Okuyama T, et al. Factors correlated with dyspnea in advanced lung cancer patients: organic causes and what else? J Pain Symp-tom Manage. 2002;23:490–500.
14. Nield M, Kim MJ, Patel M. Use of magnitude estimation for estimating the parameters of dyspnea. Nurs Res 1989;38:77–80.
15. Burdon JGW, Juniper EF, Killian FE, et al. The perception of breathlessness in asthma. Am Rev Respir Dis 1982;126:825–828.
16. Carrieri VK, Janson BS, Jacobs S. The sensation of dyspnea: a review. Heart Lung 1984;13:436–447. 17. Ripamonti C, Bruera E. Dyspnea: pathophysiol-ogy and assessment. J Pain Symptom Manage 1997; 13:220–232.
18. Wilson RC, Jones PW. A comparison of the visual analogue scale and modified Borg scale for the mea-surement of dyspnea during exercise. Clin Sci 1989; 76:277–282.
19. Hung FC, Cheng YR, Chiu TY, et al. Psychosocial problem, coping strategies, and negative feeling in terminal cancer patients. [in Chinese]. Res App Psy-chol 1999;3:79–104.
20. Sprusinska E. The family APGAR index: study on relationship between family function, social sup-port, global stress and mental health perception in women. Int J Occup Med Environ Health 1994;7: 23–32.
21. Bhikkhuni TT, Bhikkhuni MS, Chen CY, et al. Spiritual care for terminal patients with head and neck cancer. [in Chinese]. Taiwan J Hosp Palliat Care 2002;7:269–282.
22. Cohen SR, Mount BM, Bruera E, et al. Validity of the McGill Quality of Life Questionnaire in the palliative care setting: a multi-centre Canadian study demonstrating the importance of the existential domain. Palliat Med 1997;11:3–20.
23. Hu WY, Dai YT, Berry D, et al. Psychometric testing of the translated McGill quality of life ques-tionnaire-Taiwan version in patients with terminal cancer. J Formos Med Assoc 2003;102:97–104. 24. Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimeters. Pain 1997;72:95–97.
25. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singa-pore 1994;23:129–138.
26. Craven J, Sutherland A. Buspirone for anxiety dis-orders in patients with severe lung disease. Lancet 1991;338:249.
27. Patrick H, Heather L, Rudolph M. Antimicrobial use in patients with advanced cancer receiving hos-pice care. J Pain Symptom Manage 2003;25:438–443. 28. Vitetta L, Kenner D, Sali A. Bacterial infections in terminally ill hospice patients. J Pain Symptom Manage 2000;20:326–334.