Ion transport and storage of ionic liquids in ionic polymer conductor
network composites
Yang Liu,1Sheng Liu,1Junhong Lin,2Dong Wang,3Vaibhav Jain,4Reza Montazami,5 James R. Heflin,3Jing Li,6Louis Madsen,6and Q. M. Zhang1,2,a兲
1
Department of Electrical Engineering, Pennsylvania State University, University Park, Pennsylvania 16802, USA
2
Department of Materials Science and Engineering, Pennsylvania State University, University Park, Pennsylvania 16802, USA
3
Department of Physics, Virginia Tech, Blacksburg, Virginia 24061, USA 4
Macromolecular Science and Engineering, Virginia Tech, Blacksburg, Virginia 24061, USA 5
Department of Materials Science and Engineering, Virginia Tech, Blacksburg, Virginia 2406, USA 6
Department of Chemistry, Virginia Tech, Blacksburg, Virginia 24061, USA
共Received 24 February 2010; accepted 16 April 2010; published online 2 June 2010兲
We investigate ion transport and storage of ionic liquids in ionic polymer conductor network composite electroactive devices. Specifically, we show that by combining the time domain electric and electromechanical responses, one can gain quantitative information on transport behavior of the two mobile ions in ionic liquids共i.e., cation and anion兲 in these electroactive devices. By employing a two carrier model, the total excess ions stored and strains generated by the cations and anions, and their transport times in the nanocomposites can be determined, which all depend critically on the morphologies of the conductor network nanocomposites. © 2010 American Institute of Physics. 关doi:10.1063/1.3432664兴
Ionic liquids 共ILs兲, due to many unique and attractive features as electrolytes, have shown a great promise for ap-plications in energy storage, conversion devices, and other electroactive devices.1–11 For example, the negligible vapor pressure of ILs enables these electroactive devices to operate at ambient atmosphere with long life cycles; the wide elec-trochemical window allows these devices to work at higher voltage; and the high ion mobility can lead to fast device response time. All of these properties are highly desirable for electroactive devices such as ionic polymer actuators,1,5–8 supercapacitors,2 batteries,10 fuel cells,11 and dye-sensitized solar cells.9Figure1 presents schematically a typical device configuration for such an electroactive device, which basi-cally has a three-layer structure. The two porous composite electrodes separated by an ion conducting membrane provide high specific electrode area for ion storage under an applied voltage while allowing easy ion transport. One critical issue for ILs in these electroactive devices is the transport process, where fast transport speed is highly desirable. Moreover, the strain generated by the mobile ions in these devices is also of great concern.
This letter investigates the transport and storage behav-iors of ILs in electroactive devices, referred to as the ionic polymer conductor network composites 共IPCNCs兲, as illus-trated in Fig.1. Distinct from traditional electrolytes, ILs are salts in the liquid state with low bonding energy between the cation and anion, which leads to high ion concentration and ionic conductivity. A challenge in the device characterization is how to distinguish transport and other electroactive prop-erties of the two different ions in a composite device con-figuration. Here we show that by combining the time domain electrical impedance and electromechanical response, one
can gain quantitative information of the ion transport and storage in these devices.
A broad range of IPCNCs were selected for the study, which include IPCNCs fabricated with Nafion™ and Aquivion™ ionomers via the direct assembly method6,7,12 and IPCNCs by the layer-by-layer 共LbL兲 self-assembly method.8,13 Two ILs were examined as electrolytes, where 1-ethyl-3-methylimidazolium 共关EMI+兴兲 served as the cation and tetrafluoroborate 共关BF4−兴兲 and trifluoromethanesulfonate 共关Tf−兴兲 served as the anions.14
For the IPCNCs fabricated with the directly assembly method, either the commercial Nafion film NR-211 of thick-ness 25 m or Aquivion from Solvay was chosen as the spacer layer in Fig. 1. RuO2/Nafion 共or RuO2/Aquivion兲 composite was fabricated by mixing RuO2 nanoparticles 共from Alfa Aesar with 13–19 nm diameter兲 with 20% Nafion dispersion from Aldrich 共or 20% Aquivion dispersion from Solvay兲. In this study, a CNC layer with 40 vol % of RuO2 nanoparticles was used. IPCNCs fabricated by the LbL method were made by alternately immersing the Nafion film into two aqueous solutions containing the polycation poly共al-lylamine hydrochloride兲 共PAH兲 as the polyanion and polyca-tion and anionic gold nanoparticles 共2 nm, from Purest Col-loids兲 or CNT 共from Carbon Solutions Inc.兲.8,14
The LbL CNCs comprise 100 bilayers of PAH/Au and 30 bilayers of PAH/CNT, respectively. The lateral dimensions of the
IPC-a兲Electronic mail: qxz1@psu.edu. FIG. 1. 共Color online兲 Schematic of a three layer IPCNC actuator.
APPLIED PHYSICS LETTERS 96, 223503共2010兲
0003-6951/2010/96共22兲/223503/3/$30.00 96, 223503-1 © 2010 American Institute of Physics Author complimentary copy. Redistribution subject to AIP license or copyright, see http://apl.aip.org/apl/copyright.jsp
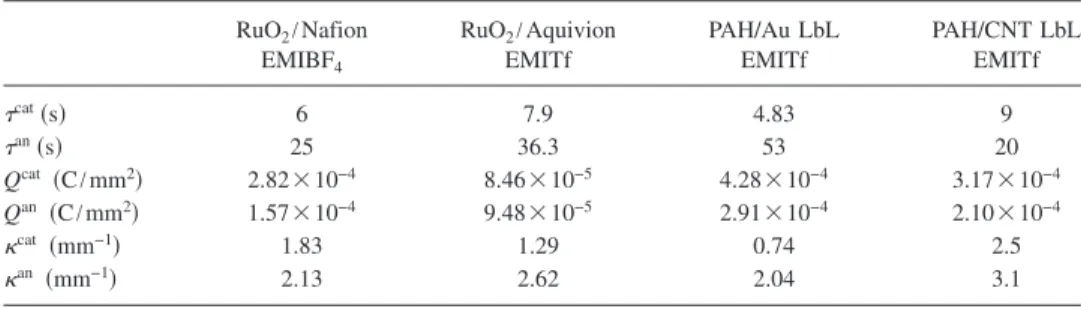
Equations 共1兲 and 共2兲 are used to fit the experimental data 共solid curves in Fig. 3兲 and the fitting parameters are
summarized in TableI. The fitting results indicate thatcatis smaller thanan. That is, the cations have fast transport speed compared with the anions, which causes the initial bending actuation from the expansion of cathode and contraction of the anode. As time progresses, the anions will generate strain in the opposite direction and eventually cause the IPCNCs to bend in the opposite direction. cat and an, as well as the ratio of an/catdepend highly on the electrode morphology and the coupling between the ions and the composite elec-trodes. an/cat for the IPCNCs with RuO
2/Nafion 共=4.16兲 and RuO2/Aquivion 共=4.6兲 are similar. For the IPCNCs with LbL electrode layers,an/catis 10.97 for PAH/Au and 2.22 for PAH/CNT, respectively.
For comparison, the diffusion coefficient from the NMR data and the mobility deduced from the Einstein relation for neat ILs and the EMI-Tf IL in Nafion共NR-212兲 are listed in TableII. Notably, the cation共EMI+兲 has higher mobility than that of anions 共BF4− and Tf−兲, which is consistent with the experimental results presented s. The ratio ofcat/anis 1.27 in neat EMI+BF
4
−and 1.5 in neat EMI+Tf−, which are much smaller than the ratio ofan/catfrom the ion transport in the IPCNCs. These results indicate the crucial role played by the CNC morphology in determining the ion transport in the porous electrodes. Moreover, the large increase in an com-pared withcatsuggests the possible large size共ions plus the solvent or aggregation shells兲 of the anions in the transport process in these composites electrodes.
A comparison of the ratio ofan/Qan withcat/Qcat re-veals that the anions are more effective in generating strain
in these porous electrodes. This again suggests that the an-ions when they are stored in the porous electrodes have much larger solvent or aggregation shells, or that they asso-ciate more strongly with the ionic electrode matrix, resulting in a larger effective ion size and higher strain generated. The results here demonstrate a simple means to compare the ef-fective ion size of the cation and anion of an IL in electro-active devices.
The authors thank Ralph Colby and Wenjuan Liu for discussions. This material is based upon work supported in part by the U.S. Army Research Office under Grant No. W911NF-07-1-0452, Ionic Liquids in Electro-Active De-vices 共ILEAD兲 MURI, and by NSF under the Grant No. CMMI 0709333.
1W. Lu, A. G. Fadeev, B. H. Qi, E. Smela, B. R. Mattes, J. Ding, G. M. Spinks, J. Mazurkiewicz, D. Z. Zhou, G. G. Wallace, D. R. MacFarlane, S. A. Forsyth, and M. Forsyth, Science 297, 983共2002兲.
2M. Ue, M. Takeda, A. Toriumi, A. Kominato, R. Hagiwara, and Y. Ito,J. Electrochem. Soc. 150, A499共2003兲.
3M. Galinski, A. Lewandowski, and I. Stepniak, Electrochim. Acta 51, 5567共2006兲.
4S. Ono, S. Seki, R. Hirahara, Y. Tominan, and J. Takeya,Appl. Phys. Lett.
92, 103313共2008兲.
5Y. Bar-Cohen and Q. M. Zhang, MRS Bull. 33, 173共2008兲.
6D. Kim, K. J. Kim, Y. Tak, D. Pugal, and I.-S. Park,Appl. Phys. Lett. 90, 184104共2007兲.
7M. D. Bennett and D. J. Leo,Sens. Actuators, A 115, 79共2004兲. 8S. Liu, R. Montazami, Y. Liu, V. Jain, M. Lin, J. R. Heflin, and Q. M.
Zhang,Appl. Phys. Lett. 95, 023505共2009兲.
9P. Wang, B. Wenger, R. Humphry-Baker, J. E. Moser, J. Teuscher, W. Kantlehner, J. Mezger, E. V. Stoyanov, S. M. Zakeeruddin, and M. Grat-zel,J. Am. Chem. Soc. 127, 6850共2005兲.
10N. Byrne, P. C. Howlett, D. R. MacFarlane, and M. Forsyth,Adv. Mater. 共Weinheim, Ger.兲 17, 2497共2005兲.
11R. F. de Souza, J. C. Padilha, R. S. Goncalves, and J. Dupont, Electro-chem. Commun. 5, 728共2003兲.
12J. Akle, D. Leo, M. Hickner, and J. McGrath, J. Mater. Sci. 40, 3715 共2005兲.
13V. Jain, H. M. Yochum, R. Montazami, and J. R. Heflin, Appl. Phys. Lett.
92, 033304共2008兲.
14S. Liu, R. Montazami, Y. Liu, V. Jain, M. Lin, X. Zhou, J. R. Heflin, and Q. M. Zhang,Sens. Actuators, A 157, 267共2010兲.
15J. Li, K. G. Wilmsmeyer, J. B. Hou, and L. A. Madsen, Soft Matter 5, 2596共2009兲.
16B. E. Conway and W. G. Pell,J. Solid State Electrochem. 7, 637共2003兲. TABLE I. Summary of fitting parameters for the IPCNCs studied.
RuO2/Nafion EMIBF4 RuO2/Aquivion EMITf PAH/Au LbL EMITf PAH/CNT LbL EMITf cat共s兲 6 7.9 4.83 9 an共s兲 25 36.3 53 20 Qcat 共C/mm2兲 2.82⫻10−4 8.46⫻10−5 4.28⫻10−4 3.17⫻10−4 Qan 共C/mm2兲 1.57⫻10−4 9.48⫻10−5 2.91⫻10−4 2.10⫻10−4 cat共mm−1兲 1.83 1.29 0.74 2.5 an共mm−1兲 2.13 2.62 2.04 3.1
TABLE II. Diffusion coefficients共measured兲 and electrophoretic mobilities 共calculated兲 from pulsed-gradient NMR for the ILs studied.
EMIBF4 EMITf EMITf in Nafiona EMI+ BF
4
− EMI+ Tf− EMI+ Tf− D共10−12 m2s−1兲 43.0 33.7 44.0 29.2 3.86 2.73 共10−9 m2V−1s−1兲 1.67 1.31 1.71 1.14 0.150 0.106 a32 wt % EMITf in Nafion with 2.1 wt % coexisting water. All data are taken at 25 ° C.
223503-3 Liu et al. Appl. Phys. Lett. 96, 223503共2010兲