Time Budget of Polyandrous Pheasant-Tailed Jacana
(Hydrophasianus chirurgus) during Breeding Seaon in Taiwan
Te-Chih Chen
(1), Yao-Sung Lin
(2)and Tzung-Su Ding
(3,4)(Manuscript received 26 November, 2007; accepted 22 April, 2008)
ABSTRACT: The mating system of the Pheasant-tailed Jacana (Hydrophasianus chirurgus) is classical
polyandry. Many hypotheses have been proposed to explain the evolution of polyandry. The replacement clutches hypothesis indicates that it would benefit both sexes if females produce more clutches as replacement when clutch lost rate is high. Females should be freed from parental duty in order to accumulate more energy for producing more replacement clutches and males should carry all or most of parental responsibility. By observing daily time budget of both males and females, we tested whether the replacement clutches hypothesis applied to Pheasant-tail Jacanas in Taiwan. Our results indicated that foraging behavior was the major activity of females in breeding season. Females spent significantly more time on foraging behavior than males during both early and late breeding stages. In males, the breeding behaviors were the dominant activity. Females increased foraging time and reduced breeding time from early to late breeding stage. Males lowered their foraging time and spent more time on breeding behaviors at the late breeding stage. The results were consistent with the predictions of the replacement clutches hypothesis. The time spent on breeding behaviors (mostly incubating behavior) of male Pheasant-tailed Jacanas peaked around noon. We suggest this was due to the high air temperature of the study site during breeding season. Males had to stay in the nests to provide eggs and chicks protection from high temperature and ultraviolet ray.
KEW WORDS: Pheasant-tailed Jacana, mating system, diurnal pattern, evolution, polyandry, parental care, reproductive strategy.
INTRODUCTION
Time budget data are useful in studying the life history and ecological adaptations of birds (Evers, 1994; Hamilton et al., 2002; Jonsson and Afton, 2006), particularly in the evolution of mating system (Andersson, 2005). The mating system of Pheasant-tailed Jacana (Hydrophasianus chirurgus) is classical polyandry (Thong-aree et al., 1995), which is a rare breeding system that the sex roles are reversed. The evolution of classical polyandry has been widely discussed but remains unclear so far (Owens, 2002). Many hypotheses have been proposed to explain the evolution of polyandry. Betts and Jenni (1991) assembled them into three main hypotheses, including female energetic stress hypothesis (Graul et al., 1977), uniparental care hypothesis (Pitelka et al., 1974), and replacement clutches hypothesis (Jenni, 1974; Emlen and Oring,
______________________________________________________________________ 1. Institute of Ecology and Evolutionary Biology, National Taiwan
University, 1, Sec. 4, Roosevelt Rd., Taipei 106, Taiwan. 2. Department of Life Science, National Taiwan University, 1, Sec.
4, Roosevelt Rd., Taipei 106, Taiwan.
3. School of Forestry and Resource Conservation, National Taiwan University, 1, Sec. 4, Roosevelt Rd., Taipei 106, Taiwan. 4.Corresponding author. Tel: 886-2-3366-5263; Email: ding@
ntu.edu.tw
1977). The energetic stress hypothesis suggests that food scarcity and laying-induced energy depletion reduce females’ parental care. If food deficiency is a long-term circumstance, clutch desertion by females will increase. If food supply fluctuates, females will produce multiple clutches for the same or a different male when food supply is abundant (Graul et al., 1977; Lenington, 1980). The uniparental care hypothesis asserts breeding success is greater by uniparental care than biparental care. Uniparental care can reduce the predation risks and food competition between parents and offspring (Pitelka et al., 1974). The replacement clutches hypothesis indicates that when clutch lost rate is high, it would benefit both sexes if females produce more clutches as replacement. In this case, females should be freed from parental duty to accumulate energy for producing replacement clutches. Males will carry all or most of parental responsibility (Jenni, 1974; Emlen and Oring, 1977).
Andersson (2005) proposed three steps that emancipated females from parental duties and prompted the evolution of classical polyandry. He pointed out that the first step was males tended eggs or reared chicks solely. The second step was females had ability to lay more eggs than a male could
accommodate. Females obtained extra energy intake by moving to habitats with rich food supply during breeding season or spending more time in foraging. Thus females had enough energy to increase fecundity. In step three, females competed to lay more clutches for different males sequentially. Females who laid more clutches could gain more reproductive benefit. Polyandrous females raised more offspring than monogamous pairs (Wiktander et al., 2000).
This study systematically collected time budget data of the Pheasant-tailed Jacana in Taiwan to test the replacement clutches hypothesis. If the replacement clutches hypothesis is correct, the reproductive strategies of males and females shall differ and be reflected in their time budgets (Maynard, 1977), particularly in foraging and breeding behaviors (Betts and Jenni, 1991). Following the explanations of Andersson (2005), we proposed and tested three predictions of the replacement clutches hypothesis. First, after laying, males will reduce their foraging time and spend most their time on incubation and tending chicks. Second, females will spend less time on incubation and tending chicks than males. Third, the foraging time of females will maintain at a steadily high level to accumulate more energy for producing more eggs after laying the first clutch.
MATERIALS AND METHODS The breeding system of Pheasant-tailed Jacana is polyandry in general. Males take most duties of incubation and parental care. The breeding season is from late April to mid-October in the study site. The copulative period usually takes three to nine days and females generally lay one egg per day. Clutch size ranges from one to five and four eggs per clutch is the most common form. Eggs take 28.1 days to incubate in average. Males have strong territoriality during breeding season. Chicks are precocious and feed with males after hatching. Males tend the chicks for four to five weeks before chicks become independent, and then males will re-mate with females if it is possible (Jung-Syuan Weng and Jiang-Ping Wang, unpublished data). We observed 391 clutches from year 2002 to 2007. The incubation rate of total clutches was 50.97% (739 chicks from 1450 eggs) and the 20 days survival rate of all chicks was 78.62% (581/739 chicks).
We observed and recorded the daily activity time budget data of the Pheasant-tailed Jacanas in Guan-Tian Pheasant-tailed Jacana Preserve (Tainan county) during the breeding seasons (from late April
to early September) of 2003 and 2004. Data were collected by continuous focal animal sampling method with continuous recording in minutes from sunrise to sunset. The observed individuals were randomly selected and their activities were continuously monitored through binoculars and spotting scopes until the birds flew out of sight. Although only one male and one female were color banded, we could identify each individual by their plumage and territorial behavior. When collecting data, the observer was always in a blind or behind fence in order to ensure the behavior of birds was not influenced by the presence of the observer.
The breeding status of the Pheasant-tailed Jacanas was divided into four reproductive periods: (1) pre-laying, the copulative period, from the first day of mating till the day the female laid the first egg; (2) laying, the laying period, from the day the female laid the first egg till the day the female laid the last egg (generally one egg per day); (3) incubation, the period after laying finished and till the first chick appeared; and (4) brooding, the period after the first chick appeared and till all chicks fledged. We combined the pre-laying and laying data into early breeding stage and grouped the incubation and brooding data as the late breeding stage for statistical comparison. For the females that mate with more than one male, the periods after laying of the first clutch finished and till re-mating with another male were grouped into late breeding stage; and the periods after re-mating and till laying of the second clutch finished were classified as early breeding stage.
The behaviors of the Pheasant-tailed Jacanas were divided into 10 categories: (1) foraging, frequently pecking at food or peering at vegetation when walking or standing; (2) alert, actively observing surroundings with hasty alarm calls; (3) aggressive, hostile interactions involving other jacanas or potential predators; (4) preening, maintenance of feathers including grooming, bathing and stretching; (5) flying, normal short-distance flight; (6) resting, standing without moving or calling; (7) courting, socially displaying toward individuals of the opposite sex or copulating comportment; (8) nest building, pulling at vegetation and push-stepping on nest platform; (9) incubating, sitting on the nest; (10) tending chicks, walking and feeding with chicks and actively observing surroundings without alarm calls; and (11) being disturbed, flying away caused by human activity or unknown reasons. For some behaviors, such as aggressive and flying, that lasted shorter than one minute, we recorded the duration of that behavior as one minute. In total, these
short-duration behaviors took less than 3% of all recorded time. In subsequent analyses, the data of being disturbed behavior were discarded; alert and aggressive behaviors were grouped as aggression; preening, flying and resting behavior were grouped as other somatic behaviors; and courting, nest building, incubating and tending behaviors were combined into breeding behaviors.
We recorded the time budget of seven males and five females in 2003 and three males and six females in 2004. The total observed time was 25,253 minutes in 2003 and 21,130 minutes in 2004. The total observed time was 1,660 minutes in 4 males and 14,505 minutes in 10 females during early breeding stage. During late breeding stage, the observed time was 23,593 minutes and 6,625 minutes in 11 males and 4 females respectively. Because of difficulties in field, we could not observe the same individual throughout the whole breeding season. Therefore, time budget data of each individual in either sex and stage were lumped to calculate the percentages of time spent on different behaviors. Nonparametric statistical tests (Wilcoxon Rank Sum Test) were used to detect the difference between sexes and stages. In order to examine the temporal variation of time budget during daytime, behavioral records of all focal birds of either sex and stage were combined every two hours and transformed into percentages. Since our field observation covered the whole breeding season and accumulated at least 1,660 minutes for each sex and breeding stage, we argue that it should be enough to effectively examine the differences in time budgets of different sexes and breeding stages.
All statistical analyses were performed by SYSTAT 11 and ORIGIN 75 software.
RESULTS Time budget difference between sexes
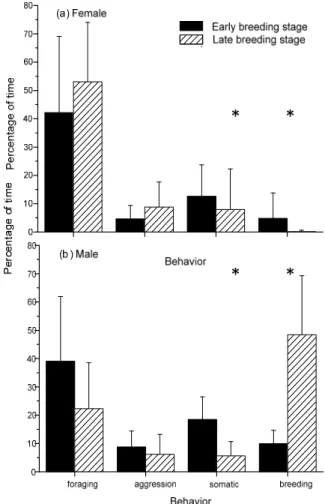
The percentage of time spent on foraging and breeding behaviors differed significantly between sexes (p<0.001). The foraging behavior constituted 45.75% of the observed time of females during the breeding seasons. In males, the breeding behaviors were the dominant activity and occupied 44.44% of the observed time. The foraging behaviors took up 24.01% of males’ time. The females spent more time on foraging behaviors than the males, which engaged in breeding activities more than the females. The females spent significantly more time foraging than males during both early and late breeding stages (early stage p=0.043; late stage p=0.003) (Fig. 1). There were significant differences in other somatic
(p=0.049) and breeding behaviors (p<0.001) between sexes during the late breeding stage. The males spent more time than the females on breeding behaviors during the late breeding stage (Fig. 1b).
Activities at different breeding stages
The percentage of foraging and aggression behaviors did not significantly differ between early and late breeding stages for both sexes. The percentage of other somatic and breeding behaviors differed significantly between early and late breeding stages for both sexes (in male other somatic behaviors, p=0.018; in female other somatic behaviors, p=0.027; in male breeding behaviors, p=0.018; in female breeding behaviors, p=0.001).
The females spent slightly more time, though not statistically significant, on foraging during the late breeding stage (p=0.355) than the early breeding stage. The breeding behaviors fell to zero during the late breeding stage in the females (Fig. 2a).
The males spent more time, with a significant level of 0.063, on foraging during the early breeding stage than the late breeding stage. The breeding behaviors increased dramatically during the late breeding stage in males (Fig. 2b).
The foraging and breeding behaviors showed an opposite trend between sexes (Fig. 2). The females increased foraging time and reduced breeding time from early to late breeding stage. However, the males lowered foraging time and spent more time on breeding behaviors in the late breeding stage. Time budgets of both sexes during a day
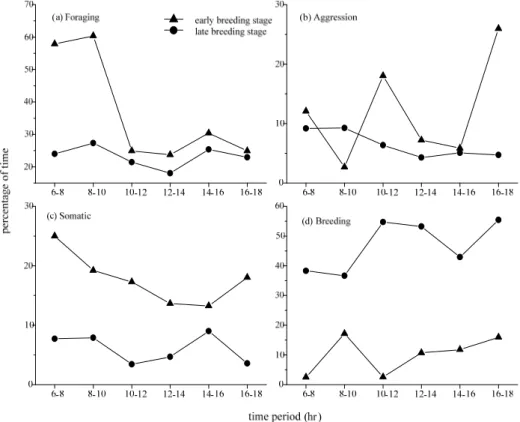
The time spent on each behavioral category in females did not fluctuate much through a day. The females spent 30–60% of time on foraging through a day during early and late breeding stage. The females spent more time foraging during late breeding stage (Fig. 3a).
The aggression and somatic behaviors took less than 20% of in females’ daytime budget and did not fluctuate much during early and late breeding stages (Fig. 3b, 3c). The females spent less than 10% of time on breeding behaviors during the daytime. During early breeding stage, females had a peak of breeding activity in mid-morning and mid-afternoon. After laying finished, the breeding activity decreased (Fig. 3d).
The time budget of the males, instead, fluctuated through a day. During early breeding stage, males spent more time feeding and had a conspicuous peak in the early morning (hour 0600–1000). The foraging time of males during late breeding stage was lower and did not fluctuate much (Fig. 4a).
*
*
*
*
Fig. 1. Time budget of male and female Pheasant-tailed Jacanas at different breeding stages: comparison between males and females. Asterisk indicates statistical significance at 0.05 level. Error bar indicates standard deviation. The number of observed females was 4 in both and late early breeding stages. The number of observed males was 10 in early breeding stage and 11 in late breeding stage.
Fig. 2. Time budget of male and female Pheasant-tailed Jacanas at different breeding stages: comparison between early and late breeding stage. Asterisk indicates statistical significance at 0.05 level. Error bar indicates standard deviation. The number of observed females was 4 in both and late early breeding stages. The number of observed males was 10 in early breeding stage and 11 in late breeding stage.
The time that males spent on aggression behaviors fluctuated greatly during early breeding stage but was relatively stable during late breeding stage. Males spent more time on other somatic behaviors during early breeding stage (Fig. 4b, 4c). The time that males spent on breeding behaviors peaked in hour 0800–1000 and remained stable in afternoon during early breeding stage. The main breeding activities were courtship and copulation during this period. Conversely, the breeding activities of the males peaked at mid-day and before sunset during late breeding stage (Fig. 3d). Incubation and brooding were the main activities at this stage.
DISCUSSION
During the whole breeding season, foraging was the most dominant activity of the female
Pheasant-tailed Jacanas. Females could increase egg production by continual feeding. Thong-aree et al. (1995) studied the breeding biology of Pheasant-tailed Jacana in Thailand. One female he studied produced four clutches in one breeding season when food resources were abundant. Abundant food supplies or effective foraging methods can reduce clutch production time and increase female reproductive ability to produce more clutches (Arnold, 1994; Andersson, 2004). Abundant food supplies also make uni-parental care more successful, thus give females greater opportunities for clutch desertion and become polyandry (Whitfield and Tomkovich, 1996). However, there may be interactions between natural food supply and parental investments, which influence the evolution of avian mating system (Graul et al., 1977). In Kentish plover (Charadrius alexandrinus), food
Fig. 3. Comparison of hourly activity pattern in female Pheasant-tailed Jacanas during early and late breeding stages.
pe
rcentage of time
quantity affected the spatial distribution of broods and the interactions between broods in turn affected the parental behavior. As the density of broods increased when food resource was centralized in one breeding site, the female parents stayed with their broods longer. This situation constrained female desertion and evolution of polyandry (Kosztolányi et al., 2006). Therefore, it is the energy requirement that acts as the limiting factor for the re-mating opportunity of females (Colwell and Oring, 1988). In this study, female Pheasant-tailed Jacanas spent more time foraging and less time on breeding behaviors than males. We conclude that once females are freed from parental responsibility they would have more time to accumulate energy for producing more eggs (Betts and Jenni, 1991). This phenomenon is very important for the evolution of polyandry (Andersson, 2005).
The energetic stress hypothesis suggests that food scarcity and laying-induced energy depletion reduce females’ parental care. In our study site, the food resources are generally abundant during breeding season. There were plenty of aquatic insects (mostly Agrinoidae, Belostomatidae Diplonychus
rusticus and Anisops spp.) and plant seeds (mostly
water lily) in the pond which Pheasant-tailed Jacanas fed (Wang et al., 1998). We suggest that energetic limitation is not an important factor for the parental investments of female Pheasant-tailed Jacanas in Taiwan.
The results showed significant difference in foraging time between sexes during the early breeding stage. It was reasonable that female Pheasant-tailed Jacanas spent more foraging time than males during the early breeding stage. Because egg laying costs lot of energy, females have to feed more to accumulate more energy before laying (Gibson, 1978, McKinney and McWiliams, 2005). The parental investment of polyandrous males has been found to be greater than females (Wesolowski, 1994, Wiktander et al., 2000). Male-only care has played an important role in the evolution of polyandrous mating system (Owens, 2002). The uniparental care hypothesis asserts breeding success by uniparental care is greater than biparental care. Polyandrous Lesser Spotted Woodpecker (Dendrocopus minor) raised 39% offspring more than monogamous pairs (Wiktander et al., 2000). Székely and Reynolds (1995) demonstrated that male care is more common than female care in shorebirds and male-only care may be ancestral state in some families (e.g., Jacanidae). In the Pheasant-tailed Jacana, males take the most
parental duties and females scarcely provide parental care, even if they are monogamous. In addition to parental investment, there would be other factors that affect breeding success, such as previous breeding experience, quality of territory and interaction with surrounding environment. Because there were few banded individuals, the contrast of breeding success in females with different harem size was lack in Pheasant-tail Jacana. The breeding success of female Bronze-winged Jacana (Metopidius indicus) is not significantly associated with harem size (Butchart, 2000). In Jacanidae, only the Lesser Jacana (Microparra capensis) is monogamous and their breeding ecology is still unclear (Hancock, 2001). It needs more detailed information about the breeding success between polyandrouos and monogamous individuals or species to discuss whether the uniparental care hypothesis is suitable for Jacanidae. Our results revealed that male Pheasant-tailed Jacanas’ foraging time decreased and the breeding behavior increased from early to late breeding season. In contrast, females’ foraging time remained high and breeding time remained low during the breeding season. The results are consistent with our predictions. Male Pheasant-tailed Jacanas take almost all parental duties and females can feed continuously to accumulate more energy to produce more clutches. The results support the replacement clutches hypothesis. In polyandrous Wattled Jacana (Jacana jacana), males decrease foraging time during incubation and brooding periods and are the primary providers of chick care (Osborne and Bourne, 1977; Osborne, 1982; Emlen and Wrege, 2004). Male Northern Jacanas (Jacana spinosa) decrease about one third of foraging time during late breeding periods (Betts and Jenni, 1991). In other species of Jacanidae, males perform almost all parental duties and this enables females to produce more replacement clutches under high nest lost situation (Osborne, 1982; Betts and Jenni, 1991; Butchart, 2000; Mace, 2000). In Black Coucal (Centropus grillii), males provide all parental care under high nest predation pressure and polyandrous females have more opportunities to re-mate (Andersson, 1995; Goymann et al, 2004). When males take very high degree of parental care, it will favor females to become polyandrous (Andersson, 2005; Pechacek et al, 2006). Our study provides useful evidence for the replacement clutches hypothesis.
One precondition of the replacement clutch hypothesis is high lost rate of clutches. In Jacanidae, the clutches lost rate was very high. In
Bronze-winged Jacana (Metopidius indicus), the predation rate of eggs was 94.4% (Butchart, 2000). Osborne (1982) reported that the lost rate of clutches was 84.6% in Wattled Jacana. In Comb-crested Jacana (Irediparra gallinacea), 80% clutches were lost before hatching (Mace, 2000). In Pheasant-tailed Jacana, the incubation rate of total clutches was 50.97% (739 chicks / 1450 eggs) and the 20 days survival rate of all chicks was 78.62% (581/739 chicks). The lost rate of clutches was lower than other species of Jacanidae. We proposed that the lower lost rate was associated with conditions of the breeding habitats. In Taiwan, the Pheasant-tailed Jacana breed in water chestnut farm and it was artificial and simple habitat. In general, there were fewer predators in such environment.
Male Pheasant-tailed Jacanas had two foraging peaks during hour 0800–1000 and 1400–1600. It is similar to the Bronze-winged Jacana (Metopidius
indicus), whose foraging time was concentrated in
0700–1100 and 1300–1500 (Ramachandran, 1998). Many bird species feed most intensively at sunrise and sunset, and rest during the middle of the day (Hamilton et al., 2002). The pattern can be regarded as an adaptation to environmental factors. The morning feeding peak may be related to the night fast and energy replenishment. The afternoon feeding peak may be associated with the stirring activity of insects that provides an optimum foraging environment (Gibson, 1978). This diurnal activity pattern, however, was not obvious in female Pheasant-tailed Jacanas. Female Pheasant-tailed Jacanas maintained a high level in foraging activity through a day, especially during late breeding stage. We presume this was related to energy accumulation for producing more clutches.
Male Pheasant-tailed Jacanas had a mid-day peak of breeding behaviors during late breeding stage, mostly incubation. We suggest the high temperature was a main factor. Air temperature and solar radiation were high during the breeding seasons in the study site. Around mid-days, males had to stay on the nest to provide protection from high temperature and ultraviolet rays.
Time budget of birds is affected by many factors, such as food abundance, air temperature, protection from exposure and human disturbance (Gibson, 1978; Mckinney and Mcwiliams, 2005). Data of time budget and daily activity pattern can be of use in environment monitoring, evaluating the habitat suitability and population management (Evers, 1994; Hamilton et al., 2002; Jonsson and Afton, 2006). Our study provided the detailed numerical data of time budget and diurnal activity of the Pheasant-tailed
Jacana and should be useful in planning the conservation and population management strategy for the Pheasant-tailed Jacana in Taiwan.
ACKNOWLEDGEMENTS
We thank two anonymous referees for their helpful suggestions on the earlier drafts. This study was supported by the Council of Agriculture, Executive Yuan of Taiwan to YSL (93AS-4.1.1-FC-R2). We are grateful to the staff of Guan-Tian Pheasant-tailed Jacana Preserve for their support during the study period.
LITERATURE CITED
Andersson, M. 1995. Evolution of reversed sex roles, sexual size dimorphism, and mating system in coucals (Centropodidae, Aves). Biol. J. Linn. Soc. 54: 173-181.
Andersson, M. 2004. Social polyandry, parental investment, sexual selection, and evolution of reduced female gamete size. Evolution 58: 24-34. Andersson, M. 2005. Evolution of classical
polyandry: three steps to female emancipation. Ethol. 111: 1-23.
Arnold, T. W. 1994. Effects of supplemental food on egg production in American Coots. Auk 111: 337-350.
Betts, B. J. and D. A. Jenni. 1991. Time budgets and adaptiveness polyandry in Northern Jacanas. Wilson Bull. 103: 578-597.
Butchart, S. H. M. 2000. Population structure and breeding system of the sex-role reversed, polyandrous Bronze-winged Jacana Metopidius
indicus. Ibis 142: 93-102.
Colwell, M. A. and L. W. Oring. 1988. Breeding biology of Wilson's Phalarope in southcentral Saskatchewan. Wilson Bull. 100: 567-582. Emlen, S. T. and L. W. Oring. 1977. Ecology, sexual
selection and the evolution of mating system. Science 197: 215-223.
Emlen, S. T. and P. H. Wrege. 2004. Size dimorphism, intrasexual competition, and sexual selection in Wattled Jacana (Jacana jacana), a sex-role-reversed shorebird in Panama. Auk. 121: 391-403.
Evers, D. C. 1994. Activity budgets of a marked Common Loon (Gavia immer) nesting population. Hydrobiologia 279-280: 415-420. Gibson, F. 1978. Ecological aspects of the time
budget of the American Avocet. Am. Midl. Nat. 99: 65-82.
Goymann, W., A. Wittenzellner and J. C. Wingfield. 2004. Competing females and caring males. Polyandry and sex-role reversal in African Black Coucals, Centropus grillii. Ethol. 110: 807-823. Graul, W. D., S. R. Derrickson and D. W. Mock.
1977. The evolution of avian polyandry. Am. Nat. 111: 812-816.
Hamilton, A. J., I. R. Taylor and G. Hepworth. 2002. Activity budgets of waterfowl (Anatidae) on a waste-stabilisation pond. Emu 102: 171-179. Hancock, P. 2001. Lesser Jacana. Toeing the line?
Africa Birds and Birding 6: 46-53.
Jenni, D. A. 1974. Evolution of polyandry in birds. Am. Zool. 14: 129-144.
Jonsson, J. E. and A. D. Afton. 2006. Different time and energy budgets of Lesser Snow Geese in rice-prairies and coastal marshes in southwest Louisiana. Waterbirds 29: 451-458.
Kosztolányi, A., T. Székely, I. C. Cuthill, K. T. Yilmaz and S. Berberoğlu. 2006. Ecological constraints on breeding system evolution: the influence of habitat on brood desertion in Kentish Plover. J. Anim. Ecol. 75: 257-265.
Lenington, S. 1980. Bi-parental care in Killdeer: an adaptive hypothesis. Wilson Bull. 92: 8-20. Mace, T. R. 2000. Time budget and pair-bond
dynamics in the Comb-crested Jacana Irediparra
gallinacean: a test of hypothesis. Emu. 100:
31-41.
Maynard, S. J. 1977. Parental investment: a prospective analysis. Anim. Behav. 25: l-9. McKinney, R. A. and S. R. McWiliams. 2005. A new
model to estimate daily energy expenditure for wintering waterfowl. Wilson Bull. 117: 44-55. Osborne, D. R. 1982. Replacement nesting and
polyandry in the Wattled Jacana. Wilson Bull. 94: 206-208.
Osborne, D. R. and G. R. Bourne. 1977. Breeding behavior and food habits of the Wattled Jacana. Condor. 79: 98-105.
Owens, I. P. F. 2002. Male only care and classical polyandry in birds: phylogeny, ecology and sex differences in remating opportunities. Philos. Trans. R. Soc. Lond. B. 357: 283-293.
Pechacek, P., K. G. Michalek, H. Winkler and D. Blomqvist. 2006. Classical polyandry found in the Three-toed Woodpecker Picoides tridactylus. J. Ornithol. 147: 112-114.
Pitelka, F. A., R. T. Holmes and S. F. MacLean, JR. 1974. Ecology and evolution of social organization in Arctic Sandpipers. Am. Zool. 14: 185-204.
Ramachandran, N. K. 1998. Activity patterns and time budgets of the Pheasant-tailed
(Hydrophaslanus chirurgus) and Bronzewinged (Metopidius indicus) Jacanas. J. Bombay Nat. Hist. Soc. 95: 234-245.
Rossmanith, E., V. Grimm, N. Blaum and F. Jeltsch. 2006. Behavioural flexibility in the mating system buffers population extinction: lessons from the Lesser Spotted Woodpecker Picoides
minor. J. Anim. Ecol. 75: 540-548.
Székely, T. and J. D. Reynolds. 1995. Evolutionary transitions in parental care in shorebirds. Proc. R. Soc. Lond. B. 262: 57-64.
Thong-aree, S., O. Khobkhet, V. Lauhachinda and S. Pong-Umpai. 1995. Breeding biology of Pheasant-tailed Jacana Hydrophasianus
chirurgus in central Thailand. Nat. Hist. Bull.
Siam. Soc. 43: 289-302.
Wang, J.-P., Y.-T. Ueng and J.-J. Perng. 1998. Water quality, aquatic plankton and insects in the habitat of Hydrophasianus chirurgus. Essays of the 4th. Conference on coastal wetlands ecology and conservation 1998: 8-23. (in chinese)
Wesolowski, T. 1994. On the origin of parental care and the early evolution of male and female parental roles in birds. Am. Nat. 143: 39-58. Whitfield, D. P. and P. S. Tomkovich. 1996. Mating
system and timing of breeding in Holarctic waders. Biol. J. Linn. Soc. 57: 277-290.
Wiktander, U., O. Olsson and S. G. Nilsson. 2000. Parental care and social mating system in the Lesser Spotted Woodpecker Dendrocopus minor. J. Avian Biol. 31: 447-456.