483
*To whom correspondence and reprint requests should be addressed. Tel and Fax: 886-2-23629842. E-mail:chilu@ntu.edu.tw
T
he sailfish (Istiophorus platypterus )(Istiophoridae) is widely distributed in the tropical and temperate oceans of the world. Based on data from longline catches, sailfish are distributed between 30
°
S and 50°
N in the Pacific Ocean, with the highest density occurring in waters influ-enced by the warm Kuroshio Current of the west-ern Pacific (Nakamura 1985). In Taiwan, sailfish are abundant from Apr. to Oct. with the peak abun-dance from May to July, and they substantially contribute to the economy of the east coast of Taiwan. Sailfish are taken primarily by drift gillnet, set nets, harpoon fisheries, and as incidental bycatches in inshore longline fisheries (Chiang et al. 2004). For the past 10 yrs, the annual landings of sailfish in Taiwan have greatly fluctuated,rang-ing from 1338 metric tons (MT) in 1994 to 574 MT in 1996, to 1021 MT in 1998 and then to 496 MT in 1999. In 2001, the landing increased to 1006 MT and then decreased to 801 MT in 2003.
In the Pacific Ocean, sailfish appear to spawn throughout the year in warm tropical waters with the peak spawning time occurring in the local sum-mer season (Beardsley et al. 1975, Nakamura 1985). Herández-Herrera et al. (2000) used the frequency of ovaries containing hydrated oocytes to measure the average intervals between 2 con-secutive spawning events as 3.6 d for the eastern Pacific sailfish off the coast of Mexico. Eldridge and Wares (1974) counted the oocytes with diam-eters ranging 0.9-1.3 mm, and estimated the fecundity to be (1.8-5.1) x 106eggs for 4 females
Spawning Frequency and Batch Fecundity of the Sailfish (Istiophorus
platypterus) (Istiophoridae) in Waters off Eastern Taiwan
Wei-Chuan Chiang1,2, Chi-Lu Sun1,*, Su-Zan Yeh1, Wei-Cheng Su3, and Don-Chung Liu3
1Institute of Oceanography, National Taiwan University, Taipei, Taiwan 106, R.O.C.
2Present address: Eastern Marine Biology Research Center of Fisheries Research Institute, Council of Agriculture, Executive Yuan,
Taitung, Taiwan 961, R.O.C.
3Fisheries Research Institute, Council of Agriculture, Executive Yuan, Keelung, Taiwan 202, R.O.C.
(Accepted December 22, 2005)
Wei-Chuan Chiang, Chi-Lu Sun, Su-Zan Yeh, Wei-Cheng Su, and Don-Chung Liu (2006) Spawning fre-quency and batch fecundity of the sailfish (Istiophorus platypterus) (Istiophoridae) in waters off eastern Taiwan.
Zoological Studies 45(4): 483-490. The sailfish (Istiophorus platypterus) (Istiophoridae) is widely distributed in
tropical and temperate oceans of the world. In Taiwan, sailfish are abundant from Apr. to Oct. (with the peak abundance from May to July) and substantially contribute to the economy of the east coast of Taiwan. In this study, we estimated the spawning frequency and batch fecundity of female sailfish off eastern Taiwan based on histological examinations of the ovaries of 246 sailfish collected at the Shinkang Fish Market in eastern Taiwan between Jan. 1999 and Sept. 2000. The proportion of mature females with ovaries containing postovulatory follicles was 0.53 on average during the spawning season of Apr. to Sept., suggesting that on average, the sail-fish spawns every 1.89 d. Sailsail-fish are multiple spawners with asynchronous oocyte development. Batch fecun-dity for 18 females with the presence of hydrated oocytes but without postovulatory follicles yielded (0.2-2.48) x 106eggs with an average of 1.3 x 106eggs. The relationships between batch fecundity in millions (BF) versus
round weight (RW, in kg) and lower jaw fork length (LJFL, in cm) are BF = 0.0944 x RW - 1.3134 (r2= 0.724)
and BF = 5.46 x 10-5LJFL6.283(r2= 0.667), respectively. http://zoolstud.sinica.edu.tw/Journals/45.4/483.pdf
(with eye fork lengths ranging 163-187 cm) from the Gulf of California. Herandez-Herrera et al. (2000) used mature ovaries with hydrated oocytes but without postovulatory follicles to estimate the batch fecundity values to be (0.42-2.5) x 106eggs
for 21 females (with eye fork lengths ranging 155-190 cm) of the eastern Pacific sailfish. Information on the western Pacific sailfish reproductive charac-teristics is, however, scarce.
Fecundity data are essential for calculating the spawning stock ratio (SSR) in order to evaluate management regulation (Cuellar et al. 1996). Female sailfish are multiple spawners with asyn-chronous oocyte development (DeSylva and Breder 1997, Hernández-Herrera et al. 2000), which results in an indeterminate fecundity pattern. Therefore, it is necessary to use the“postovulatory follicle”method (Hunter and Goldberg 1980, Hunter and Macewicz 1985a) to determine spawn-ing frequency for this species, and the“hydrated oocyte”method (Hunter et al. 1985) needs to be used to assess the batch fecundity (the number of eggs produced in a single spawning batch). To date, there has been no histological examination of the reproductive characteristics of sailfish in the northwestern Pacific Ocean. The objectives of this study were to estimate the spawning frequency and batch fecundity to quantify reproductive char-acteristics of sailfish in waters off eastern Taiwan using the histological“postovulatory follicle”and “hydrated oocyte”methods.
MATERIALS AND METHODS
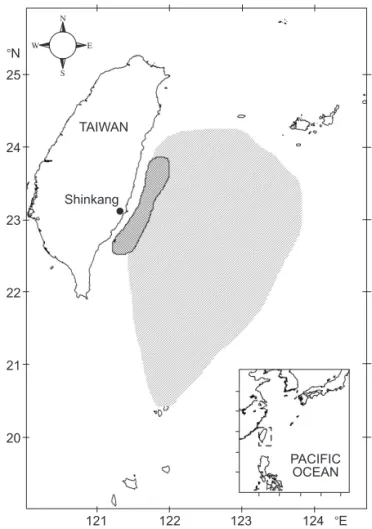
Gonad samples of sailfish were collected monthly during Jan. 1999 to Sept. 2000 from catches landed at the Shinkang Fish Market. The catches were mainly taken by gillnet with a 15.8 cm mesh during Apr. and Oct. and by offshore longline year-round. Both fisheries operate in the waters off eastern Taiwan (Fig. 1). Sailfish are caught at night with gillnets being set after sunset and hauled in around sunrise. Longlines are usu-ally set 2 times at dawn and in the late afternoon, and sailfish are caught in the morning and evening.
The size measurements taken for each sam-pled fish included the lower jaw fork length (LJFL, cm) measured from the tip of the lower jaw to the distal end of the central ray of the caudal fin, and eye fork length (EFL, cm) measured from the pos-terior margin of the eye,s bony orbit to the distal end of the central ray of the caudal fin. The weight
was measured as round weight (RW, kg), which is the total weight excluding the bill, for each fish sampled.
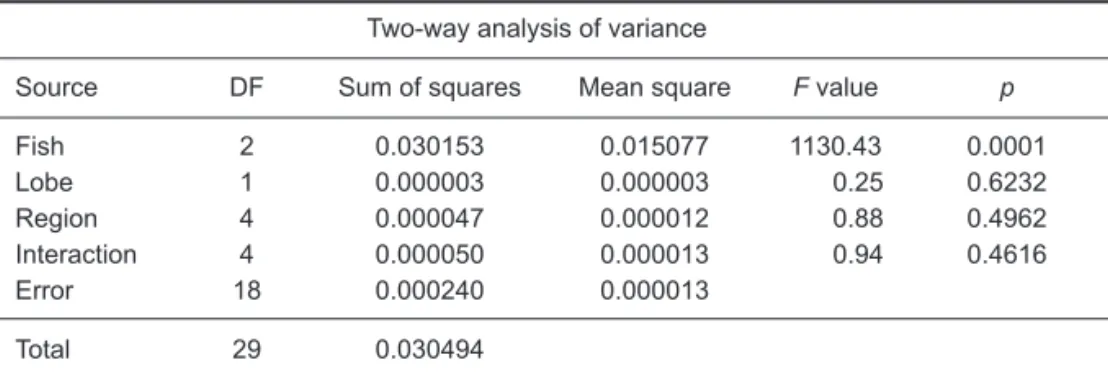
Gonads were removed and weighed to the nearest gram. Two-way analysis of variance was used to determine the appropriate locations for sub-sampling the ovaries. Data were structured by examining the ovarian lobe (left or right) and 5 regions (anterior, mid-anterior, middle, mid-posteri-or, and posterior) of 3 mature fish. No significant differences were found in the numbers or diame-ters of oocytes among the 5 regions within the ovary or between the right and left ovaries (p > 0.05; Table 1). For easy sampling, ovarian sam-ples were taken from the posterior portion of each gonad and fixed in 10% buffered formalin for later histological analyses and oocytes counting.
A portion of tissues (about 1 cm3) was
removed from the center of each ovarian sample, dehydrated in methanol, cleared in benzol, and embedded in paraffin. Tissues were sectioned at approximately a 6 µm thickness, and stained with Meyer,s hematoxylin followed by eosin
counter-TAIWAN °N °E 25 24 23 22 21 20 Shinkang PACIFIC OCEAN 121 122 123 124
Fig. 1. Fishing grounds of gillnet (cross lines) and longline (oblique lines) fishing boats based in the Shinkang fishing port, eastern Taiwan.
N
W E
staining. Histology was used to define develop-mental oocyte stages following several authors (Forberg 1982, Hunter et al. 1992, Arocha 2002). The description of the stages of postovulatory folli-cle (POF) degeneration was adapted from that given by Schaefer (1996) for yellowfin tuna (Thunnus albacares), and these stages were clas-sified as early, middle, and late stages.
Oocyte size was measured by the diameter of oocytes on histological slides using the Image-Pro Plus software after calibration against an optical micrometer. However, histological sectioning deforms the oocyte from its sphere-like shape, and 3 different types of measurements were used (Arocha 2002). Early developed oocytes were measured using the major axis crossing the nucle-us; maturing oocytes were measured across the nucleus from well-formed spheres; and the fully mature oocyte diameter (D) was calculated from D = Pπ-1, where P is the circumference of the
oocyte. Two hundred to 350 oocytes were mea-sured at various stages of maturation.
Ovaries with many translucent hydrated oocytes (i.e., enlarged by fluid uptake just prior to
ovulation) were classified in the hydrated stage, and the migratory nucleus oocytes occur just before the onset of hydration (Hunter et al. 1986). The occurrence of postovulatory follicles within the ovaries of fish is a positive indication of spawning, because they are the evacuated follicles following ovulation (Fitzhugh and Hettler 1995). The sequence of postovulatory aging described by McPherson (1991) for yellowfin tuna was applied to sailfish in the present study.
Spawning frequency was estimated by exam-ining ovarian tissues from mature and reproduc-tively active females sampled during the spawning season of Apr. to Sept. The estimate of spawning frequency was derived from the proportion of mature females with ovaries containing hydrated oocytes (the HY method; Schaefer 1987) or postovulatory follicles (the POF method; Hunter el al. 1986). The mean proportion of spawning females was calculated as the total number of spawning females divided by the total number of mature and reproductively active females in the spawning season, and the spawning frequency was the inverse of the mean proportion of
spawn-Table 1. Two-way analysis of variance for the impacts of sampling regions of
ovaries on the (A) diameters and (B) numbers of oocytes larger than 200 µm for sailfish (Istiophorus platypterus) in eastern Taiwanese waters
(A) Effect of ovary location on oocyte diameter
Two-way analysis of variance
Source DF Sum of squares Mean square F value p
Fish 2 0.030153 0.015077 1130.43 0.0001 Lobe 1 0.000003 0.000003 0.25 0.6232 Region 4 0.000047 0.000012 0.88 0.4962 Interaction 4 0.000050 0.000013 0.94 0.4616 Error 18 0.000240 0.000013 Total 29 0.030494
(B) Effect of ovary location on oocyte numbers
Two-way analysis of variance
Source DF Sum of squares Mean square F value p
Fish 2 345600.07 172800.03 77.49 0.0001 Lobe 1 8772.30 8772.30 3.93 0.0628 Region 4 1153.80 288.45 0.13 0.9697 Interaction 4 11943.53 2985.88 1.34 0.2939 Error 18 40141.27 2230.07 Total 29 407610.97
ing females (McPherson 1991).
Batch fecundity (BF, the number of oocytes released per spawning) was estimated gravimetri-cally by the hydrated oocyte method using fixed ovarian samples which contained hydrated oocytes but no early postovulatory follicles (Hunter et al. 1985, Macchi et al. 2002). For each selected ovarian sample, 3 subsamples of about 0.1 g each were weighed to 0.1 mg for counting the migratory nuclei and hydrated oocytes. Migratory nuclei and hydrated oocytes can readily be distinguished from other oocytes by their larger size and appearance. Those with migratory nuclei are less opaque than yolked oocytes, whereas hydrated oocytes are translucent. No early postovulatory follicles were present in the ovaries used for the estimation of batch fecundity (Schaefer 1996). Each of the 3 subsamples yielded an estimate of batch fecundity for each female, calculated from the product of the number of migratory nuclei or hydrated oocytes per unit weight of the subsample and the total weight of the ovaries. The mean of these 3 mates provided the spawning batch fecundity esti-mate for each fish. Regression analyses were conducted to quantify the relationships between batch fecundity and lower jaw fork length and between batch fecundity and round weight. Relative fecundity (RF), measured as hydrated oocytes per gram of body weight, was determined as the batch fecundity divided by the round weight of the fish.
RESULTS
In total, 246 ovaries were sampled from indi-viduals with LJFLs ranging 98-225 cm and RWs of
3-50 kg (Fig. 2). Among these, 117 ovaries with
LJFLs ranging 162-216 cm were mature and
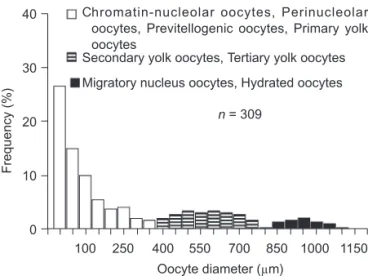
reproductively active. Based on the histological examination, the oocyte diameter distribution of gravid female sailfish (with hydrated oocytes) showed 3 distinguishable groups of oocytes (Fig. 3). The smallest group had oocytes smaller than
Table 2. Spawning fraction and spawning frequency determined by the POF
and HY methods for female sailfish (Istiophorus platypterus) in waters off eastern Taiwan. POF, postovulatory follicle; HY, hydrated oocyte
POF,s method HY,s method
Month n n with Spawning Spawning n with Spawning Spawning
POF fraction frequency HY fraction frequency
April 7 2 0.29 3.50 2 0.29 3.50 May 12 5 0.42 2.40 3 0.25 4.00 June 30 18 0.60 1.67 8 0.27 3.75 July 52 32 0.62 1.63 13 0.25 4.00 Aug. 13 4 0.31 3.25 3 0.23 4.33 Sept. 3 1 0.33 3.00 2 0.67 1.50 Total 117 62 0.53 1.89 31 0.26 3.77
Lower jaw fork length (cm)
Frequency (no. of fish)
n = 246 225 210 195 180 165 150 135 120 105 90 52 48 44 40 36 32 28 24 20 16 12 8 4 0 Round weight (kg) 30 20 10 0 40 30 20 10 0
Fig. 2. Size-frequency distributions at 5 cm (upper figure) and 2 kg intervals (lower figure) for female sailfish (Istiophorus
0.35 mm, composed of chromatin-nucleolar oocytes, perinucleolar oocytes, previtellogenic oocytes, and primary yolk oocytes. The next larg-er group was composed of advanced yolked oocytes (secondary and tertiary yolk oocytes) ranging 0.4-0.75 mm. The 3rd group was the largest and corresponded to the migratory nucleus oocytes and hydrated oocytes measuring 0.8-1.2 mm. The average hydrated oocyte diameter was 1.1 (range, 0.9-1.2) mm.
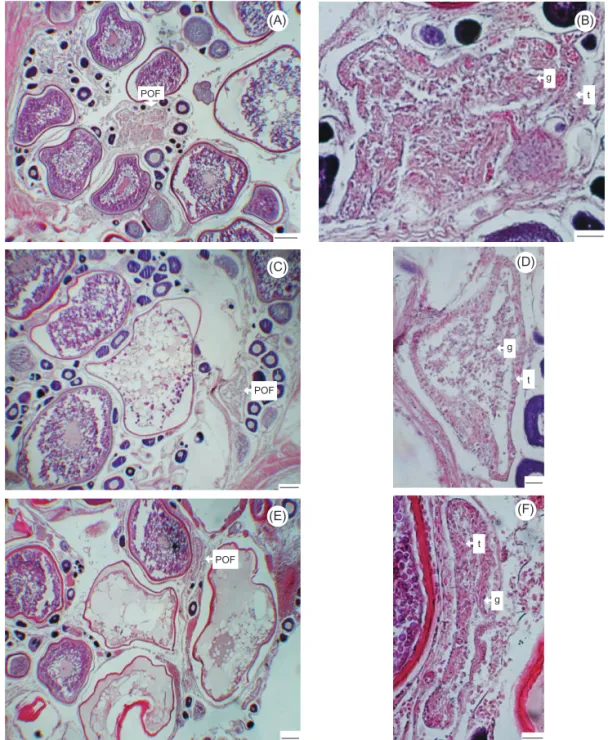
Postovulatory follicles were identified in mature females with yolked oocytes and were classified into early, middle, and late stages. Early-stage postovulatory follicles appeared con-torted or folded, the granulosa cells were aligned or cordlike, granulosa layer nuclei appeared linear in orientation, and the thecal connective tissue lay-ers was distinct (Fig. 4A, B). Middle-stage postovulatory follicles showed a degenerative process, and they were smaller and less convolut-ed. Granulosa cells no longer showed a well-orga-nized alignment of cell walls, and the thecal layer appeared thicker (Fig. 4C, D). Late-stage postovu-latory follicles were reduced in size. The granu-losa layer consisted of a few cells and was thin, and it was not clearly separable from the thicker thecal layer (Fig. 4E, F).
Of the 117 reproductively active specimens examined, about 53% had postovulatory follicles (Table 2), indicating that the average interval between the 2 consecutive spawning activities was 1.89 d. However, when the frequency of ovaries containing hydrated oocytes was used, the mean interval became 3.7 d (Table 2).
Only 18 of the mature females fit the criteria for estimating batch fecundity (hydrated oocytes present without early postovulatory follicles). The
LJFLs of these individuals ranged 167-212 (mean,
191.4) cm and RWs were 19-41 (mean, 28) kg, and their gonad weights ranged 1202-5082 (mean, 2417.2) g. The minimum sample weight used in this study to estimate BF was 0.1 g. BF estimates ranged from 0.2 x 106hydrated oocytes for a
167-cm LJFL (19 kg in RW) female to 2.48 x 106
hydrated oocytes for a 212 cm LJFL (41 kg in RW) female, with an average of 1.3 x 106 eggs. The
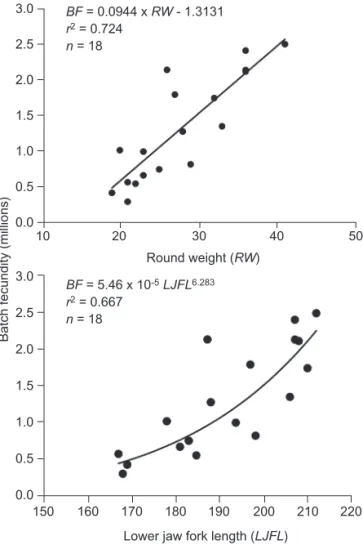
power and linear regression functions were used to describe the relationships between BF (in million eggs) versus RW (in kg) and LJFL (in cm). The 2 regression equations were: BF = 2.03 x 10-3
RW1.936 (r2 = 0.714) and BF = 0.0944 x RW
-1.3134 (r2 = 0.724) for round weight; and BF =
5.46 x 10-15LJFL6.283(r2= 0.667) and BF = 0.0394
x LJFL - 6.2469 (r2 = 0.663) for LJFL. The
rela-tionship between batch fecundity and round weight tended to be better described by the linear regres-sion functions. Power and linear functions described the BF-LJFL relationship equally well (Fig. 5). BF exhibited small variations among females of similar sizes, but increased linearly with
RW and LJFL. Relative fecundity ranged 24-76
hydrated oocytes/g of female.
DISCUSSION
The continuous oocyte size distribution of dif-ferent oocyte stages is a characteristic pattern for multiple and batch spawners (Macchi and Acha 2000). Histological analysis in this study showed the presence of different generations of oocytes in the ripening ovaries, indicating that sailfish in the study area can spawn more than once during a spawning season. A similar finding was reported by Herandez-Herrera et al. (2000). The presence of different batches of growing oocytes including hydrated eggs in the oocyte size-frequency distrib-ution of ripening females suggests that after 1 batch of egg is spawned, a new batch is devel-oped and released (Schaefer 1996, Hunter et al. 1992).
Herández-Herrera et al. (2000) used the fre-quency of ovaries containing hydrated oocytes (the HY method) to measure a mean interval of 3.6 d between 2 consecutive spawning events for the eastern Pacific sailfish. We used the same method and derived a similar value of 3.7 d for sailfish in eastern Taiwanese waters (Table 2). Chromatin-nucleolar oocytes, Perinucleolar
oocytes, Previtellogenic oocytes, Primary yolk oocytes
Secondary yolk oocytes, Tertiary yolk oocytes Migratory nucleus oocytes, Hydrated oocytes
Fig. 3. Oocyte diameter distribution of ovaries of sailfish (Istiophorus platypterus) in the gravid stage (containing hydrat-ed oocytes, black histogram). Different shadings represent the 3 groups of oocytes identified.
n = 309 40 30 20 10 0 Oocyte diameter (µm) Frequency (%) 1150 1000 850 700 550 400 250 100
However, the HY method tends to underestimate the spawning frequency of sailfish. Spawning evi-dence should be examined using the presence of postovulatory follicles not hydrated oocytes. In this study, our estimate using the POF method for the spawning frequency of sailfish in eastern Taiwanese waters was 1.89 d between 2 consecu-tive spawning events, which is similar to those reported by Tseng (2002) for blue marlin (Makaira
mazara) (1.0-2.4 d) and Arocha and Lee (1996) for
swordfish (Xiphias gladius) (2.31 d).
The degeneration of postovulatory follicles varies with species and may be largely influenced by a species, preferred spawning temperature (Hunter and Macewicz 1985b, Fitzhugh and Hettler 1995). Skipjack tuna (Katsuwonus pelamis) spawns at approximately 25
°
C, and the postovula-tory follicles disappear from the ovary within 24 h after spawning (Hunter et al. 1986). McPherson (1991) reported that the spawning of yellowfin tuna is triggered by a surface temperature of 26°
C. The degeneration of postovulatory follicles ofyel-Fig. 4. Postovulatory follicles (POFs) from female sailfish (Istiophorus platypterus) at different stages. (A, B) Early stage of POF; (C, D) middle stage of POF; (E, F) late stage of POF. g, granulosa cell layer; t, thecal cell layer. Scale bar = 100 µm in A,C,E and 10 µm in B,D,F. (A) (C) (D) (E) (F) (B) POF g POF POF t g g t t
lowfin tuna takes less than 24 h at temperatures of 28 to 29
°
C (Schaefer 1996), and many species show similar features in postovulatory follicle degeneration (Hunter and Macewicz 1985a). Chiang (2004) reported that spawning seasons of sailfish in eastern Taiwanese waters occurs from Apr. to Sept. when sea surface temperatures range 26-29°
C. We assumed that the degenera-tion of postovulatory follicles of sailfish also takes in less than 24 h. This histological evidence sug-gests that the time interval between 2 spawning batches of sailfish in eastern Taiwanese waters might be less than 1.89 d. The incidences of both postovulatory follicles and late-stage oocytes indi-cated daily spawning in yellowfin tuna (Schaefer 1996). This was also found in the present study (Fig. 4C). This result implies that reproductively active sailfish likely undergo near-daily spawning rhythms during their spring to fall spawning sea-son. In our study, fish samples were not taken atdifferent times of the day, and estimates of spawn-ing times could not be evaluated. Further studies need to be carried out to identify the spawning time of sailfish in waters off eastern Taiwan.
The fishing ground for fishermen based at the Shinkang Fish Market is located in an area bound-ed by latitude 20
°
30'-24°
10'N and longitude 121°
20'-123°
30'E. This area is adjacent to the Kuroshio Current (Nitani 1972) and is presumed to be the spawning area of sailfish. The sea surface temperature is higher than 23°
C year-round, and hydrographic surveys have shown that the Kuroshio Current off the eastern Taiwanese coast is highly variable in speed, axis position, and vol-ume transport (Yang et al. 2001). More studies need to be done for a better understanding of the vertical structure and physical characteristics of the water mass, which may point to some unique characteristics that can explain why sailfish spawn in this area during a given time of the year.Merrett (1970) found that fecundity increased with size for sailfish in eastern Africa. Herandez-Herrera et al. (2000) reported a significant correla-tion between total weight and batch fecundity for eastern Pacific sailfish. In our studies, the power function provided a good fit for the relationships of batch fecundity with lower jaw fork length and batch fecundity with round weight. The length range of spawned females collected in this study (167-212 cm LJFL; 144-184 cm EFL) was similar to that reported by Herández-Herrera et al. (2000) for eastern Pacific sailfish (155-190 cm EFL), and the batch fecundity values of (0.2-2.48) x 106eggs
per spawning were similar to their estimates of (0.42-2.52) x 106eggs per spawning.
The use of migratory nuclei or hydrated oocytes for batch fecundity determinations is cru-cial because only oocytes in these stages can be distinguished from the less-developed subsequent batch of oocytes. Results of Herández-Herrera et al. (2000) and of the present study are close to the minimum fecundity values given in Eldridge and Wares (1974) who reported (1.8-5.1) x 106 eggs
per spawning by counting the oocytes with diame-ters ranging between 0.9 and 1.3 mm for eastern Pacific sailfish. However, this difference may have resulted from the inclusion of inseparable modes for the oocyte diameter distribution in counting oocytes and the mixture of different developmental oocyte stages. Our results of (0.2-2.48) x 106
eggs per spawning was a wider range than those for Atlantic sailfish estimated by Jolley (1974 1977), which ranged (0.75-1.6) x 106 eggs per
batch spawning. The difference between Eldridge Fig. 5. Batch fecundity as a function of weight (upper figure)
and length (lower figure) for sailfish (Istiophorus platypterus) collected in the waters off eastern Taiwan during May 1999 and July 2000. BF, batch fecundity (no. of oocytes); RW, round weight (kg); LJFL, lower jaw fork length (cm).
BF = 0.0944 x RW - 1.3131 r2= 0.724 n = 18 BF = 5.46 x 10-5LJFL6.283 r2= 0.667 n = 18 Round weight (RW) 220 50 40 30 20 10 210 200 190 180 170 160 150
Batch fecundity (millions)
Lower jaw fork length (LJFL) 3.0 2.5 2.0 1.5 1.0 0.5 0.0 3.0 2.5 2.0 1.5 1.0 0.5 0.0
and Wares (1974) and Jolley (1974 1977) is likely to have resulted from differences in body sizes and methodologies between the 2 studies. However, a comparison of our results with those of Jolley (1974 1977) suggests that such a difference may have resulted from the Atlantic sailfish used in the studies being smaller than the Pacific sailfish (total weight range 17.2-33.4 vs. 19-41 kg) and the dif-ferent methodologies utilized. In this study, only 21 females were sampled in the estimation of batch fecundity. However, more samples are needed to yield more-reliable estimates of batch fecundity. Such estimates should be made annu-ally, and differences should be evaluated in rela-tion to environmental factors.
Acknowledgments: We thank Dr. Yong Chen of
the School of Marine Sciences, University of Maine, Orono, ME, USA, and the anonymous ref-erees for variable comments on the manuscript. This study was partially financially supported by the Fisheries Agency, Council of Agriculture, Taiwan, through a grant (92AS-9.1.1-FA-F1(20)) to CLS.
REFERENCES
Arocha F. 2002. Oocyte development and maturity classifica-tion of swordfish from the north-western Atlantic. J. Fish Biol. 60: 13-27.
Arocha F, DW Lee. 1996. Maturity at size, reproductive sea-sonality, spawning frequency, fecundity and sex ratio in swordfish from the Northwest Atlantic. Int. Comm. Conserv. Atl. Tunas Coll. Vol. Sci. Pap. 45: 350-357. Beardsley GL Jr, NR Merrett, WJ Richards. 1975. Synopsis of
the biology of the sailfish, Istiophorus platypterus (Shaw and Nodder, 1791). In RS Shomura, F Williams, eds. Proceedings of International Billfish Symposium. Part 3. Species Synopses. US Department of Commerce, National Oceanic and Atmospheric Administration Technical Report NMFS SSRF-675. Washington DC: US Government Printing Office, pp. 95-120.
Chiang WC. 2004. Population dynamics and stock assess-ment of the sailfish (Istiophorus platypterus) in waters off eastern Taiwan. PhD dissertation. National Taiwan Univ., Taipei, Taiwan.
Chiang WC, CL Sun, SZ Yeh, WC Su. 2004. Age and growth of sailfish (Istiophorus platypterus) in waters off eastern Taiwan. Fish. Bull. 102: 251-263.
Cuellar N, GR Sedberry, DM Wyanski. 1996. Reproductive seasonality, maturation, fecundity, and spawning frequen-cy of the vermilion snapper, Rhomboplites aurorubens, off the southeastern United States. Fish. Bull. 94: 635-653. DeSylva DP, PR Breder. 1997. Reproduction, gonad histology,
and spawning cycles of north Atlantic billfishes (Istiophoridae). Bull. Mar. Sci. 60: 668-697.
Eldridge MB, PG Wares. 1974. Some biological observations of billfish taken in the eastern Pacific Ocean 1967-1970.
In RS Shomura, F Williams, eds. Proceedings of
International Billfish Symposium. Part 2. Review and con-tributed papers. US Department of Commerce, National oceanic and Atmospheric Administration Technical Report NMFS SSRF-675. Washington DC: US Government Printing Office, pp. 89-101.
Fitzhugh GR, WF Hettler. 1995. Temperature influence on postovulatory follicle degeneration in Atlantic menhaden,
Brevoortia tyrannus. Fish. Bull. 93: 568-572.
Forberg KG. 1982. A histological study of development of oocytes in capelin, Mallotus villosus villosus (Müller). J. Fish Biol. 20: 143-154.
Hernández-Herrera A, M Ramírez-Rodríguez, A Muhlia-Melo. 2000. Batch fecundity and spawning frequency of sailfish (Istiophorus platypterus) off the Pacific coast of Mexico. Pac. Sci. 54: 189-194.
Hunter JR, SR Golberg. 1980. Spawning incidence and batch fecundity in north anchovy, Engraulis mordax. Fish. Bull. 77: 641-645.
Hunter JR, NC Lo, RJ Leong. 1985. Batch fecundity in multi-ple spawning fishes. In R Lasker, ed. An egg production method for estimating spawning biomass of pelagic fish: application to the northern anchovy, Engraulis mordax. US Department of Commerce, National Atmospheric and Atmospheric Administration Technical Report NMFS 36. Washington DC: Government Printing Office, pp. 67-78. Hunter JR, BJ Macewicz. 1985a. Measurement of spawning
frequency in multiple spawning fishes. In R Lasker, ed. An egg production method for estimating spawning bio-mass of pelagic fish: application to the northern anchovy,
Engraulis mordax. US Department of Commerce,
National Oceanic and Atmospheric Administration Technical Report NMFS 36. Washington DC: Govern-ment Printing Office, pp. 79-94.
Hunter JR, BJ Macewicz. 1985b. Rates of atresia in the ovary of captive and wild northern anchovy, Engraulis mordax. Fish. Bull. 83: 895-903.
Hunter JR, BJ Macewicz, NC Lo, CA Kimbrell. 1992. Fecundity, spawning, and maturity of female Dover sole
Microstomus pacificus, with an evaluation of assumptions
and precision. Fish. Bull. 90: 101-128.
Hunter JR, BJ Macewicz, JR Sibert. 1986. The spawning fre-quency of skipjack tuna, Katsuwonus pelamis, from the South Pacific. Fish. Bull. 84: 895-903.
Jolley Jr JW. 1974. On the biology of Florida east coast Atlantic sailfish (Istiophorus platypterus). In RS Shomura, F Williams, eds. Proceedings of International Billfish Symposium. Part 2. Review and contributed papers. US Department of Commerce, National Oceanic and Atmospheric Administration Technical Report NMFS SSRF-675. Washington DC: US Government Printing Office, pp. 81-88.
Jolley Jr JW. 1977. The biology and fishery of Atlantic sailfish,
Istiophorus platypterus, from southeast Florida. Fla. Mar.
Res. Publ. 28: 1-31.
Macchi GH, EM Acha. 2000. Spawning frequency and batch fecundity of Brazilian menhaden, Brevoortia aurea, in Rio de la Plata estuary off Argentina Uruguay. Fish. Bull. 98: 283-289.
Macchi GH, EM Acha, CA Lasta. 2002. Reproduction of black drum (Pogonias cromis) in Rio de la Plata estuary, Argentina. Fish. Res. 59: 83-92.
McPherson GR. 1991. Reproductive biology of yellowfin tuna in the eastern Australian fishing zone, with special refer-ence to the north-western Coral Sea. Aust. J. Mar.
Freshw. Res. 42: 465-477.
Merrett NR. 1970. Gonad development in billfish (Istiophoridae) from the Indian Ocean. J. Zool. 160: 355-370.
Nakamura I. 1985. FAO species catalogue. Vol. 5. Billfishes of the world. An annotated and illustrated catalogue of marlins, sailfishes, spearfishes, and swordfishes known to date. Rome: Food and Agricultural Organization Fisheries Synopsis no. 125, Vol. 5.
Nitani H. 1972. Beginning of the Kuroshio. In H Stommel, K Yoshida, eds. Kuroshio: its physical aspects. Tokyo: University of Tokyo Press, pp. 129-163.
Schaefer KM. 1987. Reproductive biology of black skipjack
tuna Euthynnus lineatus, an eastern Pacific tuna. Inter-Am. Trop. Tuna Comm. Bull. 16: 169-260.
Schaefer KM. 1996. Spawning time, frequency, and batch fecundity of yellowfin tuna, Thunnus albacares, near Clipperton Atoll in the eastern Pacific Ocean. Fish. Bull. 94: 98-112.
Tseng CC. 2002. Reproductive biology of blue marlin Makaira
mazara in the western Pacific. Master,s thesis. National Taiwan Univ., Taipei Taiwan.
Yang Y, CT Liu, TN Lee, W Johns, HW Li, M Koga. 2002. Sea surface slope as an estimator of the Kuroshio volume transport east of Taiwan. Geophys. Res. Lett. 28: 2461-2464.