EFFECTS OF NATURAL ORGANIC MATTER ON ADSORPTION CAPACITY FOR
ATRAZINE BY ACTIVATED CARBON
Gen-Shuh Wang* Department of Public Health
National Taiwan University Taipei 100, Taiwan
Key Words : Atrazine, adsorption, activated carbon, adsorption capacity
ABSTRACT
Adsorption capacities for atrazine on granular activated carbon (GAC) were determined in batch contactors. Conditions in the batch contactors were chosen to determine the reduction in atrazine capacity resulting from competitive adsorption with dissolved organic carbon (DOC), or due to the preadsorbed DOC on the GAC before exposure to atrazine. For aqueous atrazine at 7 µg/L, adsorption capacities in the batch contactors were generally greater than 10 mg/g, and competition with DOC was found to decrease atrazine capacities almost twice as much as preadsorbed DOC. Batch contactor experiments which resulted in low adsorption capacities for atrazine (1-2 mg/g) are considered kinetically limited or by the effects of competitive adsorption with DOC.
* To whom all correspondence should be addressed.
E-mail address: gswang@ha.mc.ntu.edu.tw
INTRODUCTION
Adsorption is defined as the increase in concentration of a particular component at the surface or interface between two phases. The high adsorbability of activated carbon comes from its high surface area (~1000 m2/g) which results from a porous structure of macropores (rp ≈ 25-1000 nm) subdivided
into meso-pores (rp ≈ 2-50 nm) and micropores (rp < 2
nm). Generally it is believed that the mesopores and micropores provide the internal surface area for adsorption of synthetic organics (r ≈ 0.3-0.4 nm), whereas the macropores control the rate of diffusion of an organic from water to the adsorptive surface [1]. Thermodynamic properties of the solute, water and adsorbent surface determine an adsorbent's capacity. Both the capacity and the rate of adsorption need to be understood when a given adsorbent is used for a specific solute.
Activated carbon adsorption remains an important process for drinking water purification. Two mechanisms involving background natural organic matter (NOM) are believed to limit adsorption capacities for hydrophobic synthetic organic contaminants (SOCs) at trace levels: competition and preadsorption. This paper presents experimental results for the effect of background NOM on adsorption of atrazine as a model compound. Atrazine is of interest as a mode-rately hydrophobic synthetic organic (log Kow 2.75; solubility 30 mg/L); atrazine is
also a widely used herbicide, commonly found in surface and ground waters receiving agricultural runoff [2]. Since atrazine is only used seasonally, it is not yet clear whether it is best removed by short-term or permanent modifications to the treatment process. Possibilities that have been examined include an elevated dose of powdered activated carbon (PAC; <45 µm) in contact basins [3], PAC applied to a contact basin with membrane filtration [4], and GAC in fixed-bed adsorbers or filter-adsorbers [5]. Atrazine adsorption by PAC or GAC is subject to competition from background NOM. Atrazine adsorption by GAC is also subject to pre-adsorption of background NOM, for the time that a contactor is in service before it receives an input of atrazine. The effects of both competition and pre-adsorbed background NOM are considered in this paper, in an effort to determine if the two processes are related in their ability to limit adsorption capacities for atrazine. Adsorption experiments were conducted in batch contactors, and their effect on the results is also considered.
At present, granular activated carbon is recognized as a best available technology for removal of SOCs from drinking water, including atrazine [6-7]. This designation was largely based on the measurement of adsorption isotherms under single-solute conditions, although it was understood that adsorption capacities would be diminished for individual compounds in a mixture. Mathematical solutions to this problem were developed by Crittenden
et al. [8], using adsorption isotherms that were
determined under single-solute conditions, for all of the compounds known to be present in a mixture. Competition from NOM is regarded as a somewhat more complex problem than competition from other SOCs, primarily because NOM is a mixture of substances with different adsorption properties that are not well characterized by existing methods of analysis. Several mathematical me-thods have been proposed to describe the dependence of a synthetic compound's adsorption isotherm on the concentration of background organic matter [1, 9]. The easiest way is treat the background as a single compound; its adsorption parameters are obtained from the overall isotherms where the organic concentration is measured as dissolved organic carbon (DOC).
The semi-empirical Freundlich equation is perhaps the most widely used nonlinear mathematical description of single solute adsorption equilibrium between aqueous phase (Ce) and solid phase (Qe):
n e F e=K C Q 1 × (1)
where KF and 1/n are Freundlich constants.
For competitive adsorption between atrazine and NOM, Ideal Adsorbed Solution Theory (IAST) can be used to describe the competitive adsorption between DOC and synthetic organic(s) such as atrazine [9-13]. Partitioning of the surface between two solutes can be described by: ] K n Q n [ Q Q -Q V M -C = ) Q , Q ( F ni Fi i j 2 1 = j j j 2 1 = j i i i0 2 1 i Σ Σ (2)
The equilibrium state is described by values of aqueous phase concentration (Ci) and solid phase
concentration (Qi) for which Fi is equal to zero. This
equation can be solved using the constants of the single-solute Freundlich isotherms KFi , 1/ni , the
initial concentration Ci0 and the carbon dosage M/V.
For atrazine and DOC as solutes, there are two nonlinear simultaneous equations to be solved for the two unknown Qi values. There should be an unique
solution to these simultaneous equations. However, it has been shown that IAST is sensitive to low solute concentrations and problems arise when the numerical calculations require extrapolation beyond the range of the single-solute isotherms.
Crittenden et al. [9] investigated the possibility of describing the adsorption of NOM in terms of fictive components, which could be used in calculations of competitive adsorption with synthetic organic contaminants. As an alternative, Najm and Snoeyink developed a procedure which used adsorption isotherms for a synthetic organic compound, measured at several different initial concentrations in the presence of NOM, to deduce the effect of background organic matter [10]. All of these methods involve the use of adsorption isotherms
which are determined in batch contactors, and which are most similar to the use of PAC in full-scale systems. Previous study showed that atrazine adsorption isotherms were not found to be strongly temperature dependent and the effect of competition with background DOC was found to be similar at temperatures ranging from 5 to 35 °C [14]. However, the rates of adsorption decrease as the temperature decrease. Atrazine adsorption capacities measured in minicolumns are noted to avoid some of the external diffusion limitations of batch contactors [15]. The results obtained show that capacities for atrazine decrease with increasing particle size and seem in reasonable agreement with concentrations of atrazine found at the top of a filter-adsorber and post-contactor previously operated for 30 weeks at Waterford Water Works [15]. However, more work is needed to establish correct scaling relationships for atrazine adsorption in minicolumns and pilot columns with different GAC particle sizes and contact times.
The effects of preadsorbed NOM on SOC capacities are primarily of concern when granular activated carbon (GAC; 0.42-2.38 mm) is used in fixed beds. Not only are these contactors typically in service for a relatively long time, on the order of one year, but individual contaminants advance through the bed at different rates, because of significant differences in their influent concentrations and affinity for the adsorbent. Results from pilot and full-scale systems have indicated that NOM moves through a fixed bed relatively rapidly, on a time scale of 100 days. In contrast, hydrophobia and weakly soluble SOCs are slow to move through a fixed bed, and generally reach saturation and breakthrough after NOM, if at all. In fixed beds, therefore, NOM and SOCs are chromatographically separated, and the NOM is in effect preadsorbed on GAC at deep bed depths in advance of SOCs. A number of studies have demonstrated that adsorption capacities for trichloroethylene are reduced approximately two-fold by NOM that has been preadsorbed on activated carbon [16-17]. Li et al. [18] reported that when the PAC was preloaded with NOM, the pore blockage effect of the NOM caused a reduction of up to two folds of magnitude in the surface diffusion rate of atrazine compared to simultaneous adsorption of atrazine and NOM with fresh PAC. A PAC with relatively large fraction of large micropores and mesopores was shown to suffer much less from the pore blockage effect. The NOM with molecular size between 200 and 700 Dalton appeared to be responsible for the pore blockage effect. However, Knappe et al. [19] showed that preloading NOM had the greatest effect on GAC capacity and the adsorption kinetics was not severely affected.
EXPERIMENTAL METHODS I. Water Supply
Bench-scale experiments were conducted using two different waters to study atrazine adsorption, a GAC-processed laboratory water with a low background of organic matter, and finished water from Waterford Water Works, Waterford, NY, with a mo-derate background of organic matter.
The laboratory water was the tap water from Albany Water Works, Albany, NY, a surface water supply from the Alcove Reservoir in upstate New York. At the time of this project, this water was further treated by passage through a fixed bed of GAC (15 cm id × 1 m long; 0.42 × 1.68 mm Calgon F400), which removed residual chlorine and reduced background organic matter to below 0.1 mg/L DOC. In the discussion of results, this water is designated organic-free.
Finished water from Waterford Water Works, Waterford, NY, is pumped from the Hudson River and treated by aeration, coagulation, flocculation, sedimentation; pre- and post-chlorination [5]. The concentration of background organic matter averages 2.4 mg/L DOC in this Waterford water [20].
II. Activated Carbon
Stocks of two different activated carbons were used to study atrazine adsorption, fresh Calgon F300 (initially 0.60-2.38 mm), and Calgon F300 collected from a control pilot column that received background organic matter for 30 weeks at Waterford Water Works [5]. Properties of the F300 activated carbon can be seen elsewhere [15]. All samples of GAC were ground in a rotary disc mill (Spex Shatterbox) for two to five minutes, and the resulting powder was sieved to obtain a 53 to 149 µm fraction (100 by 270 mesh), designated 100-µm GAC. Samples of pilot-column GAC were dried at room temperature, first in air, and then in a vacuum overnight, before grinding and sieving to collect a nominal 100-µm fraction.
For adsorption isotherm experiments in the laboratory, GAC from this pilot column was collected at bed depths of 23, 43 and 84 cm, after 21, 48, 76 and 141 days in operation: amounts of preadsorbed DOC on these samples have been calculated from breakthrough data and range from 2.1 to 31.6 mg DOC/g GAC [5, 16].
III. Adsorption Isotherm Experiments
A batch contactor procedure was used to determine Freundlich isotherms for GAC adsorption of atrazine. A desired amount of atrazine was weighed and transferred to a large glass bottle (~19 L),
half-filled with the water to be studied: additional water was added to 17 L, and the mixture was stirred overnight to reach an initial concentration C0, between
1000 and 3 µg/L. The stock solution was used to fill small batch contactors to a fixed volume (V, 0.9 L). Each batch contactor contained a known amount (M) of 100-µm GAC, preweighed on an analytical balance and typically between 1 and 40 mg. Different amounts of GAC were used in the batch contactors, so that final concentrations of atrazine in the aqueous and adsorbed phase, Ce (µg/L) and Qe (mg/g), respectively,
would be distributed over a range of values. The amounts of GAC were also chosen according to the value of C0, in order to have detectable concentrations
of atrazine remaining in the water after contact with the GAC.
Contact times of seven days were allowed for atrazine adsorption onto activated carbon. During this time, the contents of the batch contactors were continuously agitated on a plat form shaker (Eberbach Model 6010), and occasionally the contactors were inverted to resuspend settled GAC. Experiments were conducted in a temperature-controlled Environmental Hotpack at 12 °C , since this was the average temperature of the pilot-column study of atrazine adsorption at Waterford, NY [5]. At the end of the 7-day contact time, water samples were filtered (0.45 µm) to remove the GAC and then extracted to determine the aqueous-phase atrazine concentration Ce.
The concentration of atrazine in the adsorbed-phase, Qe, was calculated by assuming a mass balance with
atrazine in the aqueous phase:
M V C C Q e e × − = ( 0 ) (3) Linear regression analysis was used to find the
coefficients KF and l/n of the Freundlich isotherm
which fit the experimental data:
e F e C n K Q log 1log log = + (4)
IV. Analysis for Atrazine
The concentration of atrazine (C8H14N5Cl) in
water was determined by solvent extraction and analysis using capillary-column gas chromatography with nitrogen-phosphorus detection, similar to a procedure developed by Graves [21]. Atrazine was first extracted from water samples (800 mL) in methylene chloride, which was exchanged for methyl t-butyl ether (MtBE) and concentrated to 1 mL using a Kuderna-Danish evaporator attached to a Snyder column. Compounds of interest were analyzed using a Hewlett Packard 5880 gas chromatograph: extracts were injected (2 µL) on a capillary column (30 m by 0.25 mm id, 0.31 µm DB5), which was temperature-programmed from 60 °C to 275 °C at 4 °C/min, with a
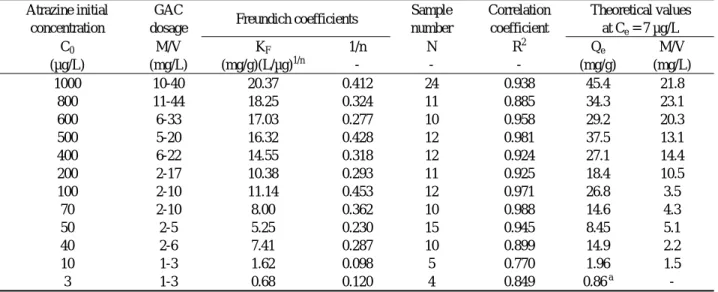
Freundlich coefficients for GAC adsorption of atrazine, without and with background DOC, are given in Tables 1 and 2, respectively. In both cases, values for the Freundlich KF generally decrease as the
initial atrazine concentration decreases. A similar trend is evident in values for the Freundlich l/n, but only with DOC present. From results in Tables 1 and 2, it can also be seen that experiments at low initial concentrations C0 were conducted using a lower dose
(M/V) of GAC. By decreasing the GAC dose at low C0, it was found possible to allow the system to
equilibrate and still have final concentrations Ce in the
range established for quantitation of atrazine. In the presence of DOC, adsorption capacities at 7 µg/L could be determined using GAC doses on the order of 20, 4 and 1 mg/L for initial concentrations C0 of 1000,
100 and 10 µg/L, respectively. However, in the absence of DOC, GAC doses on the order of 10, 1 and 0.1 mg/L were required for initial concentrations C0 of
1000, 100 and 10 µg/L. 1-min hold at the initial and final temperatures. The
injector and detector temperatures were 250 and 300 °C, respectively. Ametryn (C9H17N5S) and tripropyl
phosphate ((C3H7O)3PO4) were added to sample
extracts and calibration mixtures as internal standards at 500 µg/L.
II. Effect of Preadsorbed DOC
GAC samples from a pilot column operated at Waterford Water Works, NY, were used to determine the effect of preloaded background organics of atrazine adsorption [5]. Detail description on the effects of preadsorbed DOC on atrazine adsorption and coefficients of Freundlich isotherms obtained with preloaded GAC are presented elsewhere [16].
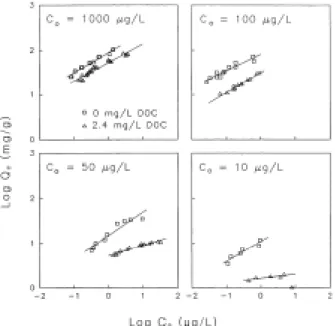
Fig. 1. Isotherms for adsorption of atrazine to 100 µm GAC at 12 oC, with and without competition from background DOC.
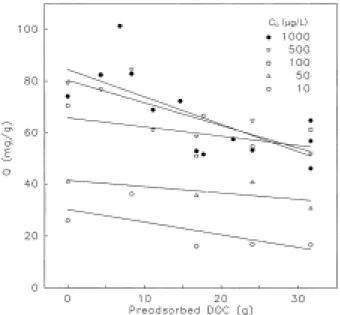
RESULTS AND DISCUSSION Figure 3 attempts to make a more direct analysis of the atrazine capacity, calculated at an aqueous phase concentration of 7 µg/L: at each initial concentration C0, capacities are fitted to a linear
function of preadsorbed DOC. Negative slopes are obtained, consistently indicating a decrease in adsorption capacity with increased preloading; however, the correlation coefficients are weak, decreasing from R2 of 0.54 at C0 of 1000 µg/L to an
R2 of 0.46 at C0 of 10 µg/L. The maximum loss in
atrazine capacity is 40 percent, based on experiments at an initial concentration of 1000 µg/L atrazine, using GAC with 31.6 mg/g pre-adsorbed DOC. In other work, the same GAC samples have been used to determine adsorption capacities for trichloroethylene: decreasing capacities for adsorption of trichloroethylene were strongly correlated with increased preloading of DOC, as indicated by an R2 value of 0.78; the decrease in the adsorption capacity for trichloroethylene reached a maximum of 60 percent at 31.6 mg/g preadsorbed DOC [5].
I. Effect of Background DOC
A reduction in the GAC capacity for atrazine is expected in systems where atrazine is competitively adsorbed with DOC. As shown in Fig. 1, Freundlich isotherms for adsorption of atrazine in the presence of DOC generally lies below those obtained in organic-free water. However Fig. 1 also reveals a more subtle tendency for adsorption capacities to decrease as the initial concentration C0 of atrazine decreases, even
when atrazine is adsorbed from organic-free water. This effect is seen more clearly by calculating the concentration of atrazine on the GAC, Qe, at a
specific aqueous phase concentration Ce, for the
isotherm coefficients specific to each C0. In Fig. 2,
GAC capacities are calculated at an aqueous phase concentration of 7 µg/L (the average influent concentration in pilot column studies [5]). As expected for competitive adsorption, GAC capacities in the presence of 2.4 mg/L DOC are consistently below capacities predicted from isotherms in organic-free water. However, both sets of data show a dramatic decrease in the isotherm capacity as the value of C0 decreases to below 200 µg/L. In this
region, adsorption is labeled particle-limited, for reasons discussed in a later section.
Figure 4 analyzes capacities from the preloading experiments as a function of initial concentration C0:
as expected from Fig. 2, the data show a dramatic decrease in the isotherm capacity as the value of C0
decreases to below 200 µg/L. In Fig. 4, it can also be seen that capacities for atrazine on GAC with 8 mg DOC/g GAC are essentially the same as on GAC with
no DOC. At 16.8, 24.1 and 31.6 mg DOC/g GAC, capacities for atrazine are significantly below those on GAC with no preadsorbed DOC: the inability to relate capacities to the precise amount of preadsorbed DOC is the reason that weak correlation coefficients were
obtained for the interpretations in Fig. 3. A difference in adsorption capacity for atrazine can only be resolved for GAC with a low versus high amount of
Table 1. Experimental conditions and coefficients of Freundich isotherms for adsorption of atrazine by 100-µm GAC in organic-free laboratory water.
Atrazine initial concentration
GAC
dosage Freundich coefficients
Sample number Correlation coefficient Theoretical values at Ce = 7 µg/L C0 M/V KF 1/n N R2 Qe M/V (µg/L) (mg/L) (mg/g)(L/µg)1/n - - - (mg/g) (mg/L) 1000 11-44 30.97 0.449 14 0.975 74.2 13.4 500 6-27 33.19 0.448 14 0.990 79.3 6.2 100 2-6 34.36 0.370 12 0.901 70.6 1.3 50 1-4 14.93 0.519 9 0.914 41.0 1.0 10 0.5-1.7 10.89 0.447 6 0.893 26.0a 0.1 3 0.3-1.7 5.64 0.510 6 0.849 15.2a - a
The value of Qe is extrapolated. For a C0 of 10 µg/L, the experimental isotherm only goes to Ce 1 µg/L, rather than 7 µg/L, and the
dose M/V is below the experimental range. A C0 of 3 µg/L is less than 7 µg/L; a value can be calculated for Qe, but the dose M/V is
mathematically undefined.
Table 2. Experimental conditions and coefficient of Freundich isotherms for adsorption of atrazine by 100-µm GAC in Waterford water with 2.4±0.8 mg/L DOC.
Atrazine initial concentration
GAC
dosage Freundich coefficients
Sample number Correlation coefficient Theoretical values at Ce = 7 µg/L C0 M/V KF 1/n N R 2 Qe M/V (µg/L) (mg/L) (mg/g)(L/µg)1/n - - - (mg/g) (mg/L) 1000 10-40 20.37 0.412 24 0.938 45.4 21.8 800 11-44 18.25 0.324 11 0.885 34.3 23.1 600 6-33 17.03 0.277 10 0.958 29.2 20.3 500 5-20 16.32 0.428 12 0.981 37.5 13.1 400 6-22 14.55 0.318 12 0.924 27.1 14.4 200 2-17 10.38 0.293 11 0.925 18.4 10.5 100 2-10 11.14 0.453 12 0.971 26.8 3.5 70 2-10 8.00 0.362 10 0.988 14.6 4.3 50 2-5 5.25 0.230 15 0.945 8.45 5.1 40 2-6 7.41 0.287 10 0.899 14.9 2.2 10 1-3 1.62 0.098 5 0.770 1.96 1.5 3 1-3 0.68 0.120 4 0.849 0.86 a - a
The value of Qe is extrapolated. Since C0 < 7 µg/L, the dose M/V is mathematically undefined.
preadsorbed DOC.
III. Reduction in Adsorption Capacities from Competition and Preadsorbed DOC
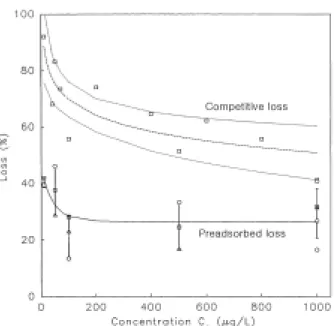
The interpretations in Figs. 2 and 4 provide a basis for comparing the loss in atrazine capacity from competitive adsorption of DOC to the loss in atrazine capacity when DOC is preadsorbed on the GAC. As shown in Fig. 5, the greatest loss in capacity is observed when atrazine is adsorbed competitively with DOC: the loss in capacity increases from 51 percent at C0 of 1000 µg/L to 92 percent at C0 of 10
µg/L, with a mean standard deviation of 14 percent. For preadsorbed DOC, maximum losses are calculated
from results obtained for 16.8 to 31.6 mg DOC/g GAC: the loss in capacity increases from 26 percent at C0 of 1000 µg/L to 41 percent at C0 of 10 µg/L, with a
mean relative standard deviation of 8 percent. In the batch contactors, therefore, competition with DOC decreases atrazine capacities almost twice as much as preadsorbed DOC.
Additional isotherm experiments were conducted in Waterford water using GAC with preadsorbed DOC: coefficients of the adsorption isotherms are given in Table 3. Results of these experiments do not simply represent the combined effects of competition and preadsorbed DOC, since the use of GAC with preadsorbed DOC should inhibit adsorption of additional DOC and its ability to actively compete
with atrazine. Similar to the experiments already described, the results can be evaluated quantitatively for the case that atrazine decreases from an initial concentration of 1000 µg/L to an equilibrium concentration of 7 µg/L. Based on a comparison of results in Table 3 to those in Table 2, the reduction in
capacity that can be attributed to preadsorption alone is found to be 24 percent. A comparison of results in Table 3 to those in Table 1 indicates a total reduction in capacity of 54 percent. Therefore, the net reduction in capacity that can be at-
Table 3. Experimental conditions and coefficients of Freundich isotherms for atrazine adsorption from Waterford water (2.4±0.8 mg/L DOC) by 100-µm GAC perloaded with organic matters.
Preloaded DOC Atrazine initial concentration GAC dosage Freundich coefficients Sample number Correlation coefficient Theoretical values at Ce = 7 µg/L C0 M/V KF 1/n N R2 Qe M/V (mg/g) (µg/L) (mg/L) (mg/g)(L/µg)1/n - - - (mg/g) (mg/L) 4.3 1000 11-44 25.53 0.308 10 0.956 46.5 21.3 6.8 1000 11-44 26.00 0.322 11 0.960 48.6 20.4 8.3 1000 11-44 23.17 0.357 12 0.943 46.3 21.4 16.8 1000 11-44 16.93 0.352 11 0.940 33.5 29.7 24.1 1000 11-44 18.71 0.328 12 0.965 35.4 28.0 24.1 10 1-3 1.46 0.216 7 0.915 2.22 1.4 31.6 1000 11-44 19.50 0.296 10 0.985 34.7 28.7 31.6 10 1-3 1.26 0.132 8 0.669 1.62 1.9
Fig. 2. Effect of competition from background DOC on the GAC capacity for atrazine, at an equilibrium concentration Ce of 7 µg/L, and as a function of
the initial concentration Co.
tributed to competition is 30 percent: this is still a surprising amount of the 51 percent reduction expected from the competitive adsorption experiment using clean GAC (Table 2). By implication, preadsorbed DOC does not entirely inhibit adsorption of additional DOC.
It should be noted that the ability to compare the effects of competition and preadsorbed DOC is li-mited by the precision of experimentally-determined capacities for adsorption of atrazine. For unused Calgon F300 in organic-free water and Waterford water with 2.4 mg/L DOC, the relative deviation of
experimental capacities from the Freundlich isotherm is found to be 13.4 and 31.4 percent, respectively. Similarly, using Calgon F300 with 16.8, 24.1 and 31.6 mg DOC/g GAC (in organic-free water), the relative deviation is found to be 14.3, 20.8, and 23.8 percent, respectively.
Fig. 3. Effect of preadsorbed DOC on GAC capacity for atrazine at an equilibrium concentration Ce of 7
µg/L.
IV. Kinetic Limitations in Batch Isotherm Experiments
Conditions in the batch contactors also affect the ability to observe the effect of competition and pre-adsorption. It is generally assumed that experiments in batch contactors are designed to avoid limitations
from bulk diffusion processes or the frequency of co-llisions between particles. In some cases, however, these processes are thought to have limited atrazine adsorption: for example, in experiments where the capacity for atrazine decreased sharply as the initial concentration of atrazine was lowered, even in organic-free water (Figs. 2 and 4). As the initial concentration of atrazine was lower down, the molecular
numbers of atrazine were reduced; in the mean time, the number of activated carbon particles were also lower than normal (Tables 1 and 2). Since the concen- trations of the background organic matter was not changed, we can expect that DOC should have a sig-
Fig. 4. Effect of preadsorbed DOC on GAC capacity for atrazine at an equilibrium concentration Ce of 7
µg/L, as a function of initial concentration Co.
Fig. 5. Comparison of the loss in GAC capacity for atrazine (Ce 7 µg/L), due to competition with 2.4
mg/L DOC and due to preadsorbed DOC. For competitive loss, the dash line shows the regression of the data and the dotted lines indicate the upper and lower 95% confidence intervals of the regression. For preadsorbed loss, see Figure 4 for symbol descriptions.
nificant competitive advantage relative to atrazine. At low doses of GAC, used with initial atrazine concentrations of 100 µg/L or less, there is reason to believe that adsorption of both atrazine and DOC is dominated by the limited frequency of their collisions with GAC particles. The relatively low temperature (12 °C) used for batch adsorption experiments also limits the kinetics of the adsorption processes [14, 22]. For atrazine, and compounds which are even more hydrophobic, there remains a need for bench-scale methods that can be reliably used to determine adsorption isotherms, and support the development of predictive models for pilot and full-scale GAC contactors [15].
CONCLUSIONS
Adsorption isotherm capacities were found to decrease strongly with a decrease in initial concentration of atrazine. The decrease in adsorption capacity for atrazine due to competition with background DOC is quantified by comparing isotherms obtained with different concentrations of DOC but the same initial concentration of atrazine and the same contact time. The effect of competition with background organics is determined by comparing adsorption capacities for atrazine in organic-free water ( <0.1 mg/L DOC) to those in Waterford water (2.4 mg/L DOC). At an initial atrazine concentration of 10 µg/L, competition with background organics in Waterford water results in a 90 percent decrease in adsorption capacity. Similar results are obtained for the effect of competition, regardless of whether the activated carbon used in adsorption isotherm experiments is fresh or preloaded with background DOC: competition with background DOC results in an 84 percent decrease in adsorption capacities for atrazine on GAC preloaded with 32 mg DOC/g GAC, compared to the 90 percent decrease observed using fresh GAC.
Several results suggest that the bulk of DOC in treated natural water is not responsible for competitive reductions in GAC capacity for atrazine. Regardless of whether the activated carbon used in adsorption isotherm experiments is fresh or preloaded with background DOC, similar results are obtained for the effect of competition: an 84 percent reduction in adsorption capacities for atrazine is obtained in experiments using GAC preloaded with 32 mg DOC/g GAC, compared to a 90 percent reduction using fresh
GAC. At low initial atrazine concentrations, adsorption of both atrazine and DOC is dominated by the limited frequency of their collisions with GAC particles. Therefore analytical attention needs to be focused on identifying a specific fraction or component of background organics in treated natural waters, which has transport and adsorption properties similar to atrazine.
REFERENCES
1. Sontheimer, H., J. C. Crittenden and R. S. Summers, Activated Carbon for Water Treatment, Am. Water Works Assoc. Research Foundation, Denver, CO (1989).
2. Howard, P. H., Handbook of Environmental Fate
and Exposure Data for Organic Chemicals. Vol III,
Lewis, Chelsea, MI (1991).
3. Miltner, R. J., D. B. Baker, T. F. Speth and C. A. Fronk, “Treatment of Seasonal Pesticides Surface Waters”, Jour. Am. Water Works Assoc., 81(1), 43-52 (1989).
4. Li, Q. L., V. L. Snoeyink, B. J. Marinas and C. Campos, “Pore Blockage Effect of NOM on Atrazine Adsorption Kinetics of PAC: the Roles of PAC Pore Size Distribution and NOM Molecular Weight”, Water Res., 37(20), 4863-4872 (2003). 5. Alben, K. T., E. Shpirt, L. Mathevet and J.
Kaczmarczyk, GAC Adsorption of SOCs Under
Dynamic Conditions in Surface Water Treatment,
Am. Water Works Assoc. Research Foundation, Denver, CO, (1994).
6. US EPA, National Primary and Secondary Drinking Water Regulations, Federal Register, 54, 22062-22160 (1989).
7. US EPA, National Primary Drinking Water Regulations; Final Rule, Federal Register, 56, 3526-3597 (1991).
8. Crittenden, J. C., P. Luft and D. Hand, “Prediction of Fixed-Bed Adsorber Removal of Organics in Unknown Mixtures”, J. Env. Eng. ASCE, 113(3), 486-498 (1987).
9. Crittenden, J. C., P. Luft and D. W. Hand, “Prediction of Multicomponent Adsorption Equilibria in Background Mixtures of Unknown Composition”, Water Res., 19(12), 1537-1548 (1985).
10. Najm, I. N., V. L. Snoeyink and Y. Richard, “Effect of Initial Concentration of a SOC in Natural Water on Its Adsorption by Activated Carbon”, Jour. Am. Water Works Assoc., 83(8), 57-63 (1991).
11. Baudu, M., D. Raveau and G. Guibaud, “Application of the IAS Theory Combining to a Three Compartments Description of Natural Organic Matter to the Adsorption of Atrazine or Diuron on Activated Carbon”, Environ. Technol., 25(7), 763-773 (2004).
12. Matsui, Y, D. R. U. Knappe and R. Takagi, “Pesticide Adsorption by Granular Activated Carbon Adsorbers. 1. Effect of Natural Organic Matter Preloading on Removal Rates and Model Simplification”, Environ. Sci. Technol., 36(15), 3426-3431 (2002)
13. Knappe, D. R. U., Y. Matsui, V. L. Snoeyink, P. Roche, M. L. Prados and M. M. Bourbigot, “Predicting the Capacity of Powered Activated Carbon for Trace Organic Compounds in Natural Water”, Environ. Sci. Technol., 32(11), 1694-1698 (1998)
14. Wang, G., “Temperature Dependence on Activated Carbon Adsorption of Atrazine”, J. Chinese Inst.
Environ. Eng.(Taiwan), 13(1), 1-9 (2003).
15. Wang, G., “Predicting Amount of Atrazine Adsorbed on Activated Carbon with GAC Minicolumns”, J. Chinese Inst. Environ. Eng. (Taiwan), 9(3), 153-162 (1999).
16. Wang, G. and K. Alben, “Effects of Preadsorbed Natural Organic Matter on Granular Activated Carbon Adsorption of Atrazine”, Sci. Total
Environ., 224(1-3), 221-226 (1998).
17. Carter, M. C., W. J. Weber and K. P. Olmstead, "Evaluation of the Effects of Background DOM on the Adsorption of Trichloroethylene by GAC",
Proceeding 1991 AWWA Annual Conf., Am. Water
Works Assoc., Denver, CO, pp.631-656 (1991). 18. Li, Q. L., V. L. Snoeyink, B. J. Marinas and C.
Campos, “Pore Blockage Effect of NOM on Atrazine Adsorption Kinetics of PAC: the Roles of PAC Pore Size Distribution and NOM Molecular Weight”, Water Res., 37(20), 4863-4872 (2003). 19. Knappe, D. R. U., V. L. Snoeyink, P. Roche, M. J.
Prados and M. M. Bourbigot, “Atrazine Removal by Preloaded GAC”, J. Am. Water Works Assoc., 91(10), 97-109 (1999).
20. Wang, G. S. “Removal of Atrazine from Drinking Water by Activated Carbon Adsorption”, Ph.D. Dissertation, State University of New York at Albany, Albany, NY. 1994.
21. Graves, R. L., Method 507: Determination of
Nitrogen and Phosphorus-Containing Pesticides in Water by Gas Chromatography with a Nitrogen-Phosphorus Detector, Revision 2.0, United States
1989.
22. Alben, K. T., E. Shpirt and J. Kaczmarczyk, “Temperature Dependence of Trihalomethane Adsorption on Activated Carbon: Implications for Systems with Seasonal Variations in Temperature and Concentration”, Environ. Sci. Technol., 22(4), 406-412 (1988).
Discussions of this paper may appear in the discussion section of a future issue. All discussions should be submitted to the Editor-in-chief within six months.
Manuscript Received: August 16, 2004 Revision Received: January 26, 2005 and Accepted: January 27, 2005