REVIEW ARTICLE
First-line Systemic Therapy for Metastatic Non-small-cell Lung Cancer
e A Review
Yuh-Min Chen
1
,2 *
, Jacqueline Whang-Peng
2
,3
, Chien-Ming Chen
2
,3
1Chest Department, Taipei Veterans General Hospital, School of Medicine, National Yang-Ming University, Taipei, Taiwan 2Center of Excellence in Cancer Research, School of Medicine, Taipei Medical University, Taipei, Taiwan3Division of Cancer Center, Shaun-Ho Hospital, Taipei Medical University, Taipei, Taiwan
a r t i c l e i n f o
Article history: Received: Jan 18, 2011 Revised: Mar 28, 2011 Accepted: Apr 4, 2011 KEY WORDS: chemotherapy;non-small-cell lung cancer (NSCLC); targeted therapy
Our aim was to review and update the current status of systemic therapy for metastatic non-small-cell lung cancer (NSCLC). We reviewed Phase II or Phase III clinical trials offirst-line third-generation chemotherapy regimens (docetaxel, gemcitabine, paclitaxel, pemetrexed, and vinorelbine) and targeted agents (bevacizumab, cetuximab, erlotinib, and gefitinib) identified through Medline, international conferences, and websites of related organizations. We found effort should be taken tofind out whether patients have a tumor epidermal growth factor receptor (EGFR)-active mutation. EGFR-tyrosine kinase inhibitor could be given as first-line treatment for patients with active EGFR mutations (Exon 19 deletions and Exon 21 L858R), whereas patients with a good performance status and wild-type or unknown EGFR mutation status should be treated with platinum-based doublets (platinum plus a third-generation chemotherapy agent). No specific third-third-generation agent is clearly superior for use in combination with a platinum agent. However, pemetrexed is more active in nonsquamous NSCLC. The survival advantage of platinum-based doublets over non-platinum combinations or older combinations is modest. Systemic chemotherapy beyond four to six cycles impedes quality of life without prolonging life. However, data suggest switching to maintenance with pemetrexed or erlotinib therapy is effective in prolonging patient survival. The addition of bevacizumab to carboplatin and paclitaxel has shown improved survival, and a large-scale Phase IV study showed the efficacy and safety of the combination of bevacizumab with platinum-based doublets. In conclusion, in tumor mutated NSCLC, EGFR-tyrosine kinase inhibitor is thefirst-line treatment of choice for patients with metastatic disease. The combination of a platinum agent plus a third-generation agent continues to be the standard of care for those patients with tumor EGFR wild-type or unknown status. Pemetrexed is more active in patients with non-squamous NSCLC, and bevacizumab in combination with platinum-based doublets can also be considered in patients with non-squamous NSCLC. As differences between the regimens are small, a detailed discussion with the patient regarding treatment toxicity and patient preference will help in making the regimen choice.
CopyrightÓ 2011, Taipei Medical University. Published by Elsevier Taiwan LLC. All rights reserved.
1. Introduction
Lung cancer is the leading cause of cancer death in the world and
non-small-cell lung cancer (NSCLC) accounts for the majority of
lung cancer cases.
1,2Adenocarcinoma and squamous cell carcinoma
(SCC) are two major histologic subtypes of NSCLC. The incidence of
adenocarcinoma has increased in recent decades, whereas the
incidence of SCC has reached a plateau or has decreased, mainly
because of changes in smoking behavior with the use of
filtered
cigarettes with low tar, low nicotine, and increased nitrate levels.
3,4Most NSCLC patients face the option of systemic chemotherapy or
targeted therapy. Because more than 40% of NSCLC patients present
with metastatic disease, they are suggested to receive systemic
therapy, and the majority of patients with Stage I
eIII tumors
treated with curative intent eventually develop recurrence.
5Third-generation anti-cancer drugs and their combination with
platinum have shown better response rates and survival than the
conventional regimens during the last decade.
6e8Pemetrexed,
a recently available third-generation chemotherapeutic agent, was
found to be more effective in non-squamous NSCLC.
9,10Further-more, bevacizumab, a monoclonal antibody of vascular endothelial
growth factor, was found to enhance chemotherapy ef
ficacy against
non-squamous NSCLC.
11Epidermal growth factor receptor-tyrosine kinase inhibitors
(EGFR-TKIs), such as erlotinib and ge
fitinib, are a new class of
anti-cancer agents that have been used for nearly one decade.
6It
was found that adenocarcinoma had a better response to this class
* Corresponding author. Chest Department, Taipei Veterans General Hospital,No. 201, Sec. 2, Shih-Pai Rd, Taipei 112, Taiwan. E-mail: Y.-M. Chen <ymchen@vghtpe.gov.tw>
Contents lists available at
ScienceDirect
Journal of Experimental and Clinical Medicine
j o u r n a l h o m e p a g e : h t t p : // w w w . j e c m - o n l i n e . c o m
1878-3317/$e see front matter Copyright Ó 2011, Taipei Medical University. Published by Elsevier Taiwan LLC. All rights reserved. doi:10.1016/j.jecm.2011.04.008
of targeted agent, whereas other subtypes of NSCLC had a less
satisfactory response because of the differing frequencies of tumor
epidermal growth factor receptor (EGFR) mutations.
12Recent
clinical trials have also shown the high ef
ficacy of EGFR-TKIs as
first-line treatment for EGFR-mutated NSCLC, compared with
platinum-based doublets, in terms of prolongation of
progression-free survival (PFS) and less toxicity.
13e15Together with the above
findings, patients with adenocarcinoma have had better treatment
options available and improved survival in recent years.
9,16In the present review, we brie
fly summarize the important
studies performed in recent years, review guidelines, and analyze
the feasibility of current therapy options for patients with NSCLC.
2. Systemic Chemotherapy
2.1. Platinum-based doublet chemotherapy
An updated review by a committee of the American Society of
Clinical Oncology provides evidence supporting the use of
chemotherapy in Stage IV NSCLC patients with Eastern Cooperative
Oncology Group (ECOG) performance status (PS) 0, 1, and possibly
2.
6Clinical trials have supported the use of two chemotherapy
agents rather than one, in terms of response rates and survival,
although toxicity is increased.
17No one speci
fic platinum-based
doublet containing a third-generation anti-cancer agent had
better ef
ficacy than the others, before the data of pemetrexed trials
was available.
10,18e20Drugs that may be combined with platinum
include the third-generation cytotoxic drugs docetaxel,
gemcita-bine, paclitaxel, pemetrexed, and vinorelbine. Patients
’ quality of
life is usually improved with chemotherapy, with improvements in
disease-speci
fic symptoms at a cost of some degree of worsening in
drug-induced toxicities or symptoms.
21Assessments of several
regimens of chemotherapy also have shown that chemotherapy
against NSCLC is cost effective.
22In spite of these treatment
bene-fits, it should be noted that the patients studied in most Phase II and
Phase III chemotherapy clinical trials had a good PS, typically ECOG
PS 0
e1, and only a few PS 2 patients were enrolled. In addition,
many studies restricted eligibility to patients more than 70 years
old.
2.2. Cisplatin, carboplatin, or non-platinum-based chemotherapy
Platinum combinations are preferred over non-platinum
combi-nations because they are superior in response rate and result in
a marginally/insigni
ficantly longer overall survival.
6The choice of
either cisplatin or carboplatin is acceptable. However, carboplatin is
not reimbursed in Taiwan except for patients with impaired renal
function. Cisplatin is slightly more effective than carboplatin, but
also has more adverse effects. Cisplatin combinations have a higher
response rate than carboplatin and may improve survival when
combined with third-generation agents.
6,23,24Carboplatin is less
likely to cause nausea, vomiting, neurotoxicity, and nephrotoxicity
than cisplatin, but is more likely to cause myelosuppression,
especially thrombocytopenia. Non-platinum therapy combinations
are reasonable in patients who have contraindications to the use of
platinum therapy, such as pre-existing heart failure, renal insuf
fi-ciency or failure, neuropathy, or hearing impairment. Triplet
chemotherapy showed no survival bene
fit, but had significantly
higher toxicities.
17,192.3. Treatment cycles
How many cycles of
first-line chemotherapy should be given? It is
generally accepted that
first-line cytotoxic chemotherapy should be
stopped at disease progression or after four cycles in stage IV NSCLC
patients whose disease is not responding to treatment.
6Cytotoxic
doublet chemotherapy should be administered for no more than six
cycles, even in those patients who have responded to treatment.
For patients who have stable disease or who respond to
first-line
chemotherapy, the present evidence does not support the
contin-uation of doublet chemotherapy beyond six cycles.
6,25,262.4. Maintenance therapy
With the advent of effective second-line cytotoxic drugs that
improve survival for NSCLC patients who have progressive disease
after
first-line chemotherapy, there is a renewed interest in
whether initiation of a different or non-cross-resistant drug
immediately after completion of
first-line chemotherapy, or the
initiation of a different chemotherapy before disease progression,
will improve survival.
27e30In general, most studies showed
improvement in PFS, but not overall survival, either by continuing
an effective chemotherapy beyond four cycles or by immediately
initiating alternative chemotherapy.
27e30However, the
improve-ment in PFS is tempered by an increase in adverse effects from
additional cytotoxic chemotherapy. Of the many chemotherapeutic
agents, including gemcitabine, vinorelbine, docetaxel, and
peme-trexed, used in maintenance therapy, only pemetrexed was
approved by the Food and Drug Administration on 2 July 2010 for
maintenance therapy in NSCLC patients with advanced disease.
Pemetrexed maintenance therapy has demonstrated a signi
ficant
survival bene
fit compared with the best supportive care after
first-line chemotherapy.
28Another type of maintenance therapy after
first-line
chemo-therapy is the use of EGFR-TKIs.
31Median PFS was longer with
erlotinib maintenance therapy than with placebo treatment
[2.9 months vs. 2.6 months, hazards ratio (HR) 0.71, 95% con
fidence
interval 0.62
e0.82; p < 0.0001] and overall survival was prolonged
with erlotinib maintenance treatment versus placebo treatment
(median 12 months vs. 11 months, HR 0.81, 95% con
fidence interval
0.7
e0.95, p ¼ 0.0088) in a large Phase III randomized trial.
2.5. Chemotherapy with targeted therapy
Chemotherapy in combination with targeted therapy has been
used for a decade already, although chemotherapy combined
with small molecular tyrosine kinase inhibitors has generally
failed.
32e35However, a small study showed promise with
preliminary ef
ficacy when chemotherapy was combined with
intercalated EGFR-TKIs.
36A Phase III randomized study is ongoing
to verify this treatment strategy. Chemotherapy in combination
with EGFR monoclonal antibody has also been studied in Phase II
and Phase III trials, and only the FLEX study showed a signi
ficant
1.2-month survival prolongation when adding cetuximab to
vinorelbine plus cisplatin chemotherapy.
37Cetuximab is suggested
for use in combination with vinorelbine plus cisplatin as one of the
treatment choices in treatment-naïve advanced NSCLC patients
with clinical characteristics similar to those in the FLEX study in
the USA,
6but not in Europe at present, because of the lack of a
predictor of who will respond well to this combination of
treat-ment. However, a recently published retrospective analysis of the
correlation of
first-cycle skin rash with treatment response in the
FLEX study showed
first-cycle skin rash was associated with
a better outcome. First-cycle skin rash might be a surrogate
clin-ical marker that could be used to tailor cetuximab treatment for
advanced NSCLC in those patients who would be most likely to
derive a signi
ficant benefit.
38With regard to anti-angiogenesis
targeted
therapy,
only
bevacizumab
(monoclonal
antibody
of vascular endothelial growth factor) was approved for use in
first-line NSCLC treatment in combination with pacliaxel plus
carboplatin.
6,11The recommended dose is 15 mg/kg every 3 weeks
in combination with paclitaxel and carboplatin in non-squamous
NSCLC patients with a PS of 0 or 1, and without brain
metas-tases Bevacizumab may be continued until disease progression.
This recommendation is based on a Phase III randomized trial
(ECOG4599) involving 878 non-squamous NSCLC patients. The
study showed there was a two-month improvement in overall
survival when bevacizumab was added to a paclitaxel plus
car-boplatin regimen (12.3 months vs. 10.3 months; HR
¼ 0.79,
p
¼ 0.003).
11The chemotherapy regimen was con
fined to
pacli-taxel plus carboplatin only because another Phase III randomized
trial of bevacizumab in combination with gemcitabine and
cisplatin showed an improvement in response rate and modest
PFS improvement, but not overall survival.
39However, a recently
published Phase IV study of bevacizumab in combination with
platinum-based chemotherapy (SAiL) documented its safety and
ef
ficacy, with a median survival of 12.3 months for NSCLC patients
and 14.6 months for patients with adenocarcinoma.
40The disease
control rate was 88%, and median survival was high up to
18.9 months for Asian patients with adenocarcinoma. Thus, it
seems there is no need to con
fine bevacizumab treatment to use
with paclitaxel plus carboplatin only. Patients with SCC are not
suggested to receive
bevacizumab-containing
chemotherapy
because of the risk of massive hemoptysis.
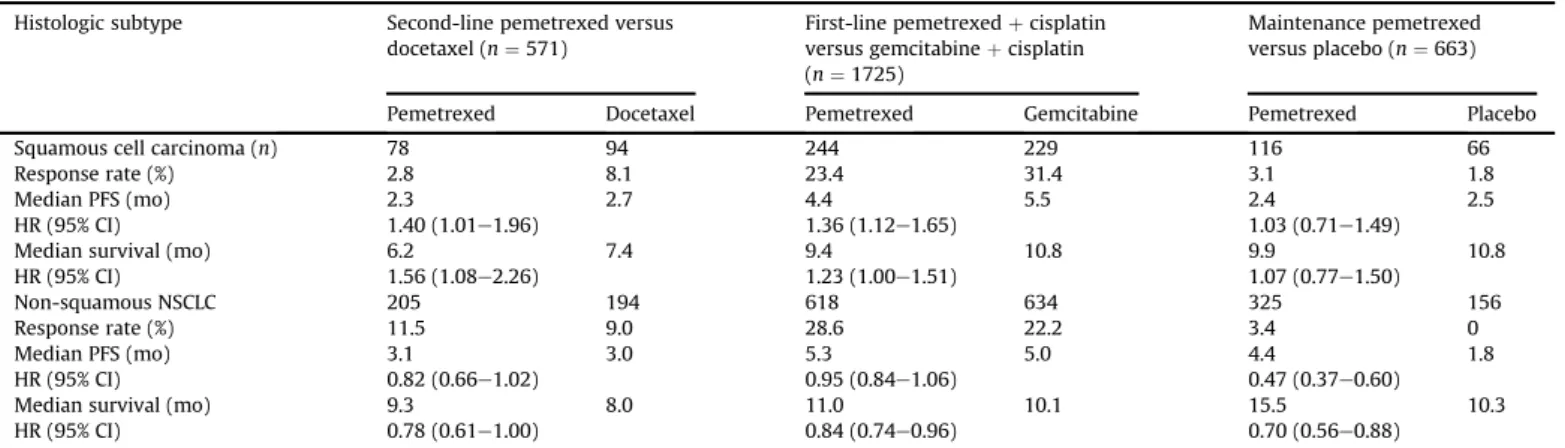
41,422.6. Histologic subtype and choice of chemotherapeutic agent
It is true that the histologic subtype of NSCLC affects the choice of
first-line chemotherapy regimen. Three large Phase III randomized
trials involving pemetrexed (second-line docetaxel vs. pemetrexed;
first-line gemcitabine þ cisplatin vs. pemetrexed þ cisplatin;
pemetrexed maintenance therapy vs. best supportive care)
20,28,43showed pemetrexed had better ef
ficacy, including PFS and overall
survival, in patients with non-squamous cell carcinoma than in
those with SCC (
Table 1
).
103. EGFR-TKI
3.1. First-line EGFR-TKI treatment in tumor EGFR-mutated NSCLC
patients
EGFR-TKIs, such as erlotinib and ge
fitinib, are a new class of
anti-cancer agents that have been used for nearly a decade.
6It was
found that the tumor EGFR mutations that occur mainly in
adenocarcinoma, especially tumor EGFR exon 19 deletions and
exon 21 L858R mutations (activating mutations), predicted a better
response to this class of targeted agents.
12,44In clinically or
molecularly unselected NSCLC patients, erlotinib or ge
fitinib should
not be used in combination with cytotoxic chemotherapy as
first-line therapy,
32e35and in clinically or molecularly unselected
patients, evidence is insuf
ficient to recommend single-agent
erlo-tinib or ge
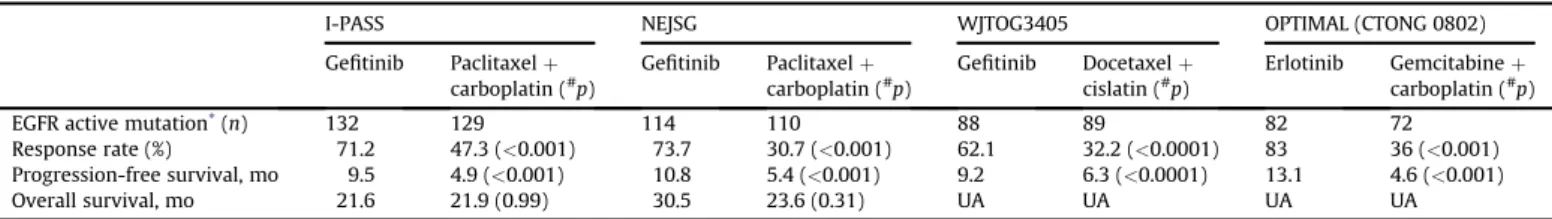
fitinib as first-line therapy. In contrast, recent Phase III
randomized clinical trials showed the high ef
ficacy of EGFR-TKIs as
first-line treatment for EGFR-mutated NSCLC, compared with
platinum-based doublets, in terms of prolongation of PFS, better
quality
of
life,
and
fewer
treatment-induced
toxicities
(
Table 2
).
13e15,45Thus,
first-line treatment with erlotinib or
gefiti-nib may be recommended for patients with activating EGFR
mutations. If the EGFR mutation status is negative or unknown,
then cytotoxic chemotherapy is preferred.
3.2. Erlotinib or ge
fitinib
Both agents are highly active in those patients whose tumors had
activating mutations, such as exon 19 deletions or exon 21 L858R
mutations.
46,47Thus, both agents can be used in
first-line treatment
of patients whose tumors have EGFR activating mutations.
However, the side effects of EGFR-TKIs, such as skin rashes and
a nausea sensation, are relatively more frequent or severe in
patients who receive erlotinib than in those taking ge
fitinib. The
occurrence of other side effects, such as diarrhea, was similar
between the two agents, or even slightly less frequent, such as
pneumonitis, with erlotinib treatment. It is also recommended that
patients with central nervous system metastases or meningeal
carcinomatosis be treated with erlotinib instead of ge
fitinib,
because of the higher blood-brain-barrier penetration rate and
higher cerebrospinal
fluid concentrations of erlotinib and its active
metabolites than with ge
fitinib.
48e513.3. Second-generation TKI
Second-generation TKI or multi-targeted agents, such as afatinib
(BIBW-2992) and vandetanib, are still in clinical trials and not
available for routine clinical use at present.
52,53In general, these
agents can be used alone or in combination with other targeted
agents if the agent belongs to the EGFR-TKI family; and they are
usually used in combination with chemotherapy when they are
anti-angiogenetic agents.
Table 1 Response rate, progression-free survival, and overall survival by histologic subtype in three pemetrexed studies Histologic subtype Second-line pemetrexed versus
docetaxel (n¼ 571)
First-line pemetrexedþ cisplatin versus gemcitabineþ cisplatin (n¼ 1725)
Maintenance pemetrexed versus placebo (n¼ 663) Pemetrexed Docetaxel Pemetrexed Gemcitabine Pemetrexed Placebo
Squamous cell carcinoma (n) 78 94 244 229 116 66
Response rate (%) 2.8 8.1 23.4 31.4 3.1 1.8
Median PFS (mo) 2.3 2.7 4.4 5.5 2.4 2.5
HR (95% CI) 1.40 (1.01e1.96) 1.36 (1.12e1.65) 1.03 (0.71e1.49)
Median survival (mo) 6.2 7.4 9.4 10.8 9.9 10.8
HR (95% CI) 1.56 (1.08e2.26) 1.23 (1.00e1.51) 1.07 (0.77e1.50)
Non-squamous NSCLC 205 194 618 634 325 156
Response rate (%) 11.5 9.0 28.6 22.2 3.4 0
Median PFS (mo) 3.1 3.0 5.3 5.0 4.4 1.8
HR (95% CI) 0.82 (0.66e1.02) 0.95 (0.84e1.06) 0.47 (0.37e0.60)
Median survival (mo) 9.3 8.0 11.0 10.1 15.5 10.3
HR (95% CI) 0.78 (0.61e1.00) 0.84 (0.74e0.96) 0.70 (0.56e0.88) CI¼ confidence interval; HR ¼ hazards ratio; NSCLC ¼ non-small-cell lung cancer; PFS ¼ progression-free survival.
4. Conclusions
In NSCLC patients with a tumor-active EGFR mutation and
meta-static disease, EGFR-TKI is the
first-line treatment of choice. The
combination of a platinum agent with a third-generation agent
continues to be the standard of care for those patients with tumor
EGFR of a wild-type or unknown status. Pemetrexed is more active
in patients with non-squamous NSCLC, and bevacizumab in
combination with platinum-based doublets can also be considered
in non-squamous NSCLC.
References
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin 2009;59:225e49.
2. Chen YM, Chao JY, Tsai CM, Shiau CY, Liang MJ, Yen SH, Perng RP. Treatment of non-small-cell lung cancer: the Chinese experience in a general teaching hospital. J Chin Med Assoc 2000;63:459e66.
3. Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:29Se55S. 4. Alberg AJ, Brock MV, Samet JM. Epidemiology of lung cancer: looking to the
future. J Clin Oncol 2005;23:3175e85.
5. Carney DN, Hansen HH. Non-small-cell lung cancere stalemate or progress? N Engl J Med 2000;343:1261e2.
6. Azzoli CG, Baker Jr S, Temin S, Pao W, Aliff T, Brahmer J, Johnson DH. American society of clinical oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 2009;27:6251e66. 7. Fathi AT, Brahmer JR. Chemotherapy for advanced stage non-small cell lung
cancer. Semin Thorac Cardiovasc Surg 2008;20:210e6.
8. Berhoune M, Banu E, Scotte F, Prognon P, Oudard S, Bonan B. Therapeutic strategy for treatment of metastatic non-small cell lung cancer. Ann Phar-macother 2008;42:1640e52.
9. Scagliotti GV, Ceppi P, Novello S, Papotti M. Chemotherapy treatment decisions in advanced non-small cell lung cancer based on histology. Edu Book Am Soc Clin Oncol 2009;27:431e5.
10. Scagliotti G, Brodowicz T, Shepherd FA, Zielinski C, Vansteenkiste J, Manegold C, Simms L, et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol 2011;6:64e70.
11. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, et al. Paclitaxelecarboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542e50.
12. Mitsudomi T, Kosaka T, Yatabe Y. Biological and clinical implications of EGFR mutations in lung cancer. Int J Clin Oncol 2006;11:190e8.
13. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. New Eng J Med 2009;361:947e57.
14. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380e8.
15. Zhou C, Wu YL, Chen G, Feng J, Liu X, Wang C, Zhang S. Efficacy results from the randomized phase III OPTIMAL (CTONG 0802) study comparing first-line erlotinib versus carboplatin (CBDCA) plus gemcitabine (GEM), in Chinese advanced non-small-cell lung cancer (NSCLC) patients (PTS) with EGFR acti-vating mutations. Ann Oncol 2010;21(Suppl. 8):LBA13 [Abstract].
16. Takano T, Fukui T, Ohe Y, Tsuta K, Yamamoto S, Nokihara H, Yamamoto N, et al. EGFR mutations predict survival benefit from gefitinib in patients with advanced lung adenocarcinoma: a historical comparison of patients treated before and after gefitinib approval in Japan. J Clin Oncol 2008;26:5589e95. 17. Delbaldo C, Michiels S, Syz N, Soria JC, Le Chevalier T, Pignon JP. Benefits of
adding a drug to a single-agent or a 2-agent chemotherapy regimen in advanced non-small cell lung cancer: a meta-analysis. JAMA 2004;292: 470e84.
18. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92e8.
19. Goffin J, Lacchetti C, Ellis PM, Ung YC, Evans WK. First-line systemic chemo-therapy in the treatment of advanced non-small cell lung cancer: a systematic review. J Thorac Oncol 2010;5:260e74.
20. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543e51.
21. Fallowfield LJ, Harper P. Health-related quality of life in patients undergoing drug therapy for advanced non-small-cell lung cancer. Lung Cancer 2005;48:365e77.
22. Dranitsaris G, Cottrell W, Evans WK. Cost-effectiveness of chemotherapy for non-small-cell lung cancer. Curr Opin Oncol 2002;14:375e83.
23. Scagliotti GV, De Marinis F, Rinaldi M, Crino L, Gridelli C, Ricci S, Matano E, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol 2002;20:4285e91. 24. Zatloukal P, Petruzelka L, Zemanova M, Kolek V, Skrickova J, Pesek M, Fojtu H.
Gemcitabine plus cisplatin vs. gemcitabine plus carboplatin in stage IIIb and IV non-small cell lung cancer: a phase III randomized trial. Lung Cancer 2003;41:321e31.
25. Park JO, Kim SW, Ahn JS, Suh C, Lee JS, Jang JS, Cho EK, et al. Phase III trial of two versus four additional cycles in patients who are non progressive after two cycles of platinum based chemotherapy in non-small-cell lung cancer. J Clin Oncol 2007;25:5233e9.
26. von Plessen C, Bergman B, Andresen O, Bremnes RM, Sundstrom S, Gilleryd M, Stephens R, et al. Palliative chemotherapy beyond three courses conveys no survival or consistent quality-of-life benefits in advanced non-small-cell lung cancer. Br J Cancer 2006;95:966e73.
27. Fidias PM, Dakhil SR, Lyss AP, Loesch DM, Waterhouse DM, Bromund JL, Chen R, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 2009;27:591e8.
28. Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, Wu YL, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009;374:1432e40.
29. Brodowicz T, Krzakowski M, Zwitter M, Tzekova V, Ramlau R, Ghilezan N, Ciuleanu T, et al, Central European Cooperative Oncology Group CECOG. Cisplatin and gemcitabinefirst-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer 2006;52:155e63.
30. Westeel V, Quoix E, Moro-Sibilot D, Mercier M, Breton JL, Debieuvre D, Richard P, et al, French Thoracic Oncology Collaborative Group (GCOT). Randomized study of maintenance vinorelbine in responders with advanced non-small-cell lung cancer. J Natl Cancer Inst 2005;97:499e506.
31. Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, Juhász E, Esteban E, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521e9.
32. Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, Natale RB, et al. Gefitinib (ZD1839) in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial (INTACT 1). J Clin Oncol 2004;22:777e84.
33. Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, Scagliotti G, et al. Gefitinib (ZD1839) in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial (INTACT 2). J Clin Oncol 2004;22:785e94.
34. Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, Kris MG, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (osi-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2005;23:5892e9.
35. Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, Milanowski J, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small cell lung cancer: the Tarceva lung cancer investigation trial. J Clin Oncol 2007;25:1545e52.
Table 2 Four phase III randomized trials comparing first-line epidermal growth factor receptor-tyrosine kinase inhibitors and chemotherapy treatment in clinically or molecularly selected non-small-cell lung cancer patients
I-PASS NEJSG WJTOG3405 OPTIMAL (CTONG 0802)
Gefitinib Paclitaxelþ carboplatin (#p) Gefitinib Paclitaxelþ carboplatin (#p) Gefitinib Docetaxelþ cislatin (#p) Erlotinib Gemcitabineþ carboplatin (#p)
EGFR active mutation*(n) 132 129 114 110 88 89 82 72
Response rate (%) 71.2 47.3 (<0.001) 73.7 30.7 (<0.001) 62.1 32.2 (<0.0001) 83 36 (<0.001) Progression-free survival, mo 9.5 4.9 (<0.001) 10.8 5.4 (<0.001) 9.2 6.3 (<0.0001) 13.1 4.6 (<0.001) Overall survival, mo 21.6 21.9 (0.99) 30.5 23.6 (0.31) UA UA UA UA
#p value when comparing epidermal growth factor receptor-tyrosine kinase inhibitors group.
EGFR¼ epidermal growth factor receptor; UA ¼ unavailable.
36. Mok T, Wu YL, Yu CJ, Zhou C, Chen YM, Zhang L, Ignacio J, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2009;27:5080e7.
37. Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, Vynnychenko I, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomized phase III trial. Lancet 2009;373:1525e31.
38. Gatzemeier U, von Pawel J, Vynnychenko I, Zatloukal P, de Marinis F, Eberhardt WEE, Paz-Ares L, et al. First-cycle rash and survival in patients with advanced non-small-cell lung cancer receiving cetuximab in combination with first-line chemotherapy: a subgroup analysis of data from the FLEX phase 3 study. Lancet Oncol 2011;12:30e7.
39. Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bev-acizumab asfirst-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009;27:1227e34.
40. Crinò L, Dansin E, Garrido P, Griesinger F, Laskin J, Pavlakis N, Stroiakovski D, et al. Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol 2010;11:733e40.
41. Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184e91.
42. Hainsworth JD, Fang L, Huang JE, Karlin D, Russell K, Faoro L, Azzoli C. BRIDGE: an open-label phase II trial evaluating the safety of bevacizumabþ carboplatin/ paclitaxel as first-line treatment for patients with advanced, previously untreated, squamous non-small cell lung cancer. J Thorac Oncol 2011;6:109e14. 43. Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemo-therapy. J Clin Oncol 2004;22:1589e97.
44. Murray S, Dahabreh IJ, Linardou H, Manoloukos M, Bafaloukos D, Kosmidis P. Somatic mutations of the tyrosine kinase domain of epidermal growth factor receptor and tyrosine kinase inhibitor response to TKIs in non-small cell lung cancer: an analytical database. J Thorac Oncol 2008;3:832e9.
45. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121e8.
46. Gandhi J, Zhang J, Xie Y, Soh J, Shigematsu H, Zhang W, Yamamoto H, et al. Alterations in genes of the EGFR signaling pathway and their relationship to EGFR tyrosine kinase inhibitor sensitivity in lung cancer cell lines. PLoS ONE 2009;4:e4576. doi:10.1371/journal.pone.0004576.
47. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169e81.
48. Jackman DM, Holmes AJ, Lindeman N, Wen PY, Kesari S, Borras AM, Bailey C, et al. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol 2006;24:4517e20.
49. Katayama T, Shimizu J, Suda K, Onozato R, Fukui T, Ito S, Hatooka S, et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol 2009;4:1415e9.
50. Togashi Y, Masago K, Fukudo M, Terada T, Ikemi Y, Kim YH, Fujita S, et al. Pharmacokinetics of erlotinib and its active metabolite OSI-420 in patients with non-small cell lung cancer and chronic renal failure who are undergoing hemodialysis. J Thorac Oncol 2010;5:601e5.
51. Fan WC, Yu CJ, Tsai CM, Huang MS, Lai CL, Hsia TC, Tien YJ, et al. Different efficacies of erlotinib and gefitinib in Taiwanese patients with advanced non-small cell lung cancer: a retrospective multi-center study. J Thor Oncol 2011;6:148e55.
52. Dempke WCM, Suto T, Reck M. Targeted therapies for non-small cell lung cancer. Lung Cancer 2010;67:257e74.
53. Somaiah N, Simon GR. Molecular targeted agents and biologic therapies for non-small cell lung cancer. J Thor Oncol 2010;5(Suppl. 6):S434e54.