行政院國家科學委員會專題研究計畫 成果報告
光動力治療對生物膜防治之研究
計畫類別: 個別型計畫 計畫編號: NSC92-2320-B-002-187- 執行期間: 92 年 08 月 01 日至 93 年 07 月 31 日 執行單位: 國立臺灣大學生化科技學系 計畫主持人: 黃慶璨 計畫參與人員: 李佳芬、涂雅屏 報告類型: 精簡報告 處理方式: 本計畫可公開查詢中 華 民 國 93 年 9 月 29 日
中文摘要
利用發光二極體為光源激發由五胺基酮戊酸轉換成的 photoporphyrin IX 可以顯示光動
力治療可抑制懸浮及生物膜狀態的 Pseudomonas aeruginosa。P. aeruginosa 懸浮細胞或 培養於旋轉圓錠反應器之生物膜,以不同濃度的五胺基酮戊酸在暗處處理一小時,再以 發光二極體照設不同時間。生物膜再生長實驗則是將一經光動力治療抑制處理的生物膜 圓錠擺回新的反應器。無論懸浮或生物膜狀態的 Pseudomonas aeruginosa,光動力治療 抑制微生物的效果隨著光感物質濃度或照光時間的增加而增強。綠膿桿菌以轉盤式流動 培養器培養 24 小時後,在不鏽鋼片上可生長為成熟之生物膜,其密度約為 109 CFU cm-2,暗培養於5、7.5、10 及 20 mM 之 ALA 溶液一小時後進行光照。在光能 240 J cm-2、 10 mM ALA 或 360 J cm-2下、7.5 mM ALA 處理之樣品可達到全殺的效果。以 20 mM ALA
進行光動力抑制生物膜,在光能量240 J cm-2下可達到完全殺菌之效果。若將此光照後
樣品以全新之轉盤式流動培養觀察其再生情況,培養 12 小時後菌數開始回升,在 48
小時後回復到原處理樣品之密度,必須經兩次光動力抑制才能避免生物膜再生。 關鍵字:生物膜、防治、光動力治療、光感物質、抗藥性
Abstract
To demonstrate photodynamic antimicrobial chemotherapy (PACT) against planktonic and biofilm cultures of Pseudomonas aeruginosa, using photoporphyrin IX which could be endogenously synthesized by administrating δ-aminolaevulinic acid (δ-ALA), and a light emitted diode (LED) array to photoactivate the photosensitizer. P. aeruginosa suspended cells or biofilms, grown on a rotating disk reactor, were treated by different concentrations of δ-ALA in the dark for 1 h, followed by LED irradiation for various time. Regrowth experiments were conducted by placed PACT-treated disks back to a sterile reactor. Viable cells were determined by serial dilution and plate counts. Both P. aeruginosa planktonic and biofilm cells were inhibited by PACT with light doses or photosensitizer concentrations increasing. Treatments of planktonic cells with 10 mM δ-ALA and incident dose 240 J cm-2 or 7.5 mM ALA and incident dose 360 J cm-2 led to completely photoinactivation. No viable biofilm cells were found after treatment of 20 mM δ-ALA and incident dose 240 J cm-2. However, regrowth was observed once PACT-treated biofilms were put back to a sterile reactor. Regrowth could be prevented only if biofilm samples were treated PACT twice. δ-ALA-mediated PACT on P. aeruginosa planktonic and biofilm cells was effective, though the detailed mechanism still required further investigation.
1.
Introduction
Antimicrobial infection has been a long-term challenge for researchers, especially when biofilms was taken into consideration. Microbial biofilms are implicated in many healthy problems such as increasing transfer resistance, deteriorating medical materials, and withstanding host immune responses [1]. It has been estimated that as many as 60% of bacterial infections treated by physicians in the developed world are related to biofilm formation [2]. Conventional methods of biofilm control relied on blocking bacteria from attachment, mechanical removal of bacteria from the surface, or applying antimicrobial agents to stop bacterial growth. However, the biofilm cells were much more recalcitrant than their planktonic counterparts [3]. Researchers have known that biofilm formation and resistance are influenced by complex genetic regulation [4]. One of the most exciting developments about biofilm genetic regulation is cell-to-cell communication or known as quorum sensing [5]. Employing quorum sensing signal molecule analogs to jam bacterial intercellular communication and consequently block biofilm formation has been proved promising in biofilm control [6]. However, more detailed investigations on global regulatory networks of biofilm remain to be comprehended before effective application to eradicate biofilm [7].
Another potential alternative of biofilm control is photodynamic antimicrobial chemotherapy (PACT). PACT involves the application of photodynamic therapy (PDT), which employs the interaction between light and certain chemical photosensitizing agents (photosensitizers, PSs). After the target cells were treated with PS, irradiation with light of a suitable wavelength would excite PS to generate reactive oxygen species (ROS), especially singlet oxygen, and lead to oxidative damage [8]. As the improvement of light source and modification of natural and synthetic photosensitizers, PACT has shown remarkable antimicrobial effects against viruses [9, 10], fungi [11], and bacteria [12]. Bertoloni et al. demonstrated that the photocytotoxic activity of methicillin-resistant strains of Staphylococcus aureus (MRSA) treated with hematoporphyrin (Hp) and 10 min irradiation, the survival fraction of MRSA fell by 99.9 % [13]. All these reports showed that both wild types and antibiotic-resistant bacteria were suppressed by PACT. Although PACT has shown effective in planktonic cultures, only a few papers have reported PACT in biofilm cultures.
Wilson et al. used aluminium disulphonated phthalocyanine (AlPcS2) as the PS to treat Streptococcus sanguis biofilms and found no viable streptococci left following PACT with 12.2 J of laser light [14]. Wood et al. provided qualitative description of PACT against the natural oral plaque biofilms by confocal laser scanning microscopy (CLSM) and showed PACT causing loss of adhesion within the biofilm and subsequent loss of bulk biofilm [15]. Employing CLSM to analysis multi-species biofilms after PACT treatment, O’Neill et al. recently reported that the lethal photosensitization occurred predominantly in the outer layers of the stack leaving some of the innermost alive bacteria [16]. This incomplete kill of PACT
on biofilms might be attributed to the inability of the PS to transmit to these inner regions or the failure of light penetration into the biofilms, rather than the biofilm resistance mechanism per se.
In this paper, we demonstrated PACT against planktonic and biofilm cultures of Pseudomonas aeruginosa, using photoporphyrin IX (PpIX) which could be endogenously synthesized by administrating δ-aminolaevulinic acid (δ-ALA) as the PS [17, 18]. The cytotoxic activity of PACT on biofilm via endogenous PS and the regrowth of biofilm cells after PACT were also investigated.
2. Materials and methods
2.1. Strain, medium, and precursor of photosensitizer
P. aeruginosa PAO1 was used in both planktonic and biofilm experiments. Tryptic Soy Broth (TSB; Difco, Detroit, MI, USA) was used as liquid medium throughout. To maintain the cell concentration at 108-109 cells ml-1, 1/10 strength TSB was used for planktonic cultures. Biofilms were cultivated by continuously feeding 1/100 strength TSB. Stock solution of 200 mM δ-ALA (Sigma Chemical Co, St. Louis, MO, USA) in phosphate buffered saline (PBS, pH 7.0) solution was prepared instantly before use.
2.2. Light source
A light emitting diode (LED) array with a major wavelength of 630 nm, designed by Industrial Technology Research Institute (Hsinchu, Taiwan), was used to excite photosensitizer. The fluence of light delivered was 100 mW cm-2.
2.3. PACT on planktonic cultures
P. aeruginosa cells from the overnight culture were harvested by centrifuging at 1,395 g for 10 min and resuspended into about 108 cells ml-1 with sterile PBS. The resultant cell suspension was treated with 0, 2.5, 5, 7.5, 10, and 12.5 mM of δ-ALA, respectively, in the dark for 1 h. After δ-ALA incubation, suspension was separated into two parts. One was irradiated by the LED subsequently for 20, 40, and 60 min, while the other without irradiation was used as control.
2.4. PACT on biofilm cultures
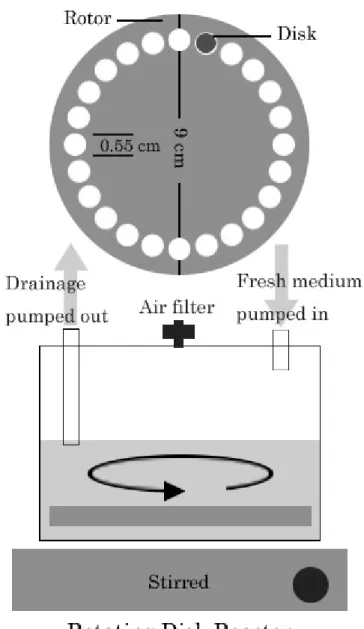
A rotating disk reactor (Figure 1), modified form the design of Pitts et al [19], was used to cultivate P. aeruginosa biofilms. The reactor was made by a 500 ml polypropylene container (Nalgene, Rochester, NY, USA) and consisted of a Teflon rotor and 24 removable 316L stainless steel disks (0.55 cm in diameter) for sampling. The Teflon rotor was embedded with a magnetic stir bar on the bottom and was driven by a stirrer at 60 rpm. For each run, one ml of P. aeruginosa seed culture was transferred into 150 ml 1/10 strength TSB in the reactor and then stirred with the rotating rotor overnight to allow cell attachment. After incubation,
the reactor was fed continuously with 1/100 strength TSB at a rate of 615 ml h-1, a dilution rate of 4.1 h-1, for 24 h to reach the steady state.
The disks with mature biofilms were placed into a 48-well multititer plate and then treated with 350 µl of 0, 5, 10, 20, and 40 mM δ-ALA, respectively, in the dark for 1 h. Four disks treated with different concentrations of δ-ALA were exposed to the LED for 20 min. All of the samples exposed to either light or drug alone were put into test tubes containing 10 ml PBS. The test tubes were vertexed for 1 min in order to remove the biofilms from the disks. The resultant suspension was used for both viable and total cell count.
2.5. Biofilm regrowth after PACT
Biofilms under PACT of 20 mM δ-ALA and 120 J cm-2 irradiation was chosen for regrowth experiments. After PACT, disks with treated biofilms were moved to a new reactor containing 150 ml sterile 1/100 strength TSB and fed at a dilution rate of 4.1 h-1 with 1/100 strength TSB. Sterile blank disks were also put in the new reactor as control. After 12 h, PACT of 10 mM δ-ALA and 20 min irradiation was applied on biofilms. The regrowth circumstances were recorded every 12 h.
2.6. Cell enumeration
Viable-cell numbers were determined by serial dilution and plating on R2A agar (Difco). For total-cell numbers, suitably diluted cell suspensions were stained with 0.01 % (w/v) acridine orange (Sigma) for 1 min and collected on black polycarbonate filter membranes (0.2 µm, Millipore, Cork, Ireland) by filtration. Direct cell counts were calculated using an E600 fluorescent microscopy (Nikon, Tokyo, Japan) by averaging ten microscopic fields.
2.7. Statistical analysis
All experiments were repeated three times. Statistical analyses were performed with Excel 2002 (Microsoft) and were based upon the log transformational means.
3. Results
3.1. PACT on planktonic cultures
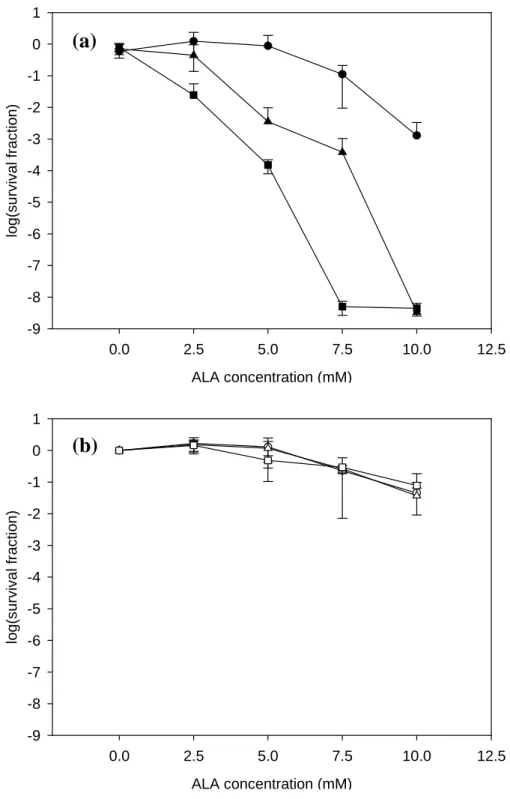
The δ-ALA mediated PACT on planktonic P. aeruginosa is shown in Figure 2. When samples treated with PS and without exposure to LED, almost all of cells remained viable up to 5 mM of δ-ALA. The survival fraction reduced to 0.26±0.03, 0.053±0.022 and 0.0042±0.0009 when treated with 7.5, 10 and 12.5 mM of δ-ALA, respectively. When samples were exposed to a light dose of 120 J cm-2, no significant reduction in survival fraction was found under 0, 2.5, 5 and 7.5 mM of δ-ALA treatment. Significant decrease in survival fraction was observed when 10 and 12.5 mM of δ-ALA were applied. When samples were under exposure to 240 J cm-2, survival fraction decreased significantly as the concentrations of δ-ALA were over 5
mM. No viable cells were detected when treated with 10 and 12.5 mM of δ-ALA. This amounted to 8 logsof the cells being inactivated and similar result was also found in the cases of 360 J cm-2 exposure. No cells were survived when the concentrations of δ-ALA were more than 7.5 mM.
3.2. PACT on biofilm cultures
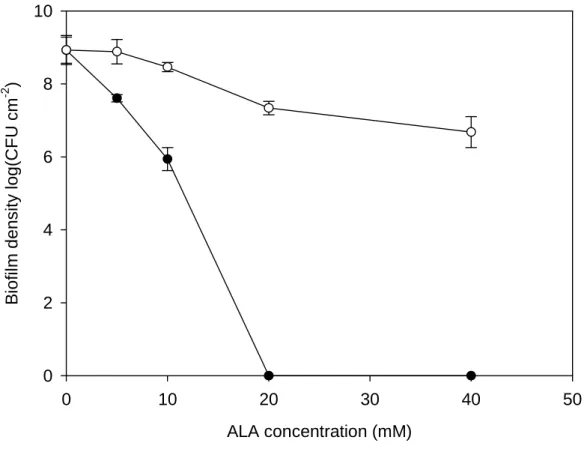
Figure 3 illustrates the δ-ALA mediated PACT on P. aeruginosa biofilms. Without exposure to LED irradiation, the biofilm density did not vary a lot when δ-ALA treatment was less than 10 mM. When treated with 20 and 40 mM δ-ALA, about 2-log reduction in biofilm density was observed. When exposed to 120 J cm-2, the biofilm density decreased with increasing concentrations of δ-ALA treatment. No viable cells were detected when 20 and 40 mM of δ-ALA were applied. It meant that biofilm density of (1.16 ± 0.17) × 109 cells cm-2 all suffered from PACT and could not recover on agar plates.
3.3. Biofilm regrowth after PACT
Figure 4 shows P. aeruginosa biofilm regrowth after δ-ALA-mediated PACT. After biofilm samples were treated with 20 mM of δ-ALA and exposed to 120 J cm-2 light dose, the disks with biofilms were put back into a sterilized reactor and the conditions of biofilm cultivation were resumed. For the disk without PACT, biofilm cell density maintained steady at c. 9.6±0.2 log CFU cm-2 during the regrowth period. No viable cell was detected on the PACT-treated disk until 24 h of regrowth. Biofilms appeared to re-accumulate thereafter and reached 7.2±0.8 log CFU cm-2 after 48 h of regrowth. In the meantime, a sterile blank disk was also placed in the regrowth reactor. No biofilm accumulation was observed until some detached cells reattached to the disk at 48 h of regrowth. To completely eliminate the possibility of regrowth, the PACT treated biofilms were allowed for recover for 12 h, followed by another PACT treatment in a separate experiment. The biofilm was completely eradicated and not a single viable cell was found at the end of experiment.
4. Discussion
Biofilms are infamous for their recalcitrance to antimicrobial agents. Mechanisms proposed to explain this enhanced resistance in bacteria within biofilms can be divided into three categories: transport limitation, physiological adaptation and cell-to-cell communication. PACT on biofilms is deemed promising since the mechanism of PDT is irrelative to biofilm recalcitrance. PACT will be effective as long as PS is able to diffuse into the biofilm cells, the light is able to penetrate the biofilm to irradiate PS, and a series of molecular energy transfers occur. Consequently, the energy transfers will lead to the liberation of cytotoxic singlet oxygen from PS, such a release being lethal to many different types of bacterial cell [20]. δ-ALA, an early intermediate in the heme biosynthesis pathway in cell, had no problem in
diffusion into P.aeruginosa. The red LED used in this study can easily penetrate at least 2 mm depth of tissue. The result of PACT on planktonic P. aeruginosa showed significant photodynamic degradation as expected and was consistent with the finding of Szocs et al. [12]. The minor reduction in viable cells without exposure to light may be due to a direct toxicity of high concentration of δ-ALA or interference with P aeruginosa homeostasis.
δ-ALA-mediated PACT on P. aeruginosa biofilms seemed potential for medical applications. Most devastating chronic infections are caused by pathogen lived as biofilms. Once a biofilm was formed, it required hundreds of times of antibiotic dose to achieve similar antimicrobial efficacy as in their planktonic counterparts. In this study, no viable biofilm cells were detected after treatment with 20 mM δ-ALA and followed by a 120 J cm-2 incident dose. When the PACT-treated disk was put back to a sterile reactor, no regrowth of P. aeruginosa biofilm cells was observed for the first 12 h. However, regrowth of biofilm cells occurred after 24 h. This observation was quite consistent with the understanding of biofilm recalcitrance. Anwar et al. found regrowth of the P. aeruginosa biofilm occurred after the termination of antibiotic therapy [21]. Svensson et al. reported that it was not possible to prevent regrowth of Staphylococcus epidermidis biofilms by increasing antibiotic concentration further [22]. In study of urinary tract infection, Sano et al. also found reproliferation of MRSA occurred immediately after withdrawal of the antimicrobial agent [23]. In our experiments, regrowth was prevented after a pulse-chase-pulse PACT treatment. In other words, it took two rounds of PACT to eliminate the possibility of biofilm regrowth. Although PACT on biofilms seemed promising, further investigation regarding the mechanism of PACT was required.
Acknowledgements
The financial support of the National Science Council of the ROC (Grant No. NSC91-2320-B-002-198) is greatly appreciated.
References
[1] D.M. Cochrane, M.R. Brown, H. Anwar, P.H. Weller, K. Lam and J.W. Costerton, Antibody response to Pseudomonas aeruginosa surface protein antigens in a rat model of chronic lung infection, J Med Microbiol 27 (1988) 255-261.
[2] J.W. Costerton, P.S. Stewart and E.P. Greenberg, Bacterial biofilms: a common cause of persistent infections, Science 284 (1999) 1318-1322.
[3] J.C. Nickel, I. Ruseska, J.B. Wright and J.W. Costerton, Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material, Antimicrob Agents Chemother 27 (1985) 619-624.
[4] J.W. Costerton, Z. Lewandowski, D.E. Caldwell, D.R. Korber and H.M. Lappin-Scott, Microbial biofilms, Annu Rev Microbiol 49 (1995) 711-745.
[5] D.G. Davies, M.R. Parsek, J.P. Pearson, B.H. Iglewski, J.W. Costerton and E.P. Greenberg, The involvement of cell-to-cell signals in the development of a bacterial biofilm, Science 280 (1998) 295-298.
[6] M. Hentzer, K. Riedel, T.B. Rasmussen, A. Heydorn, J.B. Andersen, M.R. Parsek, S.A. Rice, L. Eberl, S. Molin, N. Hoiby, S. Kjelleberg and M. Givskov, Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound, Microbiology 148 (2002) 87-102.
[7] L. Hall-Stoodley and P. Stoodley, Developmental regulation of microbial biofilms, Curr Opin Biotechnol 13 (2002) 228-233.
[8] J. Davila and A. Harriman, Photosensitized oxidation of biomaterials and related model compounds, Photochem Photobiol 50 (1989) 29-35.
[9] L.E. Bockstahler, T.P. Coohill, K.B. Hellman, C.D. Lytle and J.E. Roberts, Photodynamic therapy for herpes simplex: a critical review, Pharmacol Ther 4 (1979) 473-499.
[10] J.M. O'Brien, D.K. Gaffney, T.P. Wang and F. Sieber, Merocyanine 540-sensitized photoinactivation of enveloped viruses in blood products: site and mechanism of phototoxicity, Blood 80 (1992) 277-285.
[11] J.S. Friedberg, C. Skema, E.D. Baum, J. Burdick, S.A. Vinogradov, D.F. Wilson, A.D. Horan and I. Nachamkin, In vitro effects of photodynamic therapy on Aspergillus fumigatus, J Antimicrob Chemother 48 (2001) 105-107.
[12] K. Szocs, F. Gabor, G. Csik and J. Fidy, delta-Aminolaevulinic acid-induced porphyrin synthesis and photodynamic inactivation of Escherichia coli B, J Photochem Photobiol B 50 (1999) 8-17.
[13] G. Bertoloni, F.M. Lauro, G. Cortella and M. Merchat, Photosensitizing activity of hematoporphyrin on Staphylococcus aureus cells, Biochim Biophys Acta 1475 (2000) 169-174.
a light-activated antimicrobial agent, J Antimicrob Chemother 37 (1996) 377-381. [15] S. Wood, B. Nattress, J. Kirkham, R. Shore, S. Brookes, J. Griffiths and C. Robinson,
An in vitro study of the use of photodynamic therapy for the treatment of natural oral plaque biofilms formed in vivo, J Photochem Photobiol B 50 (1999) 1-7.
[16] J.F. O'Neill, C.K. Hope and M. Wilson, Oral bacteria in multi-species biofilms can be killed by red light in the presence of toluidine blue, Lasers Surg Med 31 (2002) 86-90. [17] M. Doss, and W.K. Philipp-Dormston, Porphyrin and heme biosynthesis from
Endogenous and exogenous delta-aminolevulinic acid in Escherichia coli, Pseudomonas aeruginosa, and Achromobacter metalcaligenes, Hoppe Seylers Z Physiol Chem, 352 (1971) 725-33.
[18] J.C. Kennedy, and R.H. Pottier, Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy, J Photochem Photobiol B, 14 (1992) 275-92.
[19] B. Pitts, A. Willse, G.A. McFeters, M.A. Hamilton, N. Zelver and P.S. Stewart, A repeatable laboratory method for testing the efficacy of biocides against toilet bowl biofilms, J Appl Microbiol 91 (2001) 110-117.
[20] S. Pervaiz, Reactive oxygen-dependent production of novel photochemotherapeutic agents, Faseb J 15 (2001) 612-617.
[21] H. Anwar, J.L. Strap, K. Chen and J.W. Costerton, Dynamic interactions of biofilms of mucoid Pseudomonas aeruginosa with tobramycin and piperacillin, Antimicrob Agents Chemother 36 (1992) 1208-1214.
[22] E. Svensson, H. Hanberger, M. Nilsson and L.E. Nilsson, Factors affecting development of rifampicin resistance in biofilm-producing Staphylococcus epidermidis, J Antimicrob Chemother 39 (1997) 817-820.
[23] M. Sano, T. Hirose, M. Nishimura, S. Takahashi, M. Matsukawa and T. Tsukamoto, Inhibitory action of clarithromycin on glycocalyx produced by MRSA, J Infect Chemother 5 (1999) 10-15.
ALA concentration (mM) 0.0 2.5 5.0 7.5 10.0 12.5 log( su rv ival fr ac tion) -9 -8 -7 -6 -5 -4 -3 -2 -1 0 1 ALA concentration (mM) 0.0 2.5 5.0 7.5 10.0 12.5 log( s u rv iva l fr acti on) -9 -8 -7 -6 -5 -4 -3 -2 -1 0 1
Figure 2. PACT on P. aeruginosa planktonic cultures with (a) and without (b) exposure to
LED. Cell concentration about 108 cells ml-1 was treated by PACT. After treatment with different δ-ALA concentrations, samples were exposed to 120 J cm-2 (solid circle), 240 J cm-2 (solid triangle), and 360 J cm-2 (solid square). For experiment without LED irradiation, samples were kept in the dark for 20 min (open circle), 40 min (open triangle), and 60 min (open square) as controls. (n=3, bars indicate standard errors.).
(a)
ALA concentration (mM) 0 10 20 30 40 50 B iofil m d ensit y l og(CF U cm -2 ) 0 2 4 6 8 10
Figure 3. PACT on P. aeruginosa biofilms. After biofilms were treated with different
concentrations of δ-ALA for 1 h, exposure to 120 J cm-2 light dose (solid circle) caused biofilm density decreasing which related to δ-ALA concentration. Samples without irradiation were as the control (open circle). (n=3, bars indicate standard errors)
Time of regrowth (h) -36 -24 -12 0 12 24 36 48 60 Bio film den s ity log( C F U cm -2 ) 0 2 4 6 8 10 12
Figure 4. P. aeruginosa biofilm regrowth after PACT treatment. (open circle): Biofilm cells
without PACT; (solid triangle): 24-h-old biofilms after one-time PACT; (open triangle): a sterile blank disk; (gray square): 24-h-old biofilms after one-time PACT , regrowth for 12 h, and followed by another PACT (arrow means the start of the second PACT). (n=3, bars indicate standard errors.)