o r i g i n a l a r t i c l e
The n e w e n g l a n d j o u r n a l of m e d i c i n eAdefovir Dipivoxil for the Treatment
of Hepatitis B e Antigen–Negative
Chronic Hepatitis B
Stephanos J. Hadziyannis, M.D., Nicolaos C. Tassopoulos, M.D., E. Jenny Heathcote, M.D., Ting-Tsung Chang, M.D., George Kitis, M.D.,
Mario Rizzetto, M.D., Patrick Marcellin, M.D., Seng Gee Lim, M.D., Zachary Goodman, M.D., Michael S. Wulfsohn, M.D., Ph.D., Shelly Xiong, Ph.D.,
John Fry, B.Sc., and Carol L. Brosgart, M.D., for the Adefovir Dipivoxil 438 Study Group*
From the Department of Medicine and Hep-atology, Henry Dunant Hospital, Athens, Greece (S.J.H.); Western Attica General Hospital, Athens, Greece (N.C.T.); Toronto Western Hospital, University of Toronto, To-ronto (E.J.H.); the Department of Internal Medicine, National Cheng Kung Universi-ty Hospital, Tainan, Taiwan (T.-T.C.); Geor-gios Papanikolaou Hospital, Thessaloniki, Greece (G.K.); Azienda Ospedaliera San Giovanni Battista, Turin, Italy (M.R.); Serv-ice d’Hépatologie, INSERM Unité 481, and Centre de Recherche Claude Bernard sur les Hépatites Virales, Hôpital Beaujon, Clichy, France (P.M.); the Division of Gastroenter-ology, National University Hospital, Sin-gapore (S.G.L.); the Armed Forces Institute of Pathology, Washington, D.C. (Z.G.); and Gilead Sciences, Foster City, Calif. (M.S.W., S.X., J.F., C.L.B.). Address reprint requests to Dr. Hadziyannis at the Department of Medicine, Henry Dunant Hospital, 107 Mesogion Ave., Athens 11526, Greece, or at hadziyannis@ath.forthnet. gr. *Other members of the Adefovir Dipivoxil
438 Study Group are listed in the Appendix. N Engl J Med 2003;348:800-7.
Copyright © 2003 Massachusetts Medical Society.
b a c k g r o u n d
Adefovir dipivoxil, a nucleotide analogue, demonstrated clinically significant antiviral activity in patients with chronic hepatitis B in phase 1 and 2 clinical trials.
m e t h o d s
We randomly assigned 185 patients with chronic hepatitis B who were negative for hep-atitis B e antigen (HBeAg) to receive either 10 mg of adefovir dipivoxil or placebo once daily for 48 weeks in a 2:1 ratio and a double-blind manner. The primary end point was histologic improvement.
r e s u l t s
At week 48, 64 percent of patients who had base-line liver-biopsy specimens available in the adefovir dipivoxil group had improvement in histologic liver abnormalities (77 of 121), as compared with 33 percent of patients in the placebo group (19 of 57, P<0.001). Serum hepatitis B virus (HBV) DNA levels were reduced to fewer than 400 copies per milliliter in 51 percent of patients in the adefovir dipivoxil group (63 of 123) and in 0 percent of those in the placebo group (0 of 61, P<0.001). The median decrease in log-transformed HBV DNA levels was greater with adefovir dipivoxil treatment than with placebo (3.91 vs. 1.35 log copies per milliliter, P<0.001). Alanine aminotransferase levels had normalized at week 48 in 72 percent of patients receiving adefovir dipivoxil (84 of 116), as compared with 29 percent of those receiving placebo (17 of 59, P<0.001). No HBV polymerase mutations associated with resistance to adefovir were identified. The safety profile of adefovir dipivoxil was similar to that of placebo.
c o n c l u s i o n s
In patients with HBeAg-negative chronic hepatitis B, 48 weeks of adefovir dipivoxil treatment resulted in significant histologic, virologic, and biochemical improvement, with an adverse-event profile similar to that of placebo. There was no evidence of the emergence of adefovir-resistant HBV polymerase mutations.
a d e f o v i r f o r h beag- neg a t i v e h e p a t i t i s b
ore than 350 million people worldwide are chronically infected with hepatitis B virus (HBV). Complications of chronic hepatitis B, such as cirrhosis, hepatocel-lular carcinoma, and end-stage liver disease, account for approximately 1 million deaths each year.1 Liver injury seems to be particularly severe and rapidly progressive in patients with chronic hepatitis B who are negative for serum hepatitis B e antigen (HBeAg) and positive for antibodies against HBeAg (anti-HBe), but in whom clinically significant HBV repli-cation persists.2 For the most part, HBeAg-negative chronic hepatitis B is due to HBV mutations that suppress synthesis of HBeAg. Most patients with HBeAg-negative, anti-HBe–positive, and HBV DNA– positive chronic hepatitis B harbor HBV variants with mutations in the precore or core promoter re-gion.3 (The core region encodes HBeAg.) This form of the disease occurs throughout the world and is more common than was previously believed.4,5 In the Mediterranean region and Southeast Asia, 50 to 80 percent of patients with chronic hepatitis B have HBeAg-negative disease.2,6 Because of its high ende-micity and chronicity, HBeAg-negative chronic hep-atitis B has become a major public health concern.
HBeAg-negative chronic hepatitis B is usually progressive. Sustained spontaneous remission is rare; the disease is characterized by persistent or in-termittent HBV replication, severe necroinflamma-tion of the liver, and progressive fibrosis.4,7,8 Cir-rhosis and hepatocellular carcinoma occur at a relatively high rate, and approximately 40 percent of patients in some studies have histologically con-firmed cirrhosis.9,10 Sustained biochemical remis-sion has been associated with a decreased rate of hepatocellular carcinoma and death among patients treated with interferon.11
No optimally effective and tolerable treatment is available for patients with HBeAg-negative chronic hepatitis B. Interferon alfa has antiviral and immu-nomodulatory effects that can induce virologic and biochemical remission in such patients, but the re-sponse is seldom sustained after the termination of treatment.12 In addition, long-term treatment with interferon alfa is problematic because of the fre-quent adverse effects and the need for parenteral administration. Lamivudine is a well-tolerated, oral-ly administered agent that suppresses HBV replica-tion in HBeAg-negative patients. Most patients who have a response to lamivudine therapy relapse once treatment is stopped.13,14 However, the benefit of long-term maintenance treatment with lamivudine
is compromised by the development of drug resist-ance. Lamivudine-resistant viral mutants have been reported in up to 32 percent of patients after one year of treatment with lamivudine.15 This compli-cation is particularly important, since patients with HBeAg-negative chronic hepatitis B are especially likely to need sustained therapy to prevent long-term sequelae, because they rarely have hepatitis B surface antigen (HBsAg) seroconversion.
Adefovir dipivoxil (Hepsera, Gilead Sciences) is an oral prodrug of adefovir, a phosphonate nucleo-tide analogue of AMP with potent activity against the polymerase activity of hepadnaviruses, retrovi-ruses, and herpesviruses.16 Preliminary clinical tri-als showed that it decreased serum HBV DNA and alanine aminotransferase levels at doses of 5, 30, and 60 mg per day in both HBeAg-positive and HBeAg-negative patients with chronic hepatitis B and resulted in HBeAg seroconversion in HBeAg-positive patients.17,18 Treatment has been found to be safe and well tolerated, with a low incidence of adverse events, and no adefovir-resistant HBV poly-merase mutations have been reported to date.
We conducted this placebo-controlled study to investigate the safety and efficacy of 48 weeks of treatment with adefovir dipivoxil at a dose of 10 mg once daily in patients with HBeAg-negative chronic hepatitis B. We report the 48-week results, but the study is ongoing and will continue for up to 5 years. When the study was designed, lamivudine was still an investigational agent; therefore, placebo was se-lected as the control.
s t u d y d e s i g n
This multicenter, double-blind, placebo-controlled trial was conducted at 32 sites in Canada, Greece, Is-rael, France, Italy, Australia, Taiwan, and Singapore. Patients were enrolled between January 10, 2000, and June 7, 2000. The study was conducted in com-pliance with the Declaration of Helsinki and ap-proved by appropriate regulatory bodies at all cen-ters. All patients gave written informed consent. Patients were randomly assigned in a double-blind manner to receive 10 mg of oral adefovir dipivoxil or placebo once daily for 48 weeks in a 2:1 ratio. The central randomization was stratified according to five geographic regions. Permuted blocks (with a block size of six) were used in each stratum.
Liver biopsies were required within six months before screening or before receiving study treatment
m
The n e w e n g l a n d j o u r n a l of m e d i c i n e
and at week 48. Virologic and biochemical assess-ments (serum HBV DNA and alanine aminotrans-ferase levels, prothrombin time, and blood chemical tests) and adverse-event monitoring were conduct-ed every four weeks. Serum creatinine and phospho-rus levels were assayed at base line and every four weeks thereafter to monitor renal function. Geno-typic analyses of HBV mutations were performed at base line and week 48. Although patients with a to-tal Knodell score below 2 were eligible, there were no such patients in the study. After week 48, patients who were assigned to adefovir dipivoxil were ran-domly reassigned to receive either continued treat-ment or placebo. Patients who had originally re-ceived placebo were assigned to receive adefovir dipivoxil. This part of the study is ongoing and re-mains blinded.
Clinical data were collected, monitored, and en-tered into a data base by Quintiles, a contract re-search organization. Laboratory tests were con-ducted by Covance. The sponsor held the data and conducted the statistical analyses. Academic inves-tigators had access to the data. Each author made a substantial contribution to the study design, the interpretation of the results, or the drafting or revis-ing of the article; all approved the final manuscript.
p a t i e n t s
Male and female patients 16 to 65 years of age who had HBeAg-negative chronic hepatitis B and com-pensated liver disease were eligible. Chronic hepa-titis B was defined by the presence of detectable HBsAg for at least six months, undetectable HBeAg, detectable anti-HBe, a serum HBV DNA level of at least 105 copies per milliliter, and an alanine amino-transferase level between 1.5 and 15 times the upper limit of the normal range. Patients had to have a to-tal bilirubin level of no more than 2.5 mg per deci-liter (42.7 µmol per deci-liter), a prothrombin time that was no more than one second above the normal range, a serum albumin level that was at least 3 g per deciliter, a serum creatinine level of no more than 1.5 mg per deciliter (133 µmol per liter), and an ad-equate blood count.
Criteria for exclusion included a coexisting seri-ous medical or psychiatric illness; immune globu-lin, interferon, or other immune- or cytokine-based therapies with possible activity against HBV disease within 6 months before screening; organ or bone marrow transplantation; recent treatment with systemic corticosteroids, immunosuppressants, or chemotherapeutic agents; a serum
alpha-fetopro-tein level of at least 50 ng per milliliter; evidence of a hepatic mass; liver disease that was not due to hep-atitis B; prior therapy for more than 12 weeks with a nucleoside or nucleotide analogue with activity against HBV; and seropositivity for human immu-nodeficiency virus or hepatitis C or D virus.
e n d p o i n t s
The primary, predefined efficacy end point was his-tologic improvement, defined as a reduction of at least 2 points in the Knodell necroinflammatory score, with no concurrent worsening of the Knodell fibrosis score.19 Knodell scores were assessed by an independent histopathologist who was unaware of the patients’ treatment assignments and the tim-ing of liver biopsy. Ranked assessments of necro-inflammatory activity and fibrosis (improved, no change, or worse) were also performed.
Secondary end points included the change from base line in serum HBV DNA levels, the effect of treatment on alanine aminotransferase levels, and the proportion of patients with HBsAg seroconver-sion. Serum HBV DNA was measured by the Roche Amplicor polymerase-chain-reaction (PCR) assay, with a lower limit of detection of 400 copies per milliliter.
s a f e t y a n a l y s i s
The primary safety analysis included all patients who received at least one dose of study medication and all events that occurred during treatment or within 30 days after the discontinuation of study drug. The severity of adverse events and laboratory abnormalities was graded according to the Com-mon Toxicity Criteria of the National Institute of Al-lergy and Infectious Diseases.20
r e s i s t a n c e s u r v e i l l a n c e
HBV DNA was isolated from serum samples at base line and week 48 and amplified by PCR. The posi-tive and negaposi-tive strands of the HBV polymerase gene spanning the polymerase–revertranscrip-tase domain (amino acids 349 to 692) were se-quenced. The HBV sequences of samples obtained at base line and week 48 from the same patient were aligned with the MegAlign program (DNAStar).
s t a t i s t i c a l a n a l y s i s
The study was designed to enroll 180 patients and to have at least 90 percent power to detect an abso-lute difference of 30 percent between groups (60 percent vs. 30 percent) with respect to the primary
a d e f o v i r f o r h beag- neg a t i v e h e p a t i t i s b
end point, assuming that 25 percent of patients would have missing biopsy specimens at week 48 or base-line Knodell scores of less than 2 and would therefore be counted as having no response and that 8 percent would have missing biopsy specimens at base line and would thus be excluded from the pri-mary efficacy analysis.
Statistical analyses included all patients who re-ceived at least one dose of study drug. The analysis of histologic end points included a subgroup of this population that had an assessable base-line bi-opsy specimen. For the primary efficacy end point, an unstratified Cochran–Mantel–Haenszel test was used, conducted at a nominal two-sided a level of 0.05. All confidence intervals, significance tests, and resulting P values were two-sided, with an a lev-el of 0.05. All HBV DNA values that were less than 400 copies per milliliter were considered to be at the lower limit of detection (400 copies per millili-ter). No interim analyses were performed on these data other than safety-data summaries, which were prepared every six months for a review by the inde-pendent, external data-monitoring committee.
c h a r a c t e r i s t i c s o f t h e p a t i e n t s
Of the 185 patients, 123 were randomly assigned to receive 10 mg of adefovir dipivoxil per day, and 62 to receive placebo. One patient who was assigned to re-ceive placebo never rere-ceived treatment and was ex-cluded from all analyses. Demographic and other base-line characteristics were similar in the two study groups (Table 1). Seventy-six patients (41 per-cent) had previously been treated with interferon alfa.
h i s t o l o g i c r e s p o n s e
The primary analysis was based on 178 patients (97 percent) with assessable base-line liver-biopsy spec-imens. A total of 167 patients (91 percent) had as-sessable pretreatment and post-treatment liver-biopsy specimens. Significantly more patients in the adefovir dipivoxil group than in the placebo group had histologic improvement, as defined by a reduction of at least two points in the Knodell necro-inflammatory score, with no worsening of fibrosis, the primary efficacy end point (77 of 121 [64 per-cent] vs. 19 of 57 [33 perper-cent]; P<0.001; absolute difference, 30.3 percent; 95 percent confidence in-terval for the difference, 15.4 to 45.2).
Treatment with adefovir dipivoxil also resulted in significant decreases in the total Knodell score
r e s u l t s
* One patient in the placebo group who never received treatment was excluded from the analysis. ULN denotes upper limit of the normal range, and HBV hepatitis B virus.
† Values were log-transformed with use of a base 10 scale. ‡ Some patients had received more than one type of medication.
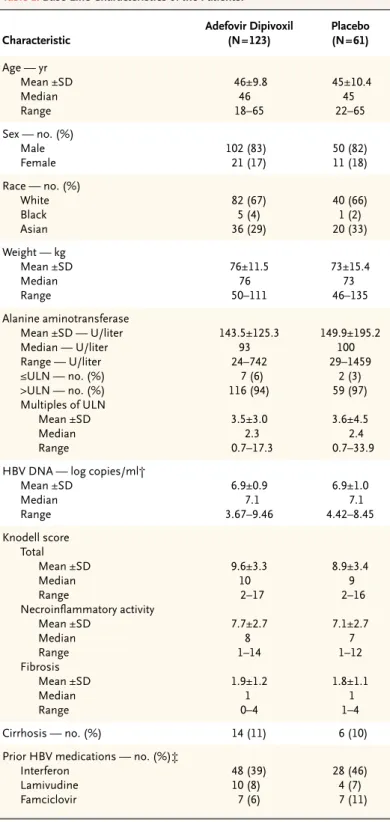
Table 1. Base-Line Characteristics of the Patients.*
Characteristic Adefovir Dipivoxil (N=123) Placebo (N=61) Age — yr Mean ±SD Median Range 46±9.8 46 18–65 45±10.4 45 22–65 Sex — no. (%) Male Female 102 (83) 21 (17) 50 (82) 11 (18) Race — no. (%) White Black Asian 82 (67) 5 (4) 36 (29) 40 (66) 1 (2) 20 (33) Weight — kg Mean ±SD Median Range 76±11.5 76 50–111 73±15.4 73 46–135 Alanine aminotransferase Mean ±SD — U/liter Median — U/liter Range — U/liter ≤ULN — no. (%) >ULN — no. (%) Multiples of ULN Mean ±SD Median Range 143.5±125.3 93 24–742 7 (6) 116 (94) 3.5±3.0 2.3 0.7–17.3 149.9±195.2 100 29–1459 2 (3) 59 (97) 3.6±4.5 2.4 0.7–33.9 HBV DNA — log copies/ml†
Mean ±SD Median Range 6.9±0.9 7.1 3.67–9.46 6.9±1.0 7.1 4.42–8.45 Knodell score Total Mean ±SD Median Range Necroinflammatory activity Mean ±SD Median Range Fibrosis Mean ±SD Median Range 9.6±3.3 10 2–17 7.7±2.7 8 1–14 1.9±1.2 1 0–4 8.9±3.4 9 2–16 7.1±2.7 7 1–12 1.8±1.1 1 1–4 Cirrhosis — no. (%) 14 (11) 6 (10) Prior HBV medications — no. (%)‡
Interferon Lamivudine Famciclovir 48 (39) 10 (8) 7 (6) 28 (46) 4 (7) 7 (11)
The n e w e n g l a n d j o u r n a l of m e d i c i n e
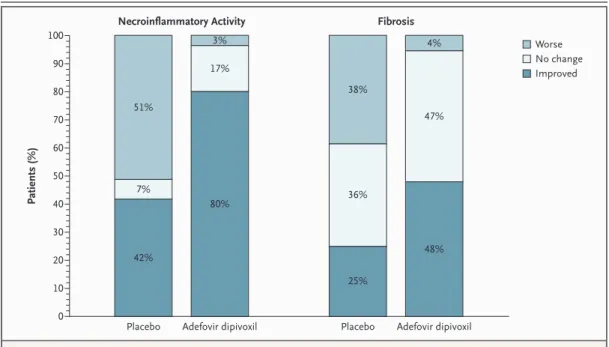
(P<0.001), the necroinflammatory score (P<0.001), and the fibrosis score (P=0.005) (Table 2). Ranked assessment showed a significant improvement in necroinflammatory activity (P<0.001) and fibrosis
(P<0.001) among patients in the adefovir dipivoxil group as compared with the placebo group (Fig. 1), and worsening of necroinflammatory activity and fibrosis was seen in a greater proportion of placebo recipients.
v i r o l o g i c r e s p o n s e
At week 48, serum HBV DNA levels were reduced by a median of 3.91 log copies per milliliter in the adefovir dipivoxil group, as compared with 1.35 log copies per milliliter in the placebo group (P<0.001) (Fig. 2). Fifty-one percent of patients in the adefovir dipivoxil group (63 of 123) had undetectable HBV DNA levels, as compared with 0 percent (0 of 61) in the placebo group (P<0.001).
b i o c h e m i c a l r e s p o n s e
At week 48, significantly more patients in the adef-ovir dipivoxil group than in the placebo group had normalized alanine aminotransferase levels (84 of 116 [72 percent] vs. 17 of 59 [29 percent], P<0.001). The median decrease in alanine aminotransferase levels from base line to 48 weeks was 55 U per liter in the adefovir dipivoxil group and 38 U per liter in the placebo group (P=0.01).
Figure 1. Ranked Assessment of Necroinflammatory Activity and Fibrosis.
Each pair of liver-biopsy specimens (one obtained at base line and one obtained at week 48) was assessed to determine the change in necroinflammatory activity and fibrosis (improved, no change, or worse). Because of rounding, percentag-es may not total 100.
Adefovir dipivoxil
Placebo Placebo Adefovir dipivoxil
Patients (%) 100 90 80 70 60 50 40 30 20 10 0
Necroinflammatory Activity Fibrosis
Worse No change Improved 51% 7% 42% 3% 17% 80% 38% 36% 25% 4% 47% 48%
* P values were calculated with the Wilcoxon rank-sum test.
Table 2. Changes in Knodell Scores from Base Line to Week 48 among
Patients with Assessable Liver-Biopsy Specimens at Base Line and Week 48.
Variable Adefovir Dipivoxil (N=112) Placebo (N=55) P Value*
Change in total Knodell score Mean ±SD Median Range ¡3.7±3.1 ¡4 ¡11 to 2 0.4±3.7 1 ¡9 to 8 <0.001
Change in Knodell necroinflammatory score Mean ±SD Median Range ¡3.4±2.9 ¡3 ¡9 to 2 0.3±3.2 0 ¡7 to 8 <0.001
Change in Knodell fibrosis score at week 48 Mean ±SD Median Range ¡0.3±0.7 0 ¡3 to 1 0.1±0.9 0 ¡2 to 2 0.005
a d e f o v i r f o r h beag- neg a t i v e h e p a t i t i s b
r e s i s t a n c e p r o f i l e
The polymerase–reverse-transcriptase domain of the HBV polymerase gene was sequenced from se-rum samples obtained at base line and week 48 from 117 patients with detectable serum HBV DNA lev-els. Four different novel substitutions occurred at conserved sites in the HBV polymerase in three pa-tients, all of whom were in the placebo group. In vi-tro phenotypic analyses showed that viruses with the mutations remained fully susceptible to adefovir.
a d v e r s e e v e n t s
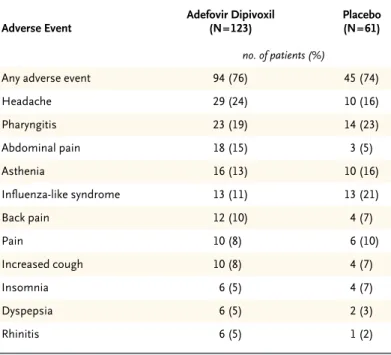
The rate of clinical adverse events was similar in the two groups: 76 percent of patients in the adefovir dipivoxil group (94 of 123 patients) and 74 percent of patients in the placebo group (45 of 61 patients) had at least one adverse event. Severe (grade 3 or 4) adverse events were reported in 7 of 123 patients (6 percent) in the group receiving adefovir dipivoxil and in 6 of 61 patients (10 percent) receiving place-bo. Headache and abdominal pain occurred more often in the adefovir dipivoxil group than in the pla-cebo group (Table 3). These events were generally mild or moderate, and none led to the discontinua-tion of study drug. One patient stopped taking adef-ovir dipivoxil after HIV infection was diagnosed.
Four patients in the placebo group (7 percent) had serious adverse events (hip abscess, transient ischemic attack, acute hepatitis, and sialadenitis), as did four patients (3 percent) in the adefovir dipiv-oxil group (perianal abscess, pain after liver biopsy, dengue fever, and renal colic). None of these events were considered to be related to treatment. With the exception of decreases in hepatic aminotransferase levels associated with adefovir dipivoxil treatment, no notable differences in laboratory values were ob-served between the two groups. There were no sig-nificant differences between the two groups in the changes in serum creatinine or serum phosphorus values. Clinically significant elevations in alanine aminotransferase levels were not associated with concurrent changes in serum bilirubin or albumin levels or the prothrombin time in patients receiving either adefovir dipivoxil or placebo.
The goal of therapy for patients with HBeAg-neg-ative chronic hepatitis B is to arrest or slow the pro-gression of HBV-associated hepatic injury, which can otherwise result in cirrhosis and hepatocellular carcinoma.9,10 In contrast to HBeAg-positive
pa-tients, in whom stopping therapy is a possibility in the event of HBeAg seroconversion associated with the normalization of alanine aminotransferase lev-d i s c u s s i o n
Figure 2. Median Change in Serum Levels of Hepatitis B Virus (HBV) DNA
from Base Line to Week 48.
Placebo P<0.001 No. Evaluated Placebo Adefovir dipivoxil 61 123 61 120 57 116 58 115 58 122 58 117 56 116 58 121 59 119 57 119 59 121 60 122 55 117 Change in HBV DNA (log copies/ml) Week of Study 0 ¡1 ¡2 ¡3 ¡4 Base line 4 8 12 16 20 24 28 32 36 40 44 48 Adefovir dipivoxil
* Adverse events were defined according to a modified Coding Symbols for The-saurus of Adverse Reaction Terms. One patient in the placebo group who nev-er received treatment was excluded from the analysis. Patients may have had more than one adverse event.
Table 3. Adverse Events Reported by at Least 5 Percent of Patients
in the Adefovir Dipivoxil Group.*
Adverse Event Adefovir Dipivoxil (N=123) Placebo (N=61) no. of patients (%)
Any adverse event 94 (76) 45 (74) Headache 29 (24) 10 (16) Pharyngitis 23 (19) 14 (23) Abdominal pain 18 (15) 3 (5) Asthenia 16 (13) 10 (16) Influenza-like syndrome 13 (11) 13 (21) Back pain 12 (10) 4 (7) Pain 10 (8) 6 (10) Increased cough 10 (8) 4 (7) Insomnia 6 (5) 4 (7) Dyspepsia 6 (5) 2 (3) Rhinitis 6 (5) 1 (2)
The n e w e n g l a n d j o u r n a l of m e d i c i n e
els and suppression of HBV DNA, the majority of patients with HBeAg-negative chronic hepatitis B are likely to require long-term therapy. In most HBeAg-negative patients, in the absence of HBsAg seroconversion, HBV DNA replication recurs and alanine aminotransferase levels become elevated after therapy is stopped.13,14 Therefore, therapy for HBeAg-negative hepatitis B must result in sustained antiviral suppression and have a low likelihood of the emergence of resistance.
We found that 48 weeks of treatment with adef-ovir dipivoxil, an orally administered nucleotide analogue, results in histologic, virologic, and bio-chemical responses without inducing viral resist-ance or clinically significant adverse events in pa-tients with HBeAg-negative chronic hepatitis B. Sequencing of the HBV polymerase gene demon-strated that there were no polymerase gene muta-tions associated with resistance to adefovir. This finding is in contrast to the experience with lamiv-udine and consistent with the findings of earlier studies in which no HBV polymerase mutations as-sociated with resistance to adefovir were identified in patients with HBeAg-positive or HBeAg-negative chronic hepatitis B who were treated with adefovir dipivoxil for up to 136 weeks.21,22
The majority of the patients who received adef-ovir dipivoxil had a decrease in necroinflammatory activity and fibrosis. Previous trials of adefovir dipiv-oxil therapy for HBeAg-positive chronic hepatitis B have had similar results.23 In contrast, many pa-tients in the placebo group had an increase in both necroinflammatory activity and fibrosis over the course of the study.
Serum HBV DNA levels decreased rapidly after the commencement of adefovir dipivoxil therapy and continued to decline throughout the 48 weeks of treatment, a result consistent with the previously
observed antiviral effect of this agent.18,23-27 Serum HBV DNA levels were below the lower limit of de-tection (fewer than 400 copies per milliliter) in 51 percent of the patients given adefovir dipivoxil, as compared with 0 percent in the placebo group. Com-parisons of the ability of various antiviral agents, including lamivudine and interferon, to suppress the activity of HBV DNA to undetectable levels are complicated by the use of assays of different sensi-tivities. Many studies of other agents have used mo-lecular hybridization assays in which the lower lim-it of detectabillim-ity is as high as 1 million copies per milliliter.
Adefovir dipivoxil was well tolerated. None of the patients withdrew from the study because of an ad-verse event attributable to treatment with adefovir dipivoxil. Except for elevations in aminotransferase levels, which occurred more often among placebo recipients, severe (grade 3 or 4) adverse events oc-curred with similar frequencies in the adefovir dipiv-oxil and placebo groups. The overall adverse-event profile of a 10-mg dose of adefovir dipivoxil was similar to that of placebo, and there were no chang-es in renal variablchang-es, such as were reported with higher doses (30 mg or more).
Treatment with adefovir dipivoxil improved his-tologic liver abnormalities, reduced serum HBV DNA levels, and normalized alanine aminotrans-ferase levels. The absence of resistance mutations during 48 weeks of therapy is a potentially impor-tant advantage, since the majority of patients with HBeAg-negative chronic hepatitis B will require long-term therapy.
Supported by Gilead Sciences.
Drs. Wulfsohn, Xiong, and Brosgart and Mr. Fry are employees of Gilead Sciences and have reported equity ownership in Gilead Scienc-es. Drs. Hadziyannis, Heathcote, Marcellin, and Goodman report hav-ing served as consultants to Gilead Sciences. Drs. Hadziyannis and Marcellin report having served as paid lecturers for Gilead Sciences.
a p p e n d i x
In addition to the authors, the Adefovir Dipivoxil International Investigator 438 Study Group includes the following persons: A. Alberti and S. Boccato (Universitá di Padova, Padua, Italy); G.D. Anagnostopoulus (Western Attica Hospital, Athens, Greece); P. Angus and R. Vaughan (Austin and Repatriation Medical Centre, Melbourne, Australia); K. Barange and J.-M. Conbis (Hôpital Purpan, Toulouse, France); F. Bon-ino and B. Coco (Azienda Ospedaliera Pisana, Pisa, Italy); C.-H. Wu and K.-C. Tseng (National Cheng Kung University Hospital, Tainan, Taiwan); G. Cooksley and G. MacDonald (Royal Brisbane Hospital, Brisbane, Australia); P. Desmond and S. Brown (St. Vincent’s Hospital, Melbourne, Australia); A. Francavilla and F. Malcangi (Azienda Ospedaliera Consorziale Policlinico, Bari, Italy); G. Papatheodoridis and V. Sevastianos (Henry Dunant Hospital, Athens, Greece); K. Kaita and G.Y. Minuk (University of Manitoba Health Sciences Centre, Winnipeg, Man., Canada); Y.-F. Liaw and R.-N. Chien (Chang Gung Memorial Hospital, Taipei, Taiwan); Y.-Y. Dan (National University Hospital, Sin-gapore); Y. Lurie and R. Pakula (Tel Aviv Sourskey Medical Center, Tel Aviv, Israel); L. Castelnau and N. Boyer (Hôpital Beaujon, Clichy, France); M. Ngu (Concord Repatriation General Hospital, Concord, Australia); E. Nussenson and O. Segol (Haemek Hospital, Afula, Israel); G. Pastore (Azienda Ospedaliera Consorziale, Bari, Italy); M. Lagget (Azienda Ospedaliera San Giovanni Battista, Turin, Italy); S. Sacks and J. Farley (Viridae Clinical Sciences, Vancouver, B.C., Canada); D. Samuel and J. Duches-Villie (Hôpital Paul Brousse, Villejuif, France); M. Sherman and A. Bartolucci (Toronto General Hospital, Toronto); W. Sievert, A. Dev, and S. Warner (Monash Medical Centre, Clayton, Aus-tralia); C. Trépo and M. Maynard (Hôtel Dieu, Lyons, France); D. Vetter and S. Metzger (Hôpital Civil de Strasbourg, Strasbourg, France); J.-P. Villeneuve and B. Willems (Centre Hospitalier Universitairé de Montréal Campus Saint-Luc, Montreal); S.S. Chen, C. James, M. Kraus, J. Ma, S. Nonaka-Wong, J. Rooney, and M. Wollman (Gilead Sciences, Foster City, Calif.); N. Virk and O. Cohen (Quintiles, Rockville, Md., and Bracknell, Berkshire, United Kingdom); and D. Hunt (Covance, Lafayette, Ind.).
a d e f o v i r f o r h beag- neg a t i v e h e p a t i t i s b
r e f e r e n c e s
1. Hepatitis B. Fact sheet WHO/204. Geneva: World Health Organization, October 2000. (Accessed January 7, 2003, at http://www. who.int/inf-fs/en/fact204.htm.)
2. Hadziyannis SJ. Hepatitis B e antigen negative chronic hepatitis B: from clinical recognition to pathogenesis and treatment. Viral Hepat Rev 1995;1:7-36.
3. Lok ASF, McMahon BJ. Chronic hepati-tis B. Hepatology 2001;34:1225-41.
4. Hadziyannis SJ, Vassilopoulos D. Hepa-titis B e antigen-negative chronic hepaHepa-titis B. Hepatology 2001;34:617-24.
5. Funk ML, Rosenberg DM, Lok AS. World-wide epidemiology of HBeAg-nega-tive chronic hepatitis B and associated pre-core and pre-core promoter variants. J Viral Hepat 2002;9:52-61.
6. Lindh M, Horal P, Dhillon AP, Furuta Y, Norkrans G. Hepatitis B virus carriers with-out precore mutations in hepatitis B e-antigen negative stage show more severe liver dam-age. Hepatology 1996;24:494-501.
7. Bonino F, Rosina F, Rizzetto M, et al. Chronic hepatitis in HBsAg carriers with serum HBV-DNA and anti-HBe. Gastroen-terology 1986;90:1268-73.
8. Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000 — sum-mary of a workshop. Gastroenterology 2001; 120:1828-53.
9. Hadziyannis S, Bramou T, Makris A, Moussoulis G, Zignego L, Papaioannou C. Interferon alfa-2b treatment of HBeAg neg-ative/serum HBV DNA positive chronic active hepatitis type B. J Hepatol 1990;11:Suppl 1: S133-S136.
10.Brunetto MR, Oliveri F, Demartini A, et al. Treatment with interferon of chronic hepatitis B associated with antibody to hep-atitis B e antigen. J Hepatol 1991;13:Suppl 1:S8-S11.
11.Lin SM, Sheen IS, Chien RN, Chu CM, Liaw YF. Long-term beneficial effect of inter-feron therapy in patients with chronic hepa-titis B virus infection. Hepatology 1999;29: 971-5.
12.Manesis EK, Hadziyannis SJ. Interferon a treatment and retreatment of hepatitis B e antigen-negative chronic hepatitis B. Gastro-enterology 2001;121:101-9.
13.Santantonio T, Mazzola M, Iacovazzi T, Miglietta A, Guastadisegni A, Pastore G. Long-term follow-up of patients with anti-HBe/HBV DNA-positive chronic hepa-titis B treated for 12 months with lamivudine. J Hepatol 2000;32:300-6.
14.Tassopoulos NC, Volpes R, Pastore G, et al. Post lamivudine treatment follow up of patients with HBeAg negative chronic hepatitis B. J Hepatol 1999;30:Suppl 1:117. abstract.
15.Dienstag JL, Schiff ER, Wright TL, et al. Lamivudine as initial treatment for chronic hepatitis B in the United Sates. N Engl J Med 1999;341:1256-63.
16.De Clercq E. Antiviral activity spectrum and target of action of different classes of nucleoside analogues. Nucl Nucl Nucleic Acids 1994;13:1271-95.
17.Heathcote EJ, Jeffers L, Wright T, et al. Loss of serum HBV DNA and HBeAg and ser-oconversion following short-term (12 weeks) adefovir dipivoxil therapy in chronic hepati-tis B: two placebo-controlled phase II studies. Hepatology 1998;28:Suppl:317A. abstract.
18.Heathcote, EJ, Jeffers L, Perrillo R, et al. Serum HBV DNA suppression and serocon-version following long-term adefovir dipiv-oxil therapy in chronic hepatitis B patients. Hepatology 2001;34:316A. abstract.
19.Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological
activity in asymptomatic chronic active hep-atitis. Hepatology 1981;1:431-5.
20.Common toxicity criteria, version 2. Bethesda, Md.: National Cancer Institute, 1999. (Accessed February 4, 2003, at http:// ctep.info.nih.gov.)
21.Lau DT-Y, Khokhar MF, Doo E, et al. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology 2000;32:828-34.
22.Yang H, Westland CE, Delaney WE, et al. Resistance monitoring in chronic hepatitis B patients exposed to adefovir dipivoxil for 72 to 136 weeks. Hepatology 2001;34:316A. abstract.
23.Marcellin P, Chang T-T, Lim SG, et al. Adefovir dipivoxil for the treatment of hepa-tits Be antigen–positive chronic hepatitis B. N Engl J Med 2003;348:808-16.
24.Perrillo R, Schiff E, Hann H-WL, et al. The addition of adefovir dipivoxil to lamivu-dine in decompensated chronic hepatitis B patients with YMDD variant HBV and reduced response to lamivudine — preliminary 24 week results. Hepatology 2001;34:349A. abstract.
25.Schiff ER, Neuhaus P, Tillmann H, et al. Safety and efficacy of adefovir dipivoxil for the treatment of lamivudine resistant HBV in patients post liver transplantation. Hepa-tology 2001;34:446A. abstract .
26.Perrillo R, Schiff E, Yoshida E, et al. Adef-ovir dipivoxil for the treatment of lamivu-dine-resistant hepatitis B mutants. Hepatol-ogy 2000;32:129-34.
27.Benhamou Y, Bochet M, Thibault V, et al. Safety and efficacy of adefovir dipivoxil in patients co-infected with HIV-1 and lamivu-dine-resistant hepatitis B virus: an open-label pilot study. Lancet 2001;358:718-23. Copyright © 2003 Massachusetts Medical Society.