科技部補助產學合作研究計畫成果精簡報告
具皮膚保護功效的蓮霧花原料開發與應用
計 畫 類 別 : 技術及知識應用型
計 畫 編 號 : NSC 102-2622-B-041-001-CC3
執 行 期 間 : 102 年 11 月 01 日至 103 年 10 月 31 日
執 行 單 位 : 嘉藥學校財團法人嘉南藥理大學化妝品應用與管理系(含化妝 品科技碩士班)
計 畫 主 持 人 : 劉家全 共 同 主 持 人 : 楊彩秀
處 理 方 式 : 1.公開資訊:立即公開
2.「本研究」是否已有嚴重損及公共利益之發現:否 3.「本報告」是否建議提供政府單位施政參考:否
中 華 民 國 104 年 01 月 26 日
中 文 摘 要 : 蓮霧生長過程,會去除過多的蓮霧花來維持蓮霧的品質。現 今蓮霧花相關的研究極少,若能開發出蓮霧花的新功效,即 能將廢棄之蓮霧花再利用,更能提升蓮霧果樹在台灣的經濟 地位。另合作企業欲尋求開發一台灣本土天然、低成本、安 全且有效的化妝品原料,加上先前研究認為蓮霧花在皮膚保 護上應深具潛力。因此本計劃欲開發蓮霧花的抗色素沉著與 防皺抗老化功效。計畫中利用蓮霧花酒精萃取物進行抗氧化 成份分析後,進一步以細胞模式研究抗紫外線傷害試驗。觀 察紫外線誘發膠原蛋白降解後,蓮霧花是否能抑制紫外線傷 害正常皮膚纖維母細胞,是否因其抗氧化特性清除了紫外線 產生的自由基,使自由基誘發的 MMP-1 生成量降低,進而抑 制膠原蛋白的分解。我們期望能提供正確科學研究數據給合 作之廠商,以利蓮霧花皮膚保護產品之開發(廠商擬推出防皺 抗老化面膜與美白保養品)。透過此合作計畫的執行,提升合 作廠商的產業競爭力與研發能力。參與本計劃的研究團隊成 員同時可獲得專案研究的實務經驗。
中文關鍵詞: 蓮霧花 皮膚老化 UVA 膠原蛋白 基質金屬蛋白酶
英 文 摘 要 : Ultraviolet A (UVA) irradiation contributes to major changes in skin aging as a result of the
downregulation of procollagen I content. This process is likely mediated by matrix metalloproteinases.
Identification of phytonutrients that can increase the amount of collagen synthesis may improve anti- aging therapy. Wax apple is one of important economic crops in in Asia, whereas the phytoprotective effect of wax apples against UV light-induced skin aging remained mostly uncertain. In the present study, we investigated the antioxidant capacity and protective effects against UVA damage from alcoholic extract of the wax apple flowers (AEWA) in human dermal
fibroblasts Hs68 cells. We then explored the
improving effects of AEWA on UVA-induced decrement of procollagen I and investigated the probable mechanism underlying those effects. Hs68 cells were treated with AEWA for the indicated times followed by UVA irradiation. The antioxidant ability of AEWA was evaluated by various in vitro assessments. The phytoprotective effect of AEWA on UVA-induced procollagen downregulation and metalloproteinase-1 (MMP-1) expression/activity were examined using
western blotting and gelatin zymography. The
malondialdehyde (MDA) accumulation measured by method of thiobarbituric acid-reactive substances (TBARS).
Levels of NF B and tissue inhibitor of
metalloproteinase-1 (TIMP-1) were assayed by ELISA.
In vitro assessment revealed that AEWA exhibited remarkable anti-oxidative potential, including scavenging capacity for oxidative radicals, inhibitory effect on lipid peroxidation, and
chelating ability of ferrous ion. Chronic exposure of UVA irradiation to Hs68 cells with AEWA prevented the UVA-induced cell death and the downregulation of procollagen I in a dose-dependent manner. Meanwhile, this AEWA treatment also ameliorated the UVA-induced the upregulation/activation of matrix
metalloproteinase-1 (MMP-1), the production of malondialdehyde (MDA), and the intracellular NF B levels. Furthermore, AEWA administration restored and increased the UVA-suppressed activity of TIMP-1, one of MMP inhibitors. Our findings highlighted the phytoprotective effect of AEWA against UV light- induced skin aging and may provide an alternative option in cosmetic and pharmaceutical applications in anti-aging skin care.
英文關鍵詞: flowers of wax apple; skin aging; UVA; procollagen I; MMP-1
科技部補助產學合作研究計畫成果精簡(進度)報告
具皮膚保護功效的蓮霧花原料開發與應用
計畫類別:□ 先導型 □ 開發型 ▓ 技術及知識應用型 計畫編號:NSC 102-2622-B-041 -001 -CC3
執行期間:102 年 11 月 01 日至 103 年 10 月 31 日 執行單位:嘉南藥理大學
計畫主持人:劉家全 共同主持人:楊彩秀
計畫參與人員: 李怡儀、賴貞羽
處理方式:
1.立即公開
(依規定,精簡報告係可供科技部立即公開之資料,並以 4
至 10 頁為原則,如有圖片或照片請以附加檔案上傳,如因涉及專利、技術移轉 案或其他智慧財產權、影響公序良俗或政治社會安定等,而不宜對外公開者,請 勿將其列入精簡報告)
2.本研究是否有嚴重損及公共利益之發現:▓否 □是
3.本報告是否建議提供政府單位參考 ▓否 □是, (請列舉提供之單位;
本部不經審議,依勾選逕予轉送。)
中 華 民 國 104 年 01 月 26 日
附件二計畫查核點自評表(請逐年填列)
一、 本表為本計畫重要審查資訊,本表之期程可視產學合作計畫執行情況予以設定。(例如按月
別、季別、半年別等均可)。
重要工作項目
查核內容概述(力求量化表示) 廠商參與情形概述
期程一 期程二 期程一 期程二
A蓮 霧 花 酒 精 萃 取物製備、分 析與細胞模式 建立
A1蓮霧花花苞收 集與乾燥粉製 作
A1-1完成分批收集 10~12月當季農民疏 花丟棄的蓮霧花花苞 50公斤以上
A1-2完成花苞進行清 洗整理烘乾,磨粉後得 到蓮霧花乾燥粉,儲存 於乾燥箱,至少得20 公斤以上乾燥粉,再分 批進行萃取
A1-1透過廠商與農會農
民聯繫蓮霧花花苞收集 A1-2廠商派遣人員觀摩
A2蓮霧花酒精萃 取流程
A2-1完成蓮霧花乾 燥粉與酒精比例1:5 或1:2萃取條件探討
A2-2完成不同時間 (2,5,10小時)萃取對 萃取率的提升評估
A2-1廠商提供一些酒精 萃取文獻討論酒精比例 條件
A2-2廠商派出研究人員 參與萃取
A3蓮霧花酒精萃 取物有效成分 分析
A3-1分析有效成分 沒食子酸的HPLC方 法移動相甲醇與 0.2%磷酸(3:97或 5:95)比例確立
A3-2建立沒食子酸標 準曲線以求得蓮霧花 酒精萃取物中每mg中 沒食子酸的定量分析
A3-1HPLC儀器由計畫 主持人提供,由廠商進 行HPLC方法確立
A3-2廠商之研究人員確 立蓮霧花酒精萃取物中 每 mg 中 沒 食 子 酸 的 含 量
A4抑制黑色素生 成細胞模式建 立
A4-1建立B16細胞生 長曲線,雙倍數時間 約8小時,細胞呈現完 整
A4-2以黑色素刺激素 300nm MSH刺激細 胞(1~3天)產生大量黑 色素
A4-1廠商派遣研究人員 學習細胞技術
A4-2廠商派出研究人員 之建立自己細胞平台
A5紫外線照射分
解膠原蛋白細 胞模式
A5-1建立Hs68細胞 生長曲線,雙倍數時 間約14小時,細胞呈 現完整
A5-2不同劑量紫外線 (5~25焦耳 )照射細胞 分解膠原蛋白,決定細 胞膠原蛋白的降低
A5-1廠商與計畫主持人 確認紫外線照射細胞模 式建立
A5-2廠商派遣人員觀摩
B蓮霧花酒精萃取 物抗色素沉著 與抗老化評估
B1細胞培養萃取
物的濃度與時 間選定
B1-1分別測試(10
~200g/ml)蓮霧花酒 精萃取物對兩種細胞 模式的濃度確立
B1-2分別測試蓮霧花 酒精萃取物對兩種細 胞模式的時間(12~48 小時)選定
B1-1廠商進行蓮霧花酒 精萃取物的培養細胞劑 量與時間確認
B1-2廠商派出研究人員 參與數據整理
B2B16 細 胞 毒 性 與黑色素生成 量評估
B2-1蓮霧花酒精萃 取物(10~200g/ml) 對B16細胞的存活率 影響
B2-2蓮霧花酒精萃取 物(10~200g/ml)對 B16細胞經MSH刺激 後黑色素生成影響與 酪胺酸酶活性評估
B2-1廠商派出研究人員 參與毒性評估試驗
B2-2廠商確認蓮霧花酒 精萃取物的抗色素沉著 功效數據
B3B16 細 胞 中 抗 氧化狀態評估
B3-1蓮霧花酒精萃 取物對B16細胞內 MDA抑制的影響
B3-2以流式細胞儀測 定 單 一 B16 細 胞 處 理 蓮霧花酒精萃取物細 胞內ROS生成的改變
B3-1廠商派遣人員觀摩 B3-1廠商派遣人員觀摩
B4 B16細胞MITF 含量與Hs68細 胞毒性測試
B4-1蓮霧花酒精萃 取物(10~200g/ml) 對細胞內MITF含量 的變化影響
B4-2蓮霧花酒精萃取 物 (5~100g/ml) 對 Hs68細胞的存活率影 響 (Hs68 細 胞 較 為 敏 感,因此選用濃度設定 範圍較低)
B4-1廠商派出研究人員 確認蓮霧花酒精萃取物 的作用機制
B4-2廠商與主持人討論 抗色素沉著數據
B5Hs68細胞中抗 氧化狀態評估
B4-1蓮霧花酒精萃 取物(5~100g/ml)對 Hs68細胞內MDA抑 制的影響
B4-2以流式細胞儀測 定單一Hs68細胞處理 蓮霧花酒精萃取物細 胞內ROS生成的改變
B4-1廠商收集細胞內抗 氧化文獻以供結果討論
B4-1廠商派遣人員觀摩 流式細胞儀
B6Hs68細胞膠原 蛋白與MMP-1 含量
B5-1蓮霧花酒精萃 取物(5~100g/ml)對 Hs68細胞內因紫外 線照射分解膠原蛋白 含量的影響
B5-2蓮霧花酒精萃取 物 (5~100g/ml) 對 Hs68細胞內因紫外線 照射MMP-1變化評估
B5-1廠商派出人員了解 膠原蛋白分析過程
B5-2廠商與主持人討論 抗紫外線數據
二、本產學合作計畫預估後續發展情形概述:
計畫執行及結束後之計畫如何配合追蹤管考、產品產出與開發規劃、預期可推廣至產業或市場之 成果、預估可授權商品、預估應用價值及產值、建立平台、主要發現等(簡要敘述成果,內容須 包含是否已有嚴重損及公共利益之發現;如已有嚴重損及公共利益之發現,請簡述可能損及之層
面及相關程度)。
本產學計畫執行過程中與廠商聯繫緊密,每個階段廠商皆派員參與計畫。同時計畫結束後計畫主持人 與廠商願隨時配合追蹤蓮霧花萃取物的應用。我們目前共同開發蓮霧花萃取物之功能性與商品化設 計,結合蓮霧花之萃取技術、功效性評估、產品配方設計、產品商品化等階段,使蓮霧花萃取物應用 於皮膚保護相關之保養品應用領域。廠商已試量產蓮霧花萃取物,並添加於保養品中,並進一步地進 行膚質相關檢測,並進行後續之產品調製與劑型設計開發工作,最後進行產品之銷售。我們的研究成 果並未嚴重損及公共利益之發現。我們也已申請專利保護,參加國內外生技展,打開市場知名度,預 期可提昇國內農產品的應用性及產值,使蓮霧花原本為無用之廢棄物,加以加工利用大幅增加其附加 價值。還可讓廠商建立細胞平台,可利用於未來公司化妝品方面的發展。
本產學合作計畫研發成 果 及 績 效 達 成 情 形 自 評 表 成果項目
本產學合作計畫預估研究成果及績效指標(作為本計畫後續管考之參據) 計畫達成情形
技術移轉 預計技轉授權 1 項 完成技轉授權 1 項
專利
國內 預估 1 件 提出申請 1 件,獲得 1 件
國外 預估 件 提出申請 件,獲得 件
人才培育
博士 人,畢業任職於業界 人 博士 人,畢業任職於業界
人
碩士 1 人,畢業任職於業界 1 人 碩士 1 人,畢業任職於業
界 0 人
其他 1 人,畢業任職於業界 1 人 其他 1 人,畢業任職於業
界 0 人
論文著作
國內
期刊論文 件 發表期刊論文 件
研討會論文 件 發表研討會論文 件
SCI論文 件 發表SCI論文 件
專書 件 完成專書 件
技術報告 件 完成技術報告 件
國外
期刊論文 件 發表期刊論文 件
學術論文 件 發表學術論文 件
研討會論文 件 發表研討會論文 件
SCI/ SSCI論文 1 件 發表SCI/ SSCI論文 1 件
專書 件 完成專書 件
技術報告 件 完成技術報告 件
其他協助產業發展
之具體績效 新公司或衍生公司 0 家 設 立 新 公 司 或 衍 生 公 司 ( 名
稱):
計畫產出成果簡 述:請以文字敘述 計畫非量化產出之 技術應用具體效 益。(限600字以內)
本計畫的成果直接驗證假說,進而增進我們瞭解蓮霧花具皮膚保護功效以及其 機制。完成探討細胞模式下蓮霧花酒精萃取物對色素沉著的抑制效果與對紫外 線傷害模式下之作用情形。協助廠商建立的細胞研究平台,促進公司的研發能 力,開創公司的未來新方向。計畫中所用的蓮霧花為現今農民疏花丟棄的花,
開發出蓮霧花的功效,即能將廢棄之蓮霧花再利用,未來便可幫助農民收入,
更能提升蓮霧果樹在台灣的經濟地位。
C012A-4 共 頁 第 頁
研究摘要(500 字以內) :
蓮霧生長過程,會去除過多的蓮霧花來維持蓮霧的品質。現今蓮霧花相關的研究極少,若能開 發出蓮霧花的新功效,即能將廢棄之蓮霧花再利用,更能提升蓮霧果樹在台灣的經濟地位。另合作 企業欲尋求開發一台灣本土天然、低成本、安全且有效的化妝品原料,加上先前研究認為蓮霧花在 皮膚保護上應深具潛力。因此本計劃欲開發蓮霧花的抗色素沉著與防皺抗老化功效。計畫中利用蓮 霧花酒精萃取物進行抗氧化成份分析後,進一步以細胞模式研究抗紫外線傷害試驗。觀察紫外線誘 發膠原蛋白降解後,蓮霧花是否能抑制紫外線傷害正常皮膚纖維母細胞,是否因其抗氧化特性清除 了紫外線產生的自由基,使自由基誘發的 MMP‐1 生成量降低,進而抑制膠原蛋白的分解。我們期望 能提供正確科學研究數據給合作之廠商,以利蓮霧花皮膚保護產品之開發(廠商擬推出防皺抗老化面 膜與美白保養品)。透過此合作計畫的執行,提升合作廠商的產業競爭力與研發能力。參與本計劃的 研究團隊成員同時可獲得專案研究的實務經驗。
人才培育成果說明:
計畫中培育一名大專生與一名碩士生,學習細胞技術、實驗數據之整理等能力。
技術研發成果說明:
採用以紫外線誘發膠原蛋白分解模擬皮膚之光老化模式下,蓮霧花酒精萃取物抑制紫外 線對正常皮膚纖維母細胞的老化及其可能作用機制;計畫的研究方法評估抗色素沉著與 抗皮膚光老化功效。此外,計畫中將技術移轉至合作之廠商和協助廠商利用以建立之平 台。
技術特點說明:
利用萃取技術加上完整的細胞皮膚保護評估平台,開發出蓮霧花皮膚保護產品將極具有 市場的競爭力。
可利用之產業及可開發之產品:
蓮霧花酒精萃取物屬於天然萃取物,成本低(由丟棄的蓮霧花而來),可屬於新型態之新 穎性原物料,擁有美白與抗老化(除皺)的作用,將增加產品之市場競爭力,因此,未 來將不再需要同時購買兩種產品,可以增加產品的購買意願。
推廣及運用的價值:
可增加廠商產值與增加蓮霧花的附加價值。
Introduction
The skin is the largest organ of the body, the immune system is the most important part, is also the first line of defense to protect the body from any bacterial invasion. Skin aging in general can be divided into natural skin aging and extrinsic skin aging. Natural skin aging can be affected by genetic factors, and
extrinsic skin aging is caused by external factors, including UV-light irradiation and environmental pollutants (cigarette, air pollution) [1, 2]. Among all extrinsic factors, the photoaging caused by UV-light irradiation accounts for the most common factors [3, 4, 5]. Due to the increasing destruction of the ozone layer on earth, the intensity of UV-light irradiation also increases. Long-term exposure to such improper environment, the skin usually becomes vulnerable to noxious stimuli and develops various UV irradiation-induced damage, including hyperpigmentation, wrinkles, or skin cancer. Therefore, prevention of UV irradiation-induced damage will be an important issue needed for the anti-aging skin care.
Wax apple (Syzygium samarangense (Blume) Merrill and Perry), also known as java apple, rose apple, bell fruit and wax jambu, is one of the important cash crops in Asia. In Taiwan, this fruit carries high economic importance and was grown mainly in the Southern Taiwan. In addition to serving as economic crop, it has been reported that wax apple modulates insulin signaling and inflammation pathway and ameliorates
insulin-stimulated glucose uptake in cultured hepatocytes [6] and has hypotriglyceridemic and hypoglycemic effects in diet-induced diabetic rats [7]. Several phytonutrients can serve as photoprotectants in culture cell, animal models and clinical trials [8]. However, whether wax apples also possess phytoprotective effect against UV light-induced skin aging remained mostly not understood.
So far the strategy of anti-aging predominantly includes local application of collagen or administration of edible collagen via an oral route to reduce wrinkles and meanwhile increase skin elasticity [1]. However, the source, stability and safety of collagen are still of very high concern. Therefore, identification of certain compound or phytonutrient that can increase the amount of collagen synthesis would be encouraged to improve anti-aging therapy. Since flower-thinning has been shown as a critical step for the maintaining the high quality of fruit during the growth stages [9], the flowers of wax apple are usually discarded and their availability for anti-aging skin care has seldom been attentioned. In the present study, we aimed to investigate whether administration of the extract from the flowers of wax apple could exhibit anti-oxidant capacity and cytoprotective effect on UVA-irradiated human skin dermal fibroblasts. We also evaluated the treatment effect of the extract from the flowers of wax apples on several UVA-induced deteriorating effects, including decreased cell viability, downregulation of procollagen I, the activation of MMP-associated pathways, and the accumulation the products of lipid peroxidation.
2. Results and Discussion
2.1. In vitro assessment of the antioxidant capacity of the flowers of wax apple
For the assessment of the antioxidant capacity of the flowers of wax apple, we extracted the flowers of wax apple with ethanol, and the resulting extract yield, total phenol, and L-ascorbic acid and trolox equivalent antioxidant capacity (TEAC) values are listed in Figure 1A. Comparing with the antioxidants butylated hydroxytoluene (BHT) and vitamin C (Vit C), the alcoholic extract from the flowers of wax apple (AEWA) also exhibited remarkable scavenging ability of AEWA on 1,1-diphenyl-2-picrylhydrazyl radical (Figure 1B), hydroxyl free radical (Figure 1C), the inhibitory effects of AEWA on lipid peroxidation (Figure 1D), and chelating ability of AEWA on Fe+2 (Figure 1E). These in vitro findings indicated that AEWA exhibited remarkable antioxidant capacity and scavenging ability against oxidative stress and free radicals.
Figure 1. Antioxidant capacity of Alcoholic extracts from the flowers of wax apple (AEWA). (A) The extract yield, total phenol, L-ascorbic acid and trolox equivalent antioxidant capacity (TEAC) values of alcoholic extract from the flowers of wax apple (AEWA). Scavenging ability of AEWA on (B)
1,1-diphenyl-2-picrylhydrazyl radical and (C) hydroxyl free radical. (D) Inhibitory effects of AEWA on lipid peroxidation. (E) Chelating ability of AEWA on Fe+2. These indicated antioxidants butylated hydroxytoluene (BHT) and vitamin C (Vit C) were used as postive control. Data shown here are the mean SD of at least three independent experiments.
(A)
(B) (C)
(D) (E)
2.2. AEWA carried no cytotoxicity and exhibited cytoprotection capacity against UVA irradiation-induced cell death in human dermal fibroblast lines
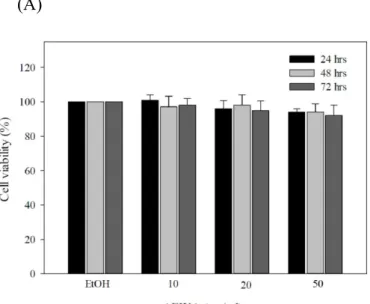
Considering the antioxidant capacity of AEWA, we attempted to assess the utility of AEWA in the treatment of skin abnormalities induced by UV irradiation. We first evaluated if long exposure to AEWA carried cytotoxicity. AEWA was sequentially diluted with ethanol and then added to the human dermal fibroblast lines Hs68 cells, followed by additional culture for 24, 48 or 72 hours. MTT assay was used to analyze the cell viability of the Hs68 lines in response to AEWA treatment. At any given dose or exposure time of AEWA, no treatment effect on cell viability (Figure 2A) and morphology (data not shown) was observed in Hs68 cells, indicating AEWA was non-toxic to Hs68 cells.
Furthermore, we attempted to assess if AEWA treatment prevent human dermal fibroblast lines from UVA-induced insults. Exposure of Hs68 cells to UVA led to the death of approximately 25% of cells, and the residual cells exhibited obvious morphological changes (Figure 2B and 2C). Notably, pretreatment of 50 μg/mL AEWA for 24 hours completely abrogated the UVA-induced cell death (Figure 2B and 2C). These data revealed that chronic treatment with AEWA prevented UVA-induced cell death in human dermal fibroblasts.
Figure 2. Cytoprotective effect of AEWA on UVA-treated human skin fibroblast cells (Hs68 cells). (A) Hs68 cells were treated with various doses of AEWA for indicated time. Cell viability was determined by MTT assay and was expressed as percent viable cells in the total number of cells counted. (B) Cell viability and (C) microscopic examination showing the cytoprotective effect of AEWA on UVA-treated human skin fibroblast cells (Hs68 cells). Briefly, the cells were pre-incubated with 50 μg/mL AEWA for 24 hrs, followed by washing with PBS and further UVA irradiation with 20 J/cm2 UVA, than harvested after 24 hrs. Data shown here are the mean SD of at least three independent experiments. *P< 0.05 vs. 0μg/mL (ethanol alone).
(A)
(B) (C)
2.3. AEWA prevented UVA-induced downregulation of the expression of type I procollagen in human dermal fibroblast lines
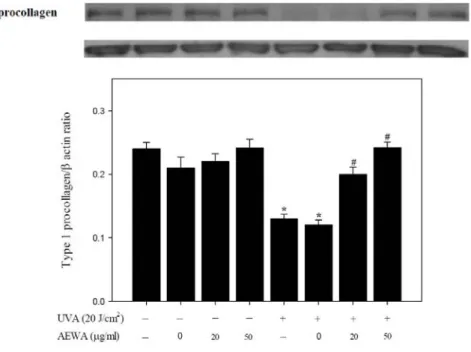
Type I collagen has been known as the most abundant collagen forming more than 90% of the dry weight of dermis and providing structural support, and UVA has been demonstrated to downregulate the expression of type I collagen [1, 10, 11]. To evaluate the utility of AEWA on UVA-induced skin
abnormalities, we analyzed the treatment effect of AEWA on the type I procollagen protein expression in the human dermal fibroblasts Hs68 cells. Chronic exposure of Hs68 cells to various doses of AEWA alone for 24 hours showed no effect on type I procollagen expression, while UVA irradiation with with 20 J/cm2 UVA
largely suppressed type I procollagen protein content as reported previously [12]. Notably, pretreatment with 0, 25, or 50 g/ml AEWA for 24 hrs prevented this UVA-induced type I procollagen downregulation in a dose-related manner (Figure 3). These findings revealed that chronic treatment of AEWA prevented both UVA-induced cell death and the downregulation of type I procollagen in human dermal fibroblasts.
Figure 3. Chronic exposure of Hs68cells to AEWA prevented UVA-induced downregulation of type I procollagen. Hs68 cells were pre-incubated with 0, 20, or 50 μg/mL AEWA for 24 hrs, followed by washing with PBS and further UVA irradiation with 20 J/cm2 UVA (+) or without UVA irradiation (-). Data shown here are the mean SD of at least three independent experiments. *P< 0.05 vs. 0 μg/mL (ethanol alone).
#P< 0.05 vs. UVA alone.
2.4. Prevention of UVA-induced upregulation of MMP-1 by AEWA.
UVA has been known to induce MMPs that lead to the transduction of various signaling pathways and other downstream effects, which eventually contribute to the pathogenesis of photoaging [13, 14, 15].
Considering the cytoprotective effect of AEWA against UVA-induced cell death, we therefore investigated whether AEWA administration also ameliorates UVA-induced MMP-1 upregulation. As detected by Western blotting, AEWA alone did not show any detectable effect on MMP-1 protein expression at any given doses of AEWA (Figure 4A). Exposure of Hs68 cells to UVA irradiation for 20 minutes significantly increased
MMP-1 protein amount. Interestingly, pretreatment of AEWA substantially decreased UVA-induced MMP-1 protein upregulation in a dose-dependent manner (Figure 4A). To further validate this findings of AEWA on MMP-1 regulation, we measured the MMP-1 activity in Hs68 cells receiving various treatment. UVA irradiation largely stimulated the MMP-1 activity. Basal and UVA-stimulated MMP-1 activity was mildly increased by the presence of vehicle ethanol, while the addition of AEWA robustly decreased the MMP-1 activity dose-dependently (Figure 4B). These results demonstrated that the AEWA-mediated cytoprotection against UVA and preservation of type I procollagen amount involves a direct inhibitory effect of AEWA on MMP-1 protein expression and activity.
Figure 4. Inhibitory effect of AEWA on UVA-stimulated MMP-1 protein expression and enzyme
activity in Hs68 cells. Hs68 cells were pre-incubated with 0, 10, 25, or 50μg/mL AEWA for 24 hrs, followed by washing with PBS and further UVA irradiation with 20 J/cm2 UVA (+) or without UVA irradiation (-). The cells were then assigned for (A) Western blotting and (B) the measurement of MMP-1 activity. Data shown here are the mean SD of at least three independent experiments. *P< 0.05 vs. 0 μg/mL (ethanol alone).
#P< 0.05 vs. UVA alone.
(A)
(B)
2.5 Prevention of UVA-induced malondialdehyde accumulation and NF
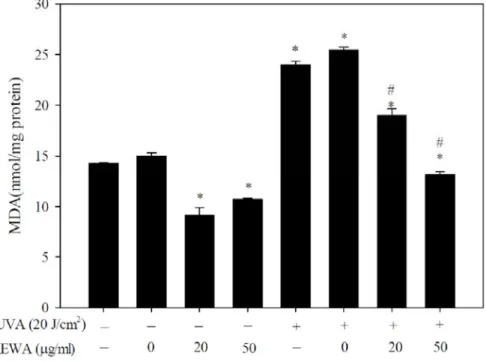
B upregulation by AEWA.Lipid peroxidation has been known as one of MMP-associated responses that could be induced by UVA irradiation [14], and malondialdehyde (MDA), one of the products of lipid peroxidation, has also been employed as the indicatives of oxidative damage [16]. Considering the treatment effect of AEWA on
UVA-mediated induction of MMP-1 expression and activation, we examined whether AEWA treatment also regulated the intracellular accumulation of MDA. Pre-incubation with AEWA alone mildly blunted basal
magnitude of MDA production (Figure 5A). UVA irradiation for 20 minutes acutely increased MDA
accumulation, and pre-incubation of AEWA suppressed UVA-induced MDA accumulation in a dose-related manner (Figure 5A).
UVA irradiation and MMP-1 upregulation were known to elicit several downstream events and activation of transcription factors such as NFB [13]. Chronic administration of AEWA alone mildly suppressed basal NFB levels (Figure 5B). In addition, UVA irradiation highly increased NFB levels and chronic administration of AEWA suppressed NFB levels in a dose-related manner, parallel to the
observations of AEWA effect on MDA accumulation (Figure 5B). These data indicated that chronic administration of AEWA suppressed both UVA-induced MDA accumulation and the elevation of NFB levels in human dermal fibroblasts.
Figure 5. Suppression of UVA-induced MDA accumulation and NFB levels by AEWA in Hs68 cells.
Hs68 cells were pre-incubated with 0, 10, 25, or 50μg/mL AEWA for 24 hrs, followed by washing with PBS and further UVA irradiation with 20 J/cm2 UVA (+) or without UVA irradiation (-). The cells were then assigned for the determination of (A) MDA levels and (B) NFB levels. Data shown here are the mean SD of at least three independent experiments. *P< 0.05 vs. 0 μg/mL (ethanol alone). #P< 0.05 vs. UVA alone.
(A)
(B)
2.6 Prevention of UVA-induced TIMP-1 upregulation by AEWA.
TIMP-1, one of matrix metalloproteinase inhibitors, has been known to have the ability to inhibit the activity of MMPs, including MMP-1, 3, and 9 [17, 18]. Considering the crucial role of TIMP-1 in the regulation of MMP-1 activity, we also examined the TIMP-1 activity in UVA-irradiated dermal fibroblasts with or without AEWA preincubation. Preincubation with AEWA for 24 hours did not affect basal TIMP-1 activity. Exposure to UVA for 20 minutes significantly suppressed basal TIMP-1 activity, and remarkably, AEWA preincubation led to the restoration and increase of the UVA-suppressed TIMP-1 activity in a dose-related manner (Figure 6). These findings indicated that the underlying mechanism of MMP-1 downregulation by AEWA may involve the AEWA-mediated activation of TIMP-1.
Figure 6. Dose-dependent elevation of UVA-suppressed TIMP-1 activity by AEWA in Hs68 cells. Hs68 cells were pre-incubated with 0, 10, 25, or 50μg/mL AEWA for 24 hrs, followed by washing with PBS and further UVA irradiation with 20 J/cm2 UVA (+) or without UVA irradiation (-). The cells were then assigned for the determination of TIMP-1 activity. Data shown here are the mean SD of at least three
independent experiments. *P< 0.05 vs. 0 μg/mL (ethanol alone). #P< 0.05 vs. UVA alone.
2.7 Discussion
The skin is the largest organ of the body as well as the most important part of immune system, and it also provides the first defense that protects the body against exogenous pathogens. The major strategy for anti-aging skin care is aimed to increase the amount of collagen production by direct application or intake of edible collagen. However, the source, quality, and safety of collagen remain still questionable. Therefore, identification of raw material that can increase the amount of collagen synthesis is a very critical issue for the development of skin care and sun care products.
Wax apples [Syzygium samarangense (Blume) Merrill and Perry] have been known as one of the important economic crops in Asia. Notably, wax apples themselves also possess some bioactive effects such as alleviation of insulin resistance, inhibition of inflammatory responses [6, 19], hypotriglyceridemic and hypoglycemic effects [7] Wax apples have been demonstrated to possess anti-inflammatory, antioxidant, anti-hyperglycemic and other effects, nevertheless, the high cost and economic value of wax apples largely restrict the development of raw materials from wax apples. In addition to the fleshy fruit of wax apples, accumulated evidences gradually revealed the biological effect of the leaves of wax apples [20, 21, 22, 23, 24, 25]. Amor et al. reported that compounds possessing inhibitory activity against propyl endopeptidase could be isolated from the leaves of wax apples [20]. In subsequent studies, flavonoids isolated from the leaves of wax apples were demonstrated to exhibit immunomodulatory effect in human peripheral blood mononuclear cells [21], spasmolytic activity [22] and calcium antagonist activity [23] in spontaneously contracting isolated rabbit jejunum, and antihyperglycaemic effect in hyperglycemic mice [24, 25]. Several active compounds have been isolated from the leaves of wax apple. For example, stercurensin [26] and aurentiacin [27] was demonstrated to have anti-inflammatory effects and inhibits nuclear factor-κB-dependent inflammatory signals in vitro and in vivo.
Comparing with the fleshy fruit, pulp [6, 7, 19], and the leaves of wax apples [20, 21, 22, 23, 24, 25], so far the biological effects of the flowers of wax apples remained mostly not understood. Considering that the flower of wax apples are usually removed for the improvement of fruit during growth [9], it is of high interests if the flowers of wax apples can be used for the development of raw materials with bio-active anti-aging capacity. In the present study, we have demonstrated that the alcoholic extract of the flowers from wax apples (AEWA) exhibited remarkable anti-oxidative capacity that can effectively remove oxidative radicals and inhibit lipid peroxidation. Chronic of human dermal fibroblasts Hs68 cells to this extract prevented UVA-induced cell death, down regulation of precollege I, the up regulation/activation of MMP-1, the production of malondialdehyde (MDA), and the intracellular NF B levels. In addition, this effect of AEWA was associated with the high activity of TIMP-1, one of MMP inhibitors. These data strongly suggested the high utility of the wax apple flower against UVA-induced photoaging and concomitant insults.
Using the wax apple flowers to develop a raw materials for anti-aging skin care or functional cosmetics are encouraged and of high feasibility. Further investigations are urgently needed to evaluate the toxicology test and therapeutic efficacy of AEWA in animal studies in vivo and in preclinical studies.
3. Experimental Section 3.1. Reagents
2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS),
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), ethylene diammine tetraacetic acid (EDTA), ascorbic acid, Folin-ciocalteu reagent, Trichloroacetic acid (TCA), thiobarbituric acid (TBA), butylated hydroxytoluene (BHT), 2,4-dinitrophenylhydrazine, tetramethyl murexide (TMM),
2-deoxy-2-ribose, hydrogen peroxide, MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenol tetrazloium bromide], 1,1-diphenyl-2-picrylhydrazyl (DPPH ) were from Sigma Chemical Co. (St. Louis, MO USA). Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), trypsin, penicillin, streptomycin, sodium pyruvate, and non-essential amino acids (NEAA) were from GIBCO/BRL (Rockville, MD USA). All chemicals used were of reagent or higher grade.
3.2. Alcoholic Extraction of the Flowers of Wax Apple
Fresh wax apple flower were obtained from the Taiwan Agricultural Research Institute, Longchuan, Pingtung County, Taiwan. Each fresh flower was air-dried in an oven at 40 °C before analysis. After a fine powder (20 mesh) was obtained using a mill (Restsch Ultra Centrifugal Mill and Sieving Machine, Haan, Germany), flower sample (50 g) was extracted by stirring with 1000 mL of alcohol at 25 °C at 150 rpm for 5 h and filtering through Whatman No. 4 filter paper. The alcoholic extracts were then rotary evaporated at
40 °C to dryness. The dried extract thus obtained was used directly for analyses of antioxidant components or redissolved in alcohol to a concentration of 10 mg/mL and stored at 4 °C for further use.
3.3. Trolox equivalent antioxidant capacity method (TEAC)
Antioxidant capacity of the extract were evaluated by ABTS•+ radical cation decolourisation assay in comparison to trolox standard [28]. The absorbance of the ABTS•+ solution was equilibrated to 0.50 (± 0.02) by diluting with water at room temperature, then 1 ml ABTS•+ solution was mixed with 10 l of the test sample and the absorbance was measured at 734 nm after 6 min. The experiment was repeated six times. The percentage inhibition of absorbance was calculated and plotted as a function of the concentration of standard and sample to determine the trolox equivalent antioxidant concentration (TEAC), calculated as the ratio of the gradients of the plots for the sample to trolox.
3.4. The total content of polyphenols
The total phenol content was determined using Folin-Ciocalteu method done earlier [28]. Briefly, 0.1 ml extract was mixed with 0.75 ml Folin-Ciocalteu reagent (previously diluted 1000-fold with distilled water), followed by the addition of 0.06% Na2CO3 (0.75 ml) solution. After incubation at 22°C for 90 min, the absorbance was taken at 725 nm. All tests were performed six times. The phenol content was evaluated from a gallic acid standard curve.
3.5. Determination of ascorbic acid
Ascorbic acid content quantification was accomplished according to the previously elucidated technique [29]. In brief, 1 ml aliquots of AEWA (1 mg/ml) in water were mixed with 1 ml of
2,4-dinitro-phenylhydrazine reagent and was incubated at 95°C for 15 min. After incubation, 5 ml of 85%
H2S04 was added drop wise to the reaction mixture in ice cold condition. After 30 min, the absorbance was measured at 520 nm. All tests were performed six times.
3.6. DPPH radical scavenging assay
The complementary study for the antioxidant capacity of the extract was confirmed by the DPPH (1,1-diphenyl-2-picrylhydrazyl) scavenging assay according to Mahakunakorn et al. Different concentrations (0-100 g/ml) of the extract and the standard ascorbic acid and BHT were mixed with equal volume of ethanol. Then 50 l of DPPH solution (1 mM) was added into the mixture and stirred thoroughly. The
resulting solution was kept standing for 2 min before the OD was measured at 517 nm. The measurement was repeated with six sets. The percentage of scavenging was calculated from the values of the control and the test samples.
3.7. Hydroxyl radical scavenging assay
The hydroxyl radical scavenging assay was performed using a standard protocol [28], based on quantification of the degradation product of 2-deoxyribose condensed with TBA. Hydroxyl radical was generated by the Fe3+-ascorbate-EDTA-H2O2 system (the Fenton reaction). In a final volume of 1 ml, various concentrations of the test sample or reference compound was mixed with 2-deoxy-2-ribose (2.8 mM);
KH2PO4-KOH buffer (20 mM, pH 7.4); FeCl3 (100 M); EDTA (100 M); H2O2 (1.0 mM); ascorbic acid (100 M) and incubated for 1 h at 37°C. 0.5 ml of the reaction mixture was added to 2.8% TCA, followed by 1% TBA and incubated at 90°C to develop the color of TBARS (Thiobarbituric acid reactive substance).
Absorbance was measured at 532 nm against an appropriate blank solution. All tests were performed six times. Percentage inhibition was evaluated by comparing the test and blank solutions.
3.8. Lipid peroxidation inhibition
The degree of lipid peroxidation was assayed by estimating the thiobarbituric acid reactive substances.
Different concentrations of AEWA were added to 1 ml liver homogenate. Liver peroxidation was initiated by adding 100 μl of 15 mM FeSO4 solution to liver homogenate. After 30 min incubation at 37 °C, 100 μl of this reaction mixture was taken in a tube containing 1.5 ml of 10% TCA. After 10 min tubes were centrifuged and supernatant was mixed with 1.5 ml of 0.67% TBA in 50% acetic acid. The mixture was heated in a water
bath for 30 min. The intensity of colored complex formed was measured at 532 nm. The percentage of inhibition of lipid peroxidation was calculated by comparing the results of test samples with those of control.
3.9. Chelating effects on ferrous ions
Chelating effect was determined according to the method of Mau et al. [30]. To 2 mL of the mixture consisting of 30 mM hexamine, 30 mM potassium chloride, and 9 mM ferrous sulfate were added 2 mL of AEWA (1−20 mg/mL) in alcohol and 200 μL of 1 mM tetramethyl murexide (TMM). After 3 min at room temperature, the absorbance of the mixture was determined at 485 nm. A lower absorbance indicates a higher chelating power.
3.10. Cell Culture and UVA Irradiation
Hs68 cells (human fibroblast cells) used in this study were obtained from Bio-resource Collection and Research Center, BCRC (BCRC, Hsinchu, Taiwan). The cells were grown in DMEM containing 10% (v/v) FBS, 0.12% NaHCO3, penicillin (100 U/ml), streptomycin (100 U/ml), and 5% CO2 in an incubator at 37°C.
For each cell line, a T-75 flask was seeded with 1x 106 cells, and cells were incubated at 37°C. The cells were harvested at ca. 90% confluence (106 cells/flask), and the survival rates were always higher than 95%
by Trypan blue assay. Cell were then incubated with AEWA at 37°C for 24~72 hours.
Irradiation was carried out in a UVA irradiation chamber (XL-1000 UV cross-linker) with an accumulated dose of 20 J/cm2. The UVA light source emits radiation at a range of 320~380 nm with main output at 365 nm. The surface of the mixture was kept at a distance of 3 cm from the filter surface where the light intensity was 2 mW/cm2 sec (or 20 W/m2 sec), as measured using a Vilber Lourmat radiometer. After irradiation, the cells were further washed once with PBS, and supplemented with new DMEM, then harvested after 24hr for western blot assay [8]. Sham-irradiated cells were treated in the same manner except that they were not irradiated.
3.11. Measurement of Cell Viability
The cytotoxic effect of AEWA on cell viability was estimated by MTT assay, as described previously [31]. Cells were cultured in 24-well plates at 1 × 104 cells/well in DMEM for 24 hours, and each well was washed and incubated with 1 ml of DMEM containing various concentration of AEWA at 37 °C for another 24 to 72 hours. After irradiation, the cells were further washed once with PBS, and supplemented with new DMEM, than harvested after 24hr for cell viability. Each well was then incubated with MTT for 1 h, after which the liquid was removed, and DMSO was added to dissolve the solid residue. The optical density at 570 nm of each well was then determined by using a microplate reader (FLUOstar OPTIMA, BMG Labtech GmbH, Germany).
3.12. Measurement of MDA production
Lipid peroxidation was measured as thiobarbituric acid-reactive substances (TBARS) released into the DMEM medium from Hs68 cells following centrifugation at 1,000 g for 10 min. TBARS were measured by mixing equal volumes of the supernatant with 0.7% TBA reagent and 2.5% TCA. BHT (0.5mM) was added to prevent sporadic lipid peroxidation during heating at 100 °C for 10 min. TBARS were extracted with an equal volume (3 ml) of butanol. After a brief centrifugation, the fluorescence of the butanol layer was measured at 515 nm excitation and 555 nm emissions [32]. TBARS were expressed as nmol
malondialdehyde (MDA) equivalent/mg protein using 1,1,3,3-tetramethoxypropane as MDA standard.
3.13. Western Blot Assay
The treated cells were harvested and lysed with 20% SDS containing 1 mM phenylmethylsulfonyl fluoride. The lysate was sonicated for 1 min on ice followed by centrifugation at 12,000×g for 30 min at 4°C.
Mitochondrial and cytosolic fractions were isolated by using the ProteoExtract® Cytosol/Mitochondria Fractionation Kit (Merck Millipore, Billerica, MA, USA). Then a sample of protein from the supernatant was resolved by SDS-PAGE and transferred onto a nitrocellulose membrane. After blocking with TBS buffer (20 mM Tris–HCl, 150 mM NaCl, pH 7.4) containing 5% nonfat milk, the membrane was incubated with
antibodies against type I procollagen, MMP-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by horseradish peroxidase–conjugated secondary antibodies and then was visualized with an ECL
chemiluminescence detection kit (PerkinElmer Life Sciences, Waltham, MA, USA). The relative density of the immunoreactive bands was quantified by using a luminescent image analyzer (LSA-100, Fujifilm, Japan).
3.14 Assay of MMP-1 activity
Culture medium was electrophoresed on a 10% SDS-PAGE gel impregnated with 0.1% gelatin (w/v).
Cells were washed two times for 30 min in 50 mM Tris-HCl (pH 7.5) plus 2.5% Triton X-100 followed by an overnight incubation at 37 °C in 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, and 0.05% NaN3. Gels were stained with Coomassie Brilliant Blue R-350 and then destained in acetic acid/methanol and dried.
Quantification of the banding pattern was performed by densitometry. Each sample lane was scanned and presented as optical density (OD) in percent of control sample.
3.15 Measurement of NFκB and TIMP-1
To determine the expression of NFκB and TIMP-1, Hs68 cells were treated with AEWA, for 24 hrs, followed by washing with PBS before incubation with 20 J/cm2 UVA. To detect NFκB and TIMP-1, the medium was collected. Levels of NFκB and TIMP-1 in the medium were determined by an enzyme-linked immunosorbent assay (ELISA, R&D, Minneapolis, MN, USA).
3.16. Data analysis
Results were expressed as mean S.D. Statistical analyses were performed by using one-way analysis of variance followed by Duncan’s multiple range tests. Results were considered statistically significant at P<0.05.
4. Conclusions
UV exposure is the major cause of skin aging. Using raw materials such as retinoid to inhibit of collagen reduction and to promote collagen synthesis in combination with antioxidant agents to neutralize oxidative substances has been considered as one strategy for preventing photo-aging [33]. In the present study, our findings have demonstrated that long-term treatment of the alcoholic extracts from the flowers of wax apples (AEWA) prevented the UVA-induced cell death, MDA accumulation and procollagen I downregulation in human dermal fibroblasts. AEWA treatment also prevented the UVA-induced MMP-1 upregulation and the activation of NFκB levels. This phytoprotective capacity of AEWA against UV light-induced deleterious effects may provide an alternative option in cosmetic and pharmaceutical applications in anti-aging skin care.
Acknowledgments
We thank Dr. Yueh Chien for critical review of the manuscript. This work was supported by grants from the National Science Council of the Republic of China(Grant nos. NSC 102-2320-B-041-002-MY2 and NSC 102-2622-B-041 -001 -CC3).
Conflicts of Interest
The authors declared no conflict of interest.
References and Notes
1. Kohl, E.; Steinbauer, J.; Landthaler, M.; Szeimies, R.M. Skin ageing. J Eur Acad Dermatol Venereol 2011, 25, 873-884.
2. Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249-256.
3. Biesalski, H.K.; Berneburg, M.; Grune, T.; Kerscher, M.; Krutmann, J.; Raab, W.; Reimann, J.; Reuther, T.; Robert, L.; Schwarz, T. Hohenheimer Consensus Talk. Oxidative and premature skin ageing. Exp Dermatol 2003, 12 Suppl 3, 3-15.
4. Battie, C.; Verschoore, M. Cutaneous solar ultraviolet exposure and clinical aspects of photodamage.
Indian J Dermatol Venereol Leprol 2012, 78 Suppl 1, S9-S14.
5. Zhao, P.; Zhu, X.; Liu, Y.; Wang, B.; Wang, C.; Burns, F.J. Solar ultraviolet radiation and skin damage:
an epidemiological study among a Chinese population. Arch Environ Health 1998, 53, 405-409.
6. Shen, S.C.; Chang, W.C.; Chang, C.L. Fraction from Wax Apple [Syzygium samarangense (Blume) Merrill and Perry] Fruit Extract Ameliorates Insulin Resistance via Modulating Insulin Signaling and Inflammation Pathway in Tumor Necrosis Factor alpha-Treated FL83B Mouse Hepatocytes. Int J Mol Sci 2012, 13, 8562-8577.
7. Shen, S.C.; Chang, W.C. Hypotriglyceridemic and hypoglycemic effects of vescalagin from Pink wax apple [Syzygium samarangense (Blume) Merrill and Perry cv. Pink] in high-fructose diet-induced diabetic rats. Food Chem 2013, 136, 858-863.
8. Evans, J.A.; Johnson, E.J. The role of phytonutrients in skin health. Nutrients 2010, 2, 903-928.
9. Solomakhin, A.A.; Blanke, M.M. Mechanical flower thinning improves the fruit quality of apples. J Sci Food Agric 2010, 90, 735-741.
10. Kim, J.A.; Ahn, B.N.; Kong, C.S.; Kim, S.K. The chromene sargachromanol E inhibits ultraviolet A-induced ageing of skin in human dermal fibroblasts. Br J Dermatol 2013, 168, 968-976.
11. Scharffetter, K.; Wlaschek, M.; Hogg, A.; Bolsen, K.; Schothorst, A.; Goerz, G.; Krieg, T.; Plewig, G.
UVA irradiation induces collagenase in human dermal fibroblasts in vitro and in vivo. Arch Dermatol Res 1991, 283, 506-511.
12. Yang, T.H.; Lai, Y.H.; Lin, T.P.; Liu, W.S.; Kuan, L.C.; Liu, C.C. Chronic exposure to Rhodobacter sphaeroides extract Lycogen prevents UVA-induced malondialdehyde accumulation and procollagen I down-regulation in human dermal fibroblasts. Int J Mol Sci 2014, 15, 1686-1699.
13. Krutmann, J. The role of UVA rays in skin aging. Eur J Dermatol 2001, 11, 170-171.
14. Wlaschek, M.; Tantcheva-Poor, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schuller, J.;
Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J Photochem Photobiol B 2001, 63, 41-51.
15. Greinert, R.; Volkmer, B.; Henning, S.; Breitbart, E.W.; Greulich, K.O.; Cardoso, M.C.; Rapp, A.
UVA-induced DNA double-strand breaks result from the repair of clustered oxidative DNA damages.
Nucleic Acids Res 2012, 40, 10263-10273.
16. Fulia, F.; Gitto, E.; Cuzzocrea, S.; Reiter, R.J.; Dugo, L.; Gitto, P.; Barberi, S.; Cordaro, S.; Barberi, I.
Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: reduction by melatonin. J Pineal Res 2001, 31, 343-349.
17. Chirco, R.; Liu, X.W.; Jung, K.K.; Kim, H.R. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev 2006, 25, 99-113.
18. Vo, N.V.; Hartman, R.A.; Yurube, T.; Jacobs, L.J.; Sowa, G.A.; Kang, J.D. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J 2013, 13, 331-341.
19. Chang, W.C.; Shen, S.C.; Wu, J.S. Protective effects of vescalagin from pink wax apple [Syzygium samarangense (Blume) Merrill and Perry] fruit against methylglyoxal-induced inflammation and carbohydrate metabolic disorder in rats. J Agric Food Chem 2013, 61, 7102-7109.
20. Amor, E.C.; Villasenor, I.M.; Yasin, A.; Choudhary, M.I. Prolyl endopeptidase inhibitors from Syzygium samarangense (Blume) Merr. & L. M. Perry. Z Naturforsch C 2004, 59, 86-92.
21. Kuo, Y.C.; Yang, L.M.; Lin, L.C. Isolation and immunomodulatory effect of flavonoids from Syzygium samarangense. Planta Med 2004, 70, 1237-1239.
22. Amor, E.C.; Villasenor, I.M.; Ghayur, M.N.; Gilani, A.H.; Choudhary, M.I. Spasmolytic flavonoids from Syzygium samarangense (Blume) Merr. & L.M. Perry. Z Naturforsch C 2005, 60, 67-71.
23. Ghayur, M.N.; Gilani, A.H.; Khan, A.; Amor, E.C.; Villasenor, I.M.; Choudhary, M.I. Presence of calcium antagonist activity explains the use of Syzygium samarangense in diarrhoea. Phytother Res 2006, 20, 49-52.
24. Resurreccion-Magno, M.H.; Villasenor, I.M.; Harada, N.; Monde, K. Antihyperglycaemic flavonoids from Syzygium samarangense (Blume) Merr. and Perry. Phytother Res 2005, 19, 246-251.
25. Shahreen, S.; Banik, J.; Hafiz, A.; Rahman, S.; Zaman, A.T.; Shoyeb, M.A.; Chowdhury, M.H.;
Rahmatullah, M. Antihyperglycemic activities of leaves of three edible fruit plants (Averrhoa carambola, Ficus hispida and Syzygium samarangense) of Bangladesh. Afr J Tradit Complement Altern Med 2011, 9, 287-291.
26. Kim, Y.J.; Kim, H.C.; Ko, H.; Amor, E.C.; Lee, J.W.; Yang, H.O. Stercurensin inhibits nuclear factor-kappaB-dependent inflammatory signals through attenuation of TAK1-TAB1 complex formation. J Cell Biochem 2011, 112, 548-558.
27. Kim, Y.J.; Kim, H.C.; Ko, H.; Amor, E.C.; Lee, J.W.; Yang, H.O. Inhibitory effects of aurentiacin from Syzygium samarangense on lipopolysaccharide-induced inflammatory response in mouse macrophages.
Food Chem Toxicol 2011, 50, 1027-1035.
28. Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and free radical scavenging activity of Spondias pinnata.
BMC Complement Altern Med 2008, 8, 63.
29. Ghate, N.B.; Chaudhuri, D.; Sarkar, R.; Sajem, A.L.; Panja, S.; Rout, J.; Mandal, N. An antioxidant extract of tropical lichen, Parmotrema reticulatum, induces cell cycle arrest and apoptosis in breast carcinoma cell line MCF-7. PLoS One 2013, 8, e82293.
30. Mau, J.L.; Lin, H.C.; Chen, C.C. Antioxidant properties of several medicinal mushrooms. J Agric Food Chem 2002, 50, 6072-6077.
31. Loveland, B.E.; Johns, T.G.; Mackay, I.R.; Vaillant, F.; Wang, Z.X.; Hertzog, P.J. Validation of the MTT dye assay for enumeration of cells in proliferative and antiproliferative assays. Biochem Int 1992, 27, 501-510.
32. Sano, M.; Motchnik, P.A.; Tappel, A.L. Halogenated hydrocarbon and hydroperoxide-induced peroxidation in rat tissue slices. J Free Radic Biol Med 1986, 2, 41-48.
33. Baumann, L. Skin ageing and its treatment. J Pathol 2007, 211, 241-251.
科技部補助計畫衍生研發成果推廣資料表
日期:2015/01/08
科技部補助計畫
計畫名稱: 具皮膚保護功效的蓮霧花原料開發與應用 計畫主持人: 劉家全
計畫編號: 102-2622-B-041-001-CC3 學門領域: 食品及農化
無研發成果推廣資料
102 年度專題研究計畫研究成果彙整表
計畫主持人:劉家全 計畫編號:102-2622-B-041-001-CC3 計畫名稱:具皮膚保護功效的蓮霧花原料開發與應用
量化
成果項目 實際已達成
數(被接受 或已發表)
預期總達成 數(含實際已
達成數)
本計畫實 際貢獻百
分比
單位
備 註 ( 質 化 說 明:如 數 個 計 畫 共 同 成 果、成 果 列 為 該 期 刊 之 封 面 故 事 ...
等)
期刊論文 0 0 100%
研究報告/技術報告 0 0 100%
研討會論文 0 0 100%
論文著作 篇
專書 0 0 100%
申請中件數 1 1 100%
專利 已獲得件數 1 1 100% 件
件數 1 0 100% 件
技術移轉
權利金 0 0 100% 千元
碩士生 1 1 100%
博士生 0 0 100%
博士後研究員 0 0 100%
國內
參與計畫人力
(本國籍)
專任助理 0 0 100%
人次
期刊論文 1 1 100%
研究報告/技術報告 0 0 100%
研討會論文 0 0 100%
論文著作 篇
專書 0 0 100% 章/本
申請中件數 0 0 100%
專利 已獲得件數 0 0 100% 件
件數 0 0 100% 件
技術移轉
權利金 0 0 100% 千元
碩士生 0 0 100%
博士生 0 0 100%
博士後研究員 0 0 100%
國外
參與計畫人力
(外國籍)
專任助理 0 0 100%
人次
其他成果
(
無法以量化表達之成果如辦理學術活動、獲 得獎項、重要國際合 作、研究成果國際影響 力及其他協助產業技 術發展之具體效益事 項等,請以文字敘述填 列。)
無
成果項目 量化 名稱或內容性質簡述
測驗工具(含質性與量性) 0
課程/模組 0
電腦及網路系統或工具 0
教材 0
舉辦之活動/競賽 0
研討會/工作坊 0
電子報、網站 0
科 教 處 計 畫 加 填 項
目 計畫成果推廣之參與(閱聽)人數 0
本產學合作計畫研發成 果 及 績 效 達 成 情 形 自 評 表 成果項目
本產學合作計畫預估研究成果及績效指標(作為本計畫後續管考之參據) 計畫達成情形
技術移轉 預計技轉授權 1 項 完成技轉授權 1 項
國內 預估 1 件 提出申請 1 件,獲得 1 件
專利
國外 預估 1 件 提出申請 0 件,獲得 0 件
博士 0人,畢業任職於業界0人 博士 0人,畢業任職於業界0人
碩士 1人,畢業任職於業界1人 碩士 1人,畢業任職於業界0人
人才培育
其他 1人,畢業任職於業界1人 其他 1人,畢業任職於業界0人
期刊論文 1 件 發表期刊論文 0 件
研討會論文 0 件 發表研討會論文 0 件
SCI論文 0 件 發表SCI論文 0 件
專書 0 件 完成專書 0 件
國內
技術報告 0 件 完成技術報告 0 件
期刊論文 0 件 發表期刊論文 0 件
學術論文 0 件 發表學術論文 0 件
研討會論文 0 件 發表研討會論文 1 件
SCI/SSCI論文 1 件 發表SCI/SSCI論文 0 件
專書 0 件 完成專書 0 件
論文著作
國外
技術報告 0 件 完成技術報告 0 件
其他協助產業發展
之具體績效 新公司或衍生公司 0 家 設立新公司或衍生公司(名稱):
計畫產出成果簡 述:請以文字敘述 計畫非量化產出之 技術應用具體效 益。(限 600 字以
內)
本計畫的成果直接驗證假說,進而增進我們瞭解蓮霧花具皮膚保護功效以及其機制。
完成探討細胞模式下蓮霧花酒精萃取物對色素沉著的抑制效果與對紫外線傷害模式 下之作用情形。協助廠商建立的細胞研究平台,促進公司的研發能力,開創公司的未 來新方向。計畫中所用的蓮霧花為現今農民疏花丟棄的花,開發出蓮霧花的功效,即 能將廢棄之蓮霧花再利用,未來便可幫助農民收入,更能提升蓮霧果樹在台灣的經濟 地位。