CLINICAL PHARMACOLOGY AND DRUG STUDIES
Assessment of Quality of Life in a Double-Blind,
Randomized Clinical Trial of Imidapril and Captopril
for Hypertensive Chinese in Taiwan
Kuo-Liong Chien, Por-Jau Huang, Ming-Fong Chen, Fu-Tien Chiang, Ling-Ping Lai,
and Yuan-Teh Lee
Department of Internal Medicine (Cardiology), National Taiwan University College of Medicine, Taipei, Taiwan
Summary. Purpose. Although the role of angiotensin-converting enzyme (ACE) inhibitors for the treatment of hypertension has been well established, no data has been generated regarding the influence of ACE inhibitors for health-related quality-of-life (QOL) dimensions for Chinese patients.
Materials. A double-blind, active-control, randomized clinical trial was used to compare the effects of two ACE inhibitors, imidapril and captopril, on quality-of-life dimen-sions in one outpatient clinic in one tertiary clinical-care fa-cility. After a 2–3 week washout period with placebo, 59 pa-tients with mild-to-moderate hypertension were randomly assigned to receive imidapril (5 to 10 mg per day) or cap-topril (25 to 50 mg twice per day) for 12 weeks. Patients completed the Short-form 36 (SF 36) health survey ques-tionnaire, which evaluates 8 QOL dimensions, just before treatment, during the 8th week, and at the end of treat-ment (12th week). ANOVA for repeated measures was used to analyze the QOL-score changes over time and compare treatments, and to assess the interaction of treatment du-ration and group on these scores.
Results. No significant differences were demonstrated for changes in blood-pressure, frequency of adverse ef-fects and withdrawal of patients from the study compar-ing the two drugs. Significant improvement, however, was demonstrated for mental-component summary scores after 12 weeks of treatment for both drugs ( P= 0.029). No sig-nificant differences were established for individual QOL di-mensions comparing the two drugs. A significantly higher baseline systolic blood pressure was found in the partici-pants who did not complete the questionnaire than in those who did.
Conclusions.Similar and significant improvements were determined for the mental-component QOL summary scores for the two ACE inhibitors, imidapril and captopril, and no significant differences were demonstrated comparing treatments.
Key Words.angiotensin-converting enzyme inhibitors, ACE, quality of life, clinical trial
Introduction
Improvements in mortality and morbidity due to hy-pertension have been confirmed in large-scale clinical
trials of antihypertensive treatments. The enhance-ment of quality of life (QOL) has also been the theme of a number of research studies. The development of angiotensin-converting enzyme (ACE) inhibitors ap-pears to have constituted the greatest advance in hy-pertension treatment, and these inhibitors have proven beneficial across a number of proposed QOL dimensions [1,2]. Various ACE inhibitors that were subsequently developed have been the subject of intensive clinical study for hypertension treatment and congestive heart failure. ACE inhibitors are recognized as one of the first treatment choices made in the step-wise therapy advo-cated for hypertension by the WHO Guidelines Sub-committee [3]. Imidapril (imidapril hydrochloride; Tan-abe Seiyaku Co., Ltd.), which contains no sulfhydryl groups in its chemical structure, is a newly developed ACE inhibitor. It is a pro-drug which becomes active as it is hydrolyzed and converted to a diacid metabolite (imidaprilat). The potency of imidaprilat for humans is about twice that of enalaprilat (the active metabolite of enalapril) and about 10 times that of captopril [4]. The efficacy and safety of imidapril for humans, admin-istered once a day, have been confirmed in a Phase I clinical trial using healthy subjects and patients diag-nosed with mild to moderate hypertension [5].
Recent research has emphasized the use of QOL measures to evaluate antihypertensive therapy [6–8], as adverse side effects and impairment of psychoso-cial function may reduce patient compliance [6,9]. Thus, QOL-guided treatment may improve this compliance and prevent deterioration of psychosocial function [10]. The QOL for hypertensive patients can be assessed us-ing generic and/or disease-specific questionnaires. In particular, it has been demonstrated that the Short-form 36 (SF-36) questionnaire is a reliable and sensitive tool for the evaluation of QOL dimensions for various
Address for correspondence: Por-Jau Huang, MD, Department of Internal Medicine (Cardiology Division), National Taiwan Uni-versity Hospital, No. 7, Chuang-San South Road, Taipei 100, Taiwan. Tel.:+886+2+2312-3456, ext. 5031; Fax: +886-2-2395-7855; E-mail: porjau@ha.mc.ntu.edu.tw
populations and diseases [11–13]. The 36-item design is used to evaluate eight QOL dimensions: physical and social functioning, role limitations due to emotional and physical problems, mental health, energy/vitality, pain level, and general-health perception. Good sensi-tivity and specificity have been demonstrated for this research instrument [14–16]. The validity of the SF-36 questionnaire for the pre-test was good. Due to the multiple correlations that are derived from the eight QOL dimensions explored in the SF-36, two composites, physical and mental component scales (PCS, MCS), are used to summarize the results [17]. These two compos-ite scores are shown to be better indicators of general health both in the general population and in disease-specific groups [17]. To date, there have been relatively few reports using the SF-36 questionnaire for QOL evaluation for hypertensive populations [2,18].
Testa et al. [1] have determined different effects for the two ACE inhibitors, captopril and enalapril, for a number of QOL dimensions. They conclude that cap-topril treatment was associated with more favourable changes for overall QOL, mental health, sleep and vi-tality dimensions. In this report, we compare captopril with the new ACE inhibitor imidapril to evaluate QOL-dimension changes for Chinese hypertensive patients treated over a 3-month study period.
The randomized, double-blind, parallel study was de-signed to compare the efficacy and safety of imidapril and captopril for the treatment of mild-to-moderate es-sential hypertension for Chinese patients in Taiwan. The SF-36 questionnaire was used to evaluate QOL changes during treatment.
Materials and Methods
Study design
This QOL-dimension clinical trial was performed con-currently with a randomized, double-blind, clinical trial, entitled “Efficacy and Safety of Imidapril, A New Angiotensin-Converting Enzyme Inhibitor, in Chinese Patients with Mild to Moderate Hypertension: A Double-Blind Comparison with Captopril” [19]. All 59 patients in the clinical trial were considered partici-pants in the QOL study. With help of an assistant, all fin-ished the initial QOL questionnaire. The study design included a placebo washout period of 2–3 weeks and an active treatment period of 12 weeks. This clinical trial was approved by the Independent Review Board of the National Taiwan University Hospital and the informed consent signed by every patient.
Adult hypertensive patients from the outpatient clinic of one university hospital were enrolled in this study. Hypertension was defined as seated diastolic blood pressure (DBP) ranging from 95–115 mm Hg during the washout period. Exclusion criteria included secondary or more severe hypertension (seated DBP over 115 mm Hg or systolic blood pressure (SBP) over 240 mm Hg during the washout period), potential
pregnancy, severe heart failure or myocardial infarc-tion during the previous three months. Further exclu-sion criteria were evident coronary heart disease, such as unstable angina pectoris with poorly controlled di-abetes, renal or hepatic disease and antihypertensive drug treatment was also excluded to prevent interfer-ence with the ACE treatment.
Patients on the imidapril regimen received a 5 mg imidapril capsule in the morning and a placebo capsule in the evening for 4 weeks. The imidapril dosage was increased to 5 mg 2 times per day for the next 8 weeks if the DBP was still>=90 mm Hg after the first phase of treatment. Patients on the captopril regimen received a 25 mg captopril capsule twice per day for 4 weeks, and the dosage was doubled for the next 8 weeks if DBP was still>=90 mm Hg after the first treatment phase. The total duration of the treatment period was 12 weeks.
Blood pressure measurements
A standard mercury sphygmomanometer was used for all BP measurements taken by the same nurse during outpatient visits. The BP was recorded after 10 minutes of rest in a sitting position. Two seated measurements were performed subsequently, separated by a 5-minute interval. The mean of the two measurements was used as the reference value.
Measurement of quality-of-life dimensions
The QOL dimensions were measured using the SF-36 questionnaire which was completed during each of the three visits [17,20]. The clinical assistant collected the self-administered questionnaires. The SF-36 was de-signed to survey health-related QOL issues for clinical research [20]. It consists of 36 questions grouped and scored on eight dimensions: physical functioning, physi-cal and emotional roles, bodily pain, general and mental health, vitality, and social functioning. The validity, re-liability and utility of the instrument have been estab-lished across various clinical trials and cohort studies [13,17,20].
Statistical analysis
As the QOL dimensions are continuous variables, data were presented as mean± standard error. Repeated-measures ANOVA was used to test the effects of drug group and treatment duration on QOL dimension scores, incorporating the interaction of treatment dura-tion and drug group. Polynomial transformadura-tions were used to fit the duration effect, and the time period as 0, 8, or 12 units. The GLM procedure of the SAS program was used for all statistical analyses [21]. Differences comparing drug group, treatment duration and their in-teraction were considered statistically significant when
P value was<0.05. The means for the various QOL
scores were also estimated from data gathered at base-line and weeks 8 and 12 to express the trend for each dimension during the two treatment phases. For the comparison of baseline data in responsive and missing
groups, we used the unpaired Student t-test to test the continuous variables and theχ2test to test the
signifi-cant level of the categorical data.
We set Type I error for the two-tailed test as 0.05 and type II error as 0.20. A deviation of 3.0 and the popu-lation standard deviation of 5.8 of PCS were assumed from literature reviews. The estimated sample size in each treatment group was 29.3, or round up to 30.
Results
A total of 59 patients were enrolled in this anti-hypertensive clinical trial and QOL study. Two patients were excluded because of abnormally high liver func-tion values. Of the remaining 57 patients, 29 received imidapril and 28 received captopril. The ages of the par-ticipants ranged from 38 to 67 (mean 52.3, standard deviation 6.9) years. No differences were determined comparing the two drugs for gender, age, history of hy-pertension, concomitant medication or adverse drug re-actions. Adverse drug reactions during the treatment period were reported by 27.6% of patients in the im-idapril group and by 46.4% of patients in the capto-pril group. Drug-related coughing occurred more in the captopril (35.7%) than in the imidapril (13.8%) group, with borderline significance ( p= 0.055). Also, one pa-tient in the imidapril group had mild proteinuria, and
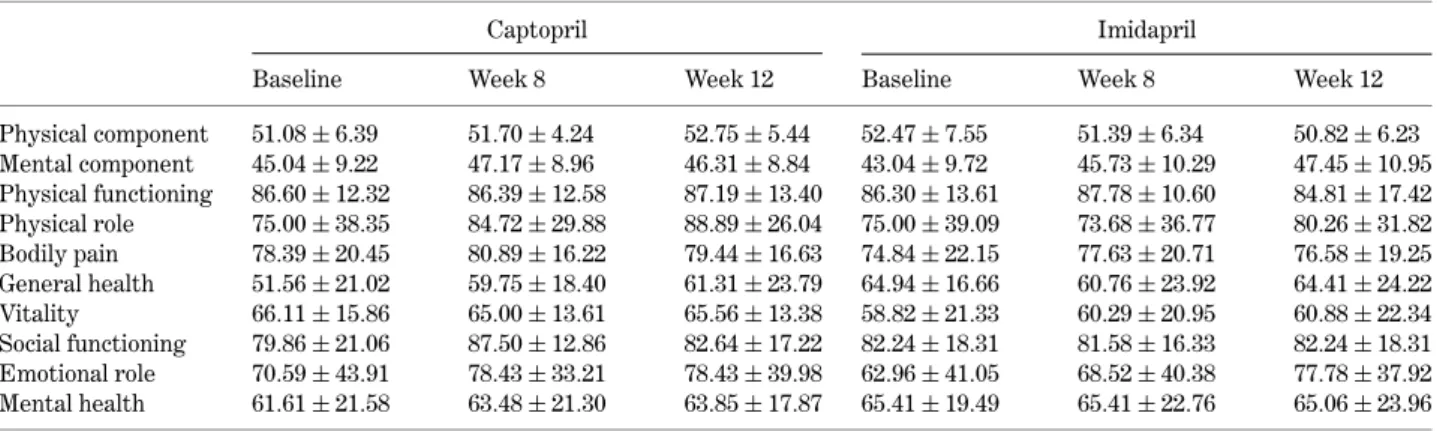
Table 1. SF-36 QOL-dimension scores specified by treatment periods and drug regimens of captopril and imidapril
Captopril Imidapril
Baseline Week 8 Week 12 Baseline Week 8 Week 12
Physical component 51.08 ± 6.39 51.70 ± 4.24 52.75 ± 5.44 52.47 ± 7.55 51.39 ± 6.34 50.82 ± 6.23 Mental component 45.04 ± 9.22 47.17 ± 8.96 46.31 ± 8.84 43.04 ± 9.72 45.73 ± 10.29 47.45 ± 10.95 Physical functioning 86.60 ± 12.32 86.39 ± 12.58 87.19 ± 13.40 86.30 ± 13.61 87.78 ± 10.60 84.81 ± 17.42 Physical role 75.00 ± 38.35 84.72 ± 29.88 88.89 ± 26.04 75.00 ± 39.09 73.68 ± 36.77 80.26 ± 31.82 Bodily pain 78.39 ± 20.45 80.89 ± 16.22 79.44 ± 16.63 74.84 ± 22.15 77.63 ± 20.71 76.58 ± 19.25 General health 51.56 ± 21.02 59.75 ± 18.40 61.31 ± 23.79 64.94 ± 16.66 60.76 ± 23.92 64.41 ± 24.22 Vitality 66.11 ± 15.86 65.00 ± 13.61 65.56 ± 13.38 58.82 ± 21.33 60.29 ± 20.95 60.88 ± 22.34 Social functioning 79.86 ± 21.06 87.50 ± 12.86 82.64 ± 17.22 82.24 ± 18.31 81.58 ± 16.33 82.24 ± 18.31 Emotional role 70.59 ± 43.91 78.43 ± 33.21 78.43 ± 39.98 62.96 ± 41.05 68.52 ± 40.38 77.78 ± 37.92 Mental health 61.61 ± 21.58 63.48 ± 21.30 63.85 ± 17.87 65.41 ± 19.49 65.41 ± 22.76 65.06 ± 23.96
Table 2. Hypothesis testing by SF-36 QOL dimension
Treatment: Duration: Week 0, 8, Interaction for treatment P value Captopril vs. imidapril and 12 duration and drug regimen
Physical functioning 0.918 0.769 0.455 Physical role 0.459 0.290 0.633 Bodily pain 0.574 0.578 0.991 General health 0.400 0.235 0.055 Vitality 0.337 0.924 0.751 Social functioning 0.804 0.241 0.130 Emotional role 0.590 0.205 0.749 Mental health 0.739 0.831 0.755 Mental component 0.793 0.029 0.337 Physical component 0.867 0.957 0.198
another patient in the captopril group had mild eleva-tion of aminotransferase. No serious adverse events were reported. Patient compliance and dosage titra-tion for both drugs were compatible. Comparing the ef-ficacy for DBP normalization, there was no significant difference between drugs. There were similar percent-ages of dose titration in both drugs (55% in imidapril vs. 46% in captopril for double dosage) after the treatment period.
The changes in QOL-dimension scores for the two treatment groups, including PCS and MCS scores de-rived for baseline, week-8 and week-12 results in the responsive 40 participants, are presented in Table 1, with the levels of significance for treatment group and duration, and interaction effects presented in Table 2. The scores for the eight QOL dimensions increased modestly or remained relatively stable for both drug groups. The PCS scores increased for captopril, and de-creased for imidapril, however, the difference was not statistically significant. The MCS scores increased for both ACE inhibitors, with significant differences from baseline demonstrated after 12 weeks of treatment (P= 0.029). No difference was demonstrated compar-ing the treatment groups (Fig. 1). We also moni-tored the other possible co-morbid disorders, such as congestive heart failure or cardiovascular events dur-ing the course of the study. We could not find any
40
45
50
55
Baseline
Week 8
Week 12
Treatment time
Scores
PCS MCS Captopril Imidapril Imidapril CaptoprilFig. 1.PCS and MCS score changes, specified by drug treatment.
co-morbid disorders that might have impacted on QOL evaluation.
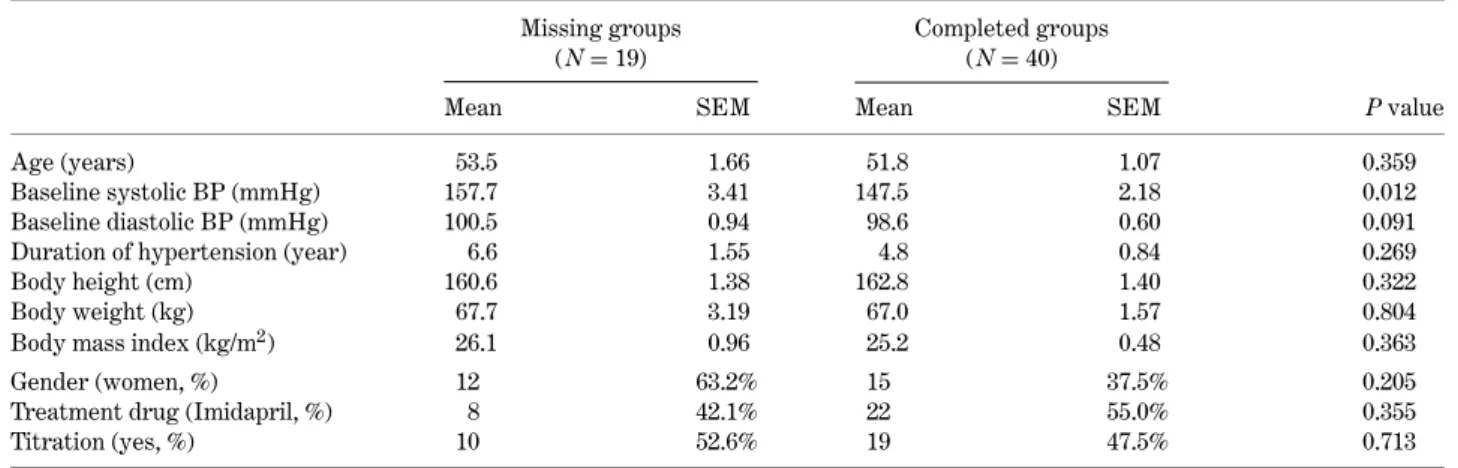
Among the 59 participants in the clinical trial, there were 19 patients who did not complete the ques-tionnaire (missing group) and 40 patients who com-pleted the questionnaire (responsive group) in this QOL trial. Table 3 shows the distribution of selected vari-ables between the two groups. We found that the pa-tients in the missing group were slightly older, had a higher baseline blood pressure, a longer duration of hypertension and higher body mass index values. Only baseline systolic blood pressure in the missing group was significantly higher than in the respon-sive group. The proportions of female gender, treat-ment drug and titration dosage were similar in both groups.
Table 3. Basic demographic, hypertension history, and blood pressure in this clinical trial, specified by completing the questionnaire or missing status
Missing groups Completed groups
(N= 19) (N= 40)
Mean SEM Mean SEM P value
Age (years) 53.5 1.66 51.8 1.07 0.359
Baseline systolic BP (mmHg) 157.7 3.41 147.5 2.18 0.012
Baseline diastolic BP (mmHg) 100.5 0.94 98.6 0.60 0.091
Duration of hypertension (year) 6.6 1.55 4.8 0.84 0.269
Body height (cm) 160.6 1.38 162.8 1.40 0.322
Body weight (kg) 67.7 3.19 67.0 1.57 0.804
Body mass index (kg/m2) 26.1 0.96 25.2 0.48 0.363
Gender (women, %) 12 63.2% 15 37.5% 0.205
Treatment drug (Imidapril, %) 8 42.1% 22 55.0% 0.355
Titration (yes, %) 10 52.6% 19 47.5% 0.713
SEM: Standard error of means.
Discussion
This clinical trial clearly demonstrates that both imidapril and captopril can improve SF-36 mental-component summary scores after 12 weeks of treat-ment. No significant difference was demonstrated for either drug for the various QOL dimensions.
Although it has been demonstrated that antihyper-tensive treatment decreases cardiovascular morbid-ity and mortalmorbid-ity, some drugs, especially methyldopa and propranolol, produce side effects and impairment of QOL dimension, such as sexual dysfunction, sleep-ing problems and depression [6,9]. To improve drug compliance for hypertensive patients it is important to monitor QOL changes and remain vigilant for ad-verse effects. Clinicians have an additional tool to aid
in drug selection for the hypertensive patient. In ad-dition to efficacy predictions and the identification of significant physical symptoms, information is available regarding the potential impact of a particular drug on patient QOL. Use of this information may help to im-prove treatment compliance and the associated eco-nomic impact by improving work performance and re-ducing drop-out as a result of side-effects.
In 1986, Croog et al. [6] documented the applicability of these techniques for the assessment of the impact of antihypertensive therapy on the QOL of patients en-rolled in a clinical trial. Adopting a standard clinical format, over 600 patients were randomly assigned to 3 treatment groups after a 4-week washout period. The results indicated that captopril might improve general well-being, work performance and cognitive function, while both methyldopa and propranolol worsened phys-ical symptoms, sexual dysfunction and life satisfaction. The baseline PCS and MCS magnitudes in this clini-cal trial were similar to the results from a survey of hy-pertensive patients [17]. After a 1-year treatment for hypertension, the PCS decreased by 0.40 and MCS in-creased by 0.20 with ACE inhibitor treatments in gen-eral clinics. The reason that mental component scores improve is supposed to be due to ACE inhibitor effects on inhibition of renangiotensin system [17]. ACE in-hibitors are preferred in some subsets of hypertensive patients, such as congestive heart failure or diabetes and can improve QOL [22]. In our clinical trial, although the changes of PCS and MCS were different for both ACE-inhibitors, these did not reach significant levels. It might be due to the small sample size and short follow-up time in our study.
The composite PCS and MCS scores, as determined by the SF-36 questionnaire, can improve measurement precision for psychometric evaluation in comparison to the eight individual SF-36 scales [17]. Also, repro-ducible and useful summaries of results for individual patients can be derived from these scores. Further, re-peated, longitudinal assessment of QOL can produce interpretable estimates of health-status change, which would otherwise not be available to clinicians [23]. Thus, our design incorporated three discrete QOL assess-ments for each patient to investigate QOL changes in-trinsic to hypertension treatment.
This clinical trial had a limitation. The power of the statistical comparisons of the drugs was limited as a consequence of the relatively short study period and small sample population. Although the recruitment number was adequate for sample size estimation, the study was still underpowered due to the fact that only 40 patients complete the study. Also, missing data in a clinical trial is an important issue. In this trial, we found significantly higher baseline systolic blood pres-sure in the missing group. It implied that patients with higher blood pressure might drop out earlier, if not treated adequately. The non-respondents did not fill out their questionnaire because they dropped out of the clinical trial. Other factors, such as age, medical
history of hypertension and treatment plans were not significantly different between responsive and missing groups. The effects of treatment, such as the degree of blood pressure decrease and percentage of achieving adequate blood pressure control did not reach a statis-tically significant level between the two groups during the course of treatment. We considered that exclusion of missing data in this trial would not impact much on the demonstrated trends.
Improvements were demonstrated for the QOL mental-component composite score for both imidapril and captopril, which were otherwise indistinguishable according to standard clinical assessments for efficacy and safety. No significant differences were demon-strated for individual quality-of-life dimensions, al-though improved QOL scores were noted.
Acknowledgment
The study was partially sponsored by Taiwan Tanabe Seiyaku Co., Ltd., Taipei, Taiwan.
References
1. Testa MA, Anderson RB, Nackley JF, Hollenberg NK. Quality of life and antihypertensive therapy in men. A comparison of captopril with enalapril. The Quality-of-Life Hypertension Study Group. New Eng J Med 1993;328:907– 913.
2. Houston MC. The management of hypertension and associ-ated risk factors for the prevention of long-term cardiac com-plications. J Cardiovasc Pharm 1993;21(Suppl 2):S2–S13. 3. Guidelines Subcommittee. 1999 World Health
Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens 1999;17:151–183.
4. Kubo M, Kato J, Ishida R, et al. Pharmacological studies on (4S)-1-methyl-3- {(2S)-2-[N-((1S)-1-ethoxycarbonyl-3-phen-ylpropyl)amino] Propionyl }-2-oxo-imidazolidine-4-carboxy-lic acid hydrochloride (TA-6366), a new inhibitor. I. ACE inhibitory and anti-hypertensive activities. Japan J Pharmacol 1990;53:201–210.
5. Omae T, Saruta T, Yoshinaga H, et al. Pilot study of ACE/TA-6366 (imidapril hydrochloride), an angiotensin-converting enzyme inhibitor, in patients with mild to moderate essential hypertension. J Clin Therap Med 1991;7:2187–2203. 6. Croog SH, Levine S, Testa MA, et al. The effects of
anti-hypertensive therapy on the quality of life. N Eng J Med 1986;314:1657–1664.
7. Ferrara LA, Di Marino L, Russo O, Marotta T, Mancini M. Doxazosin and captopril in mildly hypercholesterolemic hypertensive patients. The Doxazosin-Captopril in Hy-percholesterolemic Hypertensives Study. Hypertension 1993;21:97–104.
8. Neaton JD, Grimm RH Jr, Prineas RJ, et al. Treatment of Mild Hypertension Study Research Group. Treatment of mild hypertension study: Final results. JAMA 1993;270:713– 724.
9. Wassertheil-Smoller S, Blaufox D, Oberman A, et al. TAIM Research Group. Effect of antihypertensives on sexual
function and quality of life: The TAIM study. Ann Intern Med 1991;114:613–620.
10. Patrick DL, Deyo RA. Generic and disease-specific mea-sures in assessing health status and quality of life. Medical Care 1989;27:217–233.
11. Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: Normative data for adults of working age. British Medical Journal 1993;306:1437– 1440.
12. Jette DU, Downing J. Health status of individuals entering a cardiac rehabilitation program as measured by the medical outcomes study 36-item short-form survey (SF-36). Physi-cal Therapy 1994;4:521–527.
13. Stewart AL, Hays RD, Ware JE. The MOS short-form gen-eral health survey reliability and validity in a patient popu-lation. Medical Care 1988;26:724–732.
14. Ware JE. Standards for validating health measures: Defini-tion and content. J. Chron Dis 1987;40:473–482.
15. Wenger NK, Mattson ME, Furberg CD, et al. Assessment of quality of life in clinical trials of cardiovascular therapies. Am J Cardiol 1984;54:908–913.
16. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). Med Care 1992;30:473–483.
17. Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute, 1994.
18. Krousel-Wood MA, Re RN. Health status assessment in a hypertension section of an internal medicine clinic. Am J Med Sci 1994;308:211–217.
19. Huang PJ, Chien KL, Chen MF, Lai LP, Chiang FT. Efficacy and safety of imidapril in patients with essential hyperten-sion: A double-blind comparison with captopril. Cardiology 2001;95:146–150.
20. Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Boston, MA: The Health Institute, 1993.
21. SAS Institute Inc. SAS/STAT User’s Guide, Release 6.03 edition. 6.03 ed. Cary, NC: SAS Institute Inc. 1988. 22. Moser M. Angiotensin-converting enzyme inhibitors,
an-giotensin II receptor antagonists and calcium channel block-ing agents: A review of potential benefits and possible ad-verse reactions. JACC 1997;29:1414–1421.
23. Meyer KB, Espindle DM, DeGiacomo JM, Jenuleson CS, Kurtin PS, Davis AR. Monitoring dialysis patients’ health status. American Journal of Kidney Disease 1994;24:267– 279.