Aerosol and Air Quality Research, 12: 218–227, 2012 Copyright © Taiwan Association for Aerosol Research ISSN: 1680-8584 print / 2071-1409 online
doi: 10.4209/aaqr.2011.09.0144
Emissions of Regulated Pollutants and PAHs from Waste-cooking-oil
Biodiesel-fuelled Heavy-duty Diesel Engine with Catalyzer
Shou-Heng Liu
1, Yuan-Chung Lin
2*, Kuo-Hsiang Hsu
21 Department of Chemical and Materials Engineering, National Kaohsiung University of Applied Sciences, Kaohsiung 807,
Taiwan
2 Institute of Environmental Engineering, National Sun Yat-sen University, Kaohsiung 804, Taiwan
ABSTRACT
The development of biodiesels is being driven by the need for reducing emissions from diesel engines without modifying engines and for saving energy. The major obstacle to biodiesel commercialization is the high cost of raw materials. Biodiesel from waste cooking oil is an economical source and an effective strategy for reducing the raw material cost. Although biodiesels made from waste cooking oil have been previously investigated, PAH emissions from heavy-duty diesel engines (HDDEs) with catalyzer fueled with biodiesel from waste cooking oil and its blend with ultra-low sulfur diesel (ULSD) for the US-HDD transient cycle have seldom been addressed. Experimental results indicate that ULSD/WCOB (biodiesel made from waste cooking oil) blends had lower PM, HC, and CO emissions but higher CO2 and NOx emissions when compared
with that of ULSD. Using ULSD/WCOB blends instead of ULSD decreased PAHs by 14.1%–53.3%, PM by 6.80%–15.1%, HC by 6.76%–23.5%, and CO by 0.962%–8.65% but increase CO2 by 0.318–1.43% and NOx by 0.384–1.15%. Using WCOB
is an economical source and an effective strategy for reducing cost, and solves the problem of waste oil disposal.
Keywords: Waste cooking oil; Biodiesel; PAH; Diesel engine; Ultra-low sulfur diesel.
INTRODUCTION
Emissions from engines contain carcinogenic components such as carbonyl compounds (i.e. formaldehyde), polycyclic aromatic hydrocarbons (PAHs), and nitro-PAHs (Chen et
al., 2001; Grosjean et al., 2001; Mi et al., 2001; Lin et al.,
2005; Lin et al., 2006; Lin et al., 2006; Lin et al., 2006; Lin et al., 2006; Ho et al., 2007; Legreid et al., 2007; Ban-Weiss et al., 2008; Lin et al., 2008; Lin et al., 2008; Lin et
al., 2008; Chien et al., 2009; Lin et al., 2009; Shi et al.,
2009; Wu et al., 2009; Shi et al., 2010; Wu et al., 2010; Tsai et al., 2011; Srivastava et al., 2011; Yao et al., 2011; Wang et al., 2012). Therefore, alternative fuel is needed in the future. Biodiesel is considered as one of best alternative fuels. The development of biodiesels is being driven by the need for reducing emissions from diesel engines without modifying engines and for saving energy. Biodiesels used as alternative fuels in diesel engines reduce the emissions of hydrocarbons (HC), carbon monoxide (CO), sulfur oxide (SO2), and PAHs (Antolin et
al., 2002; Beer et al., 2002; Cardone et al., 2002; Durbin et
* Corresponding author. Tel.: +886 7 5252000 ext. 4412;
Fax: +886 7 5254412
E-mail address: yclin@faculty.nsysu.edu.tw
al., 2002; Dorado et al., 2003; Kalam et al., 2003;
Kalligeros et al., 2003; Goodrum and Geller, 2005; Hu et
al., 2005; Lin et al., 2006; Lin et al., 2006; Legreid et al.,
2007; Yuan et al., 2007; Lin et al., 2008; Lin et al., 2008; Chien et al., 2009; Pehan et al., 2009; Yuan et al., 2009; Tsai
et al., 2010; Tsai et al., 2011; Tsai et al., 2011). Previous
studies and measurements of NOx emissions from biodiesel
showed an increase in NOx emissions (Scholl and Sorenson,
1993; Graboski et al., 1996; Choi et al., 1997; Graboski and McCormick, 1998; Yoshimoto et al., 1999; McCormick et
al., 2001; Grimaldi et al., 2002; Tat and Van Gerpen, 2003;
Tat, 2004; Saravanan et al., 2010; Sun et al., 2010). The problem of high NOx emissions from biodiesel-diesel
engines can be mitigated by the use of low-temperature combustion, reformulated biodiesel, selective catalytic reduction, and exhaust gas recirculation (Hess et al., 2007; Tsolakis et al., 2007; Muncrief et al., 2008; Tsolakis et al., 2008). There are mixed results for PM reduction, however, most of results (> 90%) show PM reduction when using biodiesel (Lapuerta et al., 2005; Lapuerta et al., 2008; Cheung
et al., 2009; Karavalakis et al., 2010).
Furthermore, biodiesel has a relatively high flash point, which makes it less volatile and safer to transport or handle than petroleum diesel (Lin et al., 2006; Lin et al., 2006) and it also enhances lubrication, which can reduce engine wear and extend engine life (Goodrum and Geller, 2005; Hu et al., 2005; Pehan et al., 2009). The major obstacle to
Liu et al., Aerosol and Air Quality Research, 12: 218–227, 2012 219 biodiesel commercialization is the high cost of biodiesel.
The cost of biodiesel is approximately 1.5 times higher than that of petroleum diesel fuel due to the cost of vegetable oil (Prokop, 2002; Zhang et al., 2003; Lott, 2005; Hass et
al., 2006). Biodiesel made from waste cooking oil is an
economical source and an effective strategy for reducing raw material cost (Supple et al, 1999; Zhang et al., 2003; Kulkarni and Dalai, 2006; Lapuerta et al., 2008). Using waste cooking oil solves the problem of waste oil disposal. Therefore, biodiesel made from waste cooking oil was used in this study.
Dorado et al. (2003) investigated the effect of biodiesel made from waste olive oil for a direct injection diesel engine at several steady-state operating conditions. Results revealed that the use of biodiesel resulted in lower emissions of CO (up to 58.9%), CO2 (up to 8.6%, except for a case with a
7.4% increase), NO (up to 37.5%), and SO2 (up to 57.7%),
with an increase in emissions of NO2 (up to 81%, except
for a case with a slight reduction). Biodiesel also exhibited a slight increase in BSFC (lower than 8.5%) that may be tolerated due to the exhaust emission benefits. Kulkarni and Dalai (2006) reported that the biodiesel obtained from waste cooking oil gives better engine performance and less emissions, except for NOx, when tested on commercial diesel
engines. Lapuerta et al. (2008) tested biodiesel from waste cooking oils in a direct injection diesel commercial engine under a set of engine operating conditions corresponding to typical road conditions. When compared to conventional low sulfur diesel fuel, a sharp decrease was observed in both smoke and particulate matter emissions as the biodiesel concentration was increased. Di et al. (2009) investigated a 4-cylinder direct-injection diesel engine using ultra-low sulfur diesel, biodiesel from waste cooking oil, and their blends, to investigate emissions from the engine under five engine loads at an engine speed of 1800 rev/min. They found that HC and CO emissions decreased whereas NOx
and NO2 emissions increased with increasing biodiesel
blends. For unregulated gaseous emissions, generally, the emissions of formaldehyde, 1,3-butadiene, toluene, and xylene decreased with increasing biodiesel blends; however, acetaldehyde and benzene emissions increased. Ozsezen et al. (2009) investigated a DI diesel engine fueled with biodiesels from waste cooking oil at a constant engine speed (1500 rpm) under the full load condition of the engine. They also found that biodiesels caused reductions in CO, and HC emissions and smoke opacity, but increased NOx emissions
when compared to petroleum-based diesel fuel.
Emissions from a HDDE (heavy-duty diesel engine) under the US-HDD transient cycle test are representative because the engine is tested over a full range of load and speed conditions, including expressway, congested-urban, and uncongested-urban. Furthermore, further research on the use of biodiesel from waste cooking oil will promote its application to diesel engines. Ultra-low sulfur diesel (ULSD) is becoming increasingly popular for HDDEs. Further research on regulated and unregulated emissions from HDDEs fueled with ULSD/WCOB (waste cooking oil biodiesel) blends is required. Although biodiesels made from waste cooking oil have been previously investigated,
PAH emissions from HDDEs with catalyzer fueled with biodiesel from waste cooking oil and its blend with ULSD for the US-HDD transient cycle have seldom been addressed. This study investigated the brake specific fuel consumption and the feasibility of biodiesel blends was assessed. Emissions of regulated matters and PAHs from HDDEs with catalyzer fueled with waste-cooking-oil biodiesel were calculated and compared.
EXPERIMENTAL SECTION Test Engine and Fuels
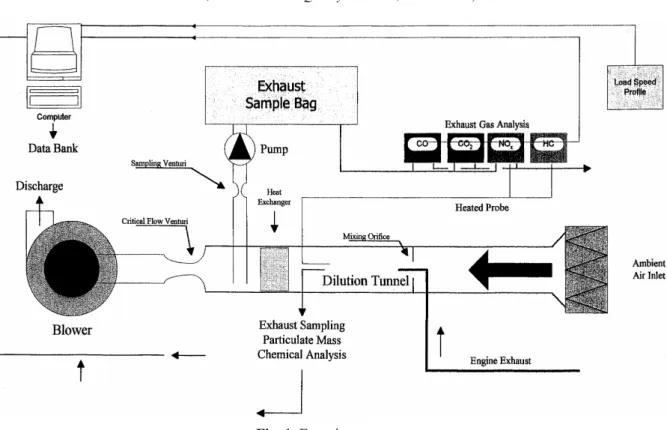
The experimental setup is shown in Fig. 1. The tested HDDE with catalyzer used in this study was a Cummins B5.9-160. Testing was conducted according to Code of Federal Regulations (CFR) 40 Part 86 Subpart N (the US-HDD transient cycle), with related mild engine loaded conditions, which represents typical urban and freeway driving conditions (Code of Federal Regulations). Cold start and hot start emissions were measured and a complex emission index was calculated by multiplying weighting factors (1/7 × cold start + 6/7 × hot start). Operation conditions of test engine varied with time under US-HDD transient cycle test. Therefore, the air/fuel ratio was not constant. In this study, the air/fuel ratio ranged from 29 to 33. The specifications of the test HDDE with catalyzer is listed in Table 1. A Schenck GS-350 dynamometer was used. A dilution tunnel and a monitoring system were installed downstream of the exhaust to supply dilute air and to facilitate continuous measurement of suspended particles (PM and particulate-phase PAHs). Gas-particulate-phase pollutants (THC, CO, CO2, NOx,
and gas-phase PAHs) were also collected and measured. In order to decrease the temperature of the original exhaust, clean ambient air was used to dilute the original exhaust. The appropriate dilution ratio is approximately 18 fold. In this study, ultra-low sulfur diesel (ULSD) was purchased from CPC Corporation in Taiwan and the biodiesel made from waste cooking oil (WCOB) was purchased from Great Green Renewable Energy Technology Corporation in Taiwan. The following five test fuels were selected for this study: ultra-low sulfur diesel (ULSD), WCOB5 (5 vol% biodiesel made from waste cooking oil + 95 vol% ULSD), WCOB10 (10 vol% biodiesel made from waste cooking oil + 90 vol% ULSD), WCOB20 (20 vol% biodiesel made from waste cooking oil + 80 vol% ULSD), and WCOB30 (30 vol% biodiesel made from waste cooking oil + 70 vol% ULSD).
Sample Collection
PAH samples of both particulate-phase and gas-phase were collected by using a PAH sampling system at a temperature below 52°C. Particulate-phase PAHs were collected on a glass-fiber filter. Filters were placed in an oven at 450°C for 8 hrs before sampling to burn off any organic compounds that might be present. The cleaned filters were stored in a desiccator for at least 8 hrs to achieve moisture equilibrium before weighing. After sampling, the filters were brought back to the laboratory and put in a desiccator for 8 hrs to remove moisture, and were weighed again to determine the net mass of particles collected. Gas-phase PAHs were
Liu et al., Aerosol and Air Quality Research, 12: 218–227, 2012 220
Fig. 1. Experiment setup. Table 1. The specifications of the test HDDE with catalyzer.
Parameters Test HDDE
Engine Model Cummins
Engine Type B5.9-160
Aspiration Turbocharged
Intercooler Water Cooler
Injection Type Direct Injection Bore × Stroke 102 mm × 120 mm Displacement 5880 cc Injection Sequence 1-5-3-6-2-4 Injection Timing 12.3°BTDCa Compression Ratio 17.9:1 Idle Speed 810 rpm
Max. Power 118 kW (at 2400 rpm) Max. Torque 534 Nm (at 1600 rpm)
a BTDC = Before Top Dead Center
collected on a three-stage glass cartridge containing a polyurethane foam (PUF) plug XAD-16 resin. The glass cartridge was packed with 5.0 cm of XAD-16 resin sandwiched between 2.5 cm top and bottom PUF plugs. Silicone glue was used to seal and hold these two pieces of PUF to prevent resin from leaking out during the sampling and extraction processes. After 8 hrs of adherence, the newly PUF/resin cartridge was cleaned up by Soxhlet extraction for one day each with distilled water, methanol, dichloromethane, and n-hexane for a total of 4 days, then placed in a vacuum oven at 60°C for 2 hrs to dry and evaporate the residual solvent in them. After drying, each PUF/resin cartridge was individually wrapped in hexane-washed aluminum foil and stored in a refrigerator at 4°C and transported in clean screw-capped jars with Teflon cap liners before sampling. Each
glass fiber filter was transported to and from the field in a glass box, which was also wrapped with aluminum foil.
Analysis
Each collected sample (including particulate and gaseous PAH samples) was extracted in a Soxhlet extractor with a mixed solvent (n-hexane and dichloromethane; vol/vol, 1:1; 500 mL each) for 24 hrs. The extract was then concentrated, cleaned up, and reconcentrated to exactly 1.0 mL. The PAH contents were determined by a Hewlett-Packard (HP) gas chromatograph (GC) (HP 5890A; Hewlett-Packard, Wilmington, DE, USA), a mass selective detector (MSD) (HP 5972), and a computer workstation (Aspire C500; Acer, Taipei, Taiwan). This GC/MSD was equipped with a capillary column (HP Ultra 2, 50 m × 0.32 mm × 0.17 μm) and an automatic sampler (HP-7673A), and operated under the following conditions: the injection volume of the GC/MSD was 1 μL; the splitless injection temperature was 310°C; the ion source temperature was 310°C; the oven was heated from 50°C to 100°C at 20 °C/min, 100°C to 290°C at 3 °C/min, then held at 290°C for 40 minutes. The mass of primary and secondary PAH ions was determined by using the scan mode for pure PAH standards. The PAHs were qualified by using the selected ion monitoring (SIM) mode. The PAH homologues grouped by the number of rings were as follows: naphthalene(Nap) for 2-ring; acenaphthylene (AcPy), acenaphthene (Acp), fluorene (Flu), phenanthrene (PA) and anthracene (Ant) for 3-ring; fluoranthene (FL), pyrene (Pyr), benzo[a]anthracene (BaA) and chrysene (CHR) for 4-ring; cyclopenta[c,d]pyrene (CYC), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[e]pyrene (BeP), benzo(a)pyrene (B[a]P), perylene (PER), dibenzo[a,h]anthracene (DBA) and
Liu et al., Aerosol and Air Quality Research, 12: 218–227, 2012 221 benzo[b]chrycene (BbC) for 5-ring; indeno[1,2,3,-cd]pyrene
(IND), benzo[ghi]perylene (Bghip) for 6-ring; and, coronene (COR) for 7-ring. The total-PAHs data for the HDDE exhaust is given by the sum of the 21 individual PAHs. The GC/MSD was calibrated with a diluted standard solution of 16 PAH compounds (PAH mixture-610M; Supelco, Bellefonte, PA, USA) plus five additional individual PAHs obtained from Merck (Darmstadt, Germany). Analysis of serial dilutions of PAHs standards showed that the detection limit (DL) for GC/MSD was between 26 pg and 308 pg for the 21 PAH compounds. The limit of quantification (LOQ) is defined as the DL divided by the sampling volume or sampling time. The LOQ for individual PAHs was between 24 pg/m3 and
277 pg/m3, while values for sampling time were between
75 pg/hr and 952 pg/hr. Ten consecutive injections of a PAH 610-M standard yielded an average relative standard deviation of the GC/MSD integration area of 6.71%, within a range of 4.18% to 9.63%. Following the same experimental procedures used for sample treatment, recovery efficiencies were determined by processing a solution containing known PAH concentrations. The experimental results showed the recovery efficiencies for the 21 PAH compounds ranged from 0.835 to 1.08, with an average value of 0.942. Analyses of field blanks, including aluminum foil, glass-fiber filters and PUF/XAD-16 cartridges, revealed no significant contamination (GC/MSD integrated area < detection limit). For particulate matter (PM) analysis, each filter sample was weighed again using an electronic analytical balance with fully automatic calibration technology (AT200, Mettler, Switzerland with accuracy of 0.001 mg) to determine the net mass of collected PM. For total hydrocarbon analysis, each sample was analyzed using a flame ionization detector (FID) (model 404, Rosemount, UK with precision of 0.5%). For carbon monoxide/carbon dioxide analysis, each sample was analyzed using a non-dispersive infrared detector (NDIR) (model 880A, Rosemount, UK with precision of 1%). For nitrogen oxides analysis, each sample was analyzed using a chemiluminescent detection (CLD) (model 404, Rosemount, UK with precision of 0.5%). Anon-touch type HBM torque meter was used to measure engine speed and engine load simultaneously. K-Type thermal couple was used to measure exhaust temperature.
RESULTS AND DISCUSSION Fuel Specifications
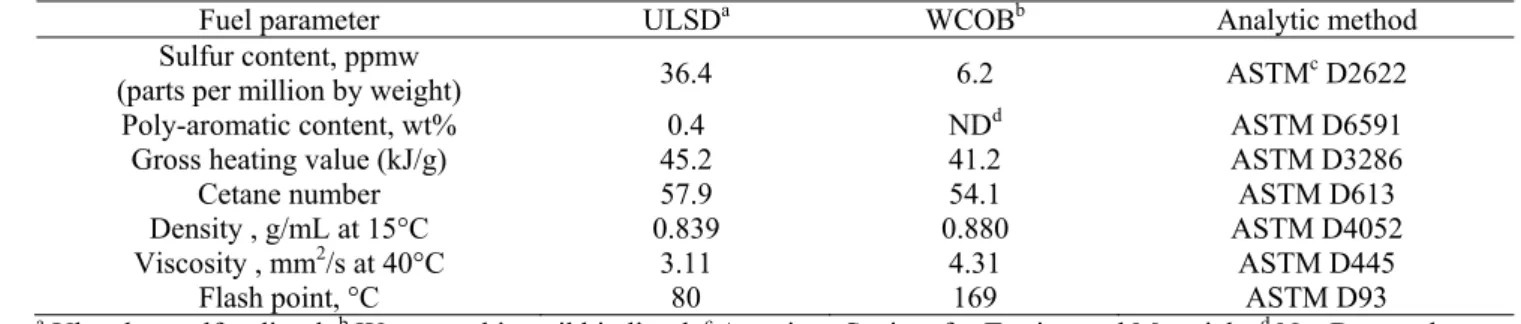
In this study, test fuels were analyzed according to the American Society for Testing and Materials (ASTM), which is one of the most frequently used methods in the US. Fuel specifications are shown in Table 2. The mean total poly-aromatic content of ULSD was 0.4 wt%, but that of WCOB was less than the detection limit (0.1 wt%). The mean sulfur content of ULSD and WCOB were 36.4 and 6.2 ppmw (parts per million by weight). Analytical results reveal that using WCOB instead of ULSD can reduce emissions of SOx and PAHs. Previous studies indicate that
the viscosity, flash points and pour points of biodiesel is higher than that of diesel (Lin et al., 2006; Lin et al., 2006; Legreid et al., 2007; Lin et al., 2008; Lin et al., 2008; Lin
et al., 2009). High viscosity is good for the lubrication of
engines, but it causes poor nebulization, resulting in poor combustion. The boiling point is important for air-fuel mixing. A high boiling point may lead to long penetration, resulting in increased fuel impingement and poor combustion (Dec et al., 1998). Although biodiesels have higher flash point, previous studies indicated that biodiesel was not suitable to be used in cold weather conditions because of its higher viscosity, cetane number, iodine value, and minor constituents such as saturated monoacylglycerols or free steryl glucosides (Dunn, 2009; Eevera et al., 2009; Tesfa
et al., 2010) which may influence pipeline transportation
and induce fuel system flushing and power loss. Therefore, biodiesels were not suitable to be used in cold weather conditions unless additive or heating system was used (Chiu et al., 2004; Tate et al., 2006).
Brake Specific Fuel Consumption
The brake specific fuel consumptions (BSFC) of ULSD, WCOB5, WCOB10, WCOB20, and WCOB30 were shown in Table 3. BSFC increased with increasing WCOB blends at cold start and hot start. The mean BSFC (1/7 cold start + 6/7 hot start) of ULSD, WCOB5, WCOB10, WCOB20, and WCOB30 were 198, 199, 199, 200, and 201 g/BHP-hr, respectively. The mean increases of BSFC were 0.505%, 0.505%, 1.01%, and 1.52% for WCOB5, WCOB10, WCOB20, and WCOB30, respectively, when compared with the BSFC for ULSD. These increases are due to the gross heat value (GHV) of WCOB (41.2 kJ/g) being lower than that of ULSD (45.2 kJ/g) (Table 2). The results indicate that BSFC was higher for biodiesel blends as a consequence of biodiesel having a lower heating value. However, this increase was compensated by higher density
Table 2. Specifications of the test fuel.
Fuel parameter ULSDa WCOBb Analytic method
Sulfur content, ppmw
(parts per million by weight) 36.4 6.2 ASTMc D2622
Poly-aromatic content, wt% 0.4 NDd ASTM D6591
Gross heating value (kJ/g) 45.2 41.2 ASTM D3286
Cetane number 57.9 54.1 ASTM D613
Density , g/mL at 15°C 0.839 0.880 ASTM D4052
Viscosity , mm2/s at 40°C 3.11 4.31 ASTM D445
Flash point, °C 80 169 ASTM D93
Liu et al., Aerosol and Air Quality Research, 12: 218–227, 2012 227 Sidhu. S., Graham. J. and Striebich, R. (2001). Semi-volatile
and Particulate Emissions from the Combustion of Alternative Diesel Fuels. Chemosphere 41: 681–690. Srivastava, D.K., Agarwal, A.K. and Gupta, T. (2011).
Effect of Engine Load on Size and Number Distribution of Particulate Matter Emitted from a Direct Injection Compression Ignition Engine. Aerosol Air Qual. Res. 11: 915–920.
Sun, J., Caton, J.A. and Jacobs, T.J. (2010). Oxides of Nitrogen Emissions from Biodiesel-fuelled Diesel Engines.
Prog. Energy Combust. Sci. 36: 677–695.
Supple, B., Howard-Hildige, R., Gonzalez-Gomez, E. and Leahy, J.J. (1999). The Effect of Steam Treating Waste Cooking Oil on the Yield of Methyl Ester. J. Am. Oil
Chem. Soc. 79: 175–178.
Tat, M.E. (2004). Investigation of Oxides of Nitrogen Emissions from Biodiesel-fueled Engines, Ph.D. Dissertation, Iowa State University, Mechanical Engineering Department.
Tat, M.E. and Van Gerpen, J.H. (2003). Fuel Property Effects on Biodiesel. SAE Paper 036034.
Tate, K.C., Watts, C.A. and Wilkie, K.I. (2006). The Viscosities of Three Biodiesel Fuels at Temperatures up to 300°C. Fuel 85: 1010–1015.
Tesfa, B., Mishra, R., Gu, F. and Powles, N. (2010). Prediction Models for Density and Viscosity of Biodiesel and Their Effects on Fuel Supply System in CI Engines.
Renewable Energy 35: 2752–2760.
Tsai, J.H., Chen, S.J., Huang, K.L., Lee, W.J., Kuo, W.C. and Lin, W.Y. (2011). Characteristics of Particulate Emissions from a Diesel Generator Fueled with Varying Blends of Biodiesel and Fossil Diesel. J. Environ. Sci. Health.,
Part A 46: 204–213
Tsai, J.H., Chen, S.J., Huang, K.L., Lin, Y.C., Lee, W.J., Lin, C.C. and Lin, W.Y. (2010). PM, Carbons, and PAH Emissions from a Diesel Generator Fuelled with Soy-biodiesel Blends. J. Hazard. Mater. 179: 237-243 Tsai, J.H., Huang, K.L., Chiu, C.H., Lin, C.C., Kuo, W.C.,
Lin, W.Y., Chaung, H.C., Yang, T.H. and Chen, S.J. (2011). Particle-bound PAHs and Particle-extract-induced Cytotoxicity of Emission from a Diesel-generator Fuelled with Soy-biodiesel. Aerosol Air Qual. Res. 11: 822–836. Tsai, Y.I., Yang, H.H., Wang, L.C., Huan, J.L., Young, L.H.,
Cheng, M.T. and Chiang, P.C. (2011). The Influences of Diesel Particulate Filter Installation on Air Pollutant Emissions for Used Vehicles. Aerosol Air Qual. Res. 11: 578–583.
Tsolakis, A., Megaritis, A. and Yap, D. (2008). Application of
Exhaust Gas Fuel Reforming in Diesel and Homogeneous Charge Compression Ignition (HCCI) Engines Fuelled with Biofuels. Energy 33: 462–470.
Tsolakis, A., Megaritis, A., Wyszynski, M.L. and Theinnoi, K. (2007). Engine Performance and Emissions of a Diesel Engine Operating on Diesel-RME (Rapeseed Methyl Ester) Blends with EGR (Exhaust Gas Recirculation). Energy 32: 2072–2080.
Wang, S.K., Cheng, C.Y., Lin, Y.C. and Chen, K.S. (2012). Emission reductions of Air Pollutants from a Heavy-duty Diesel Engine Mixed with Various Amounts of H2/O2. Aerosol Air Qual. Res. 12: 133–140.
Wu, S.P., Wang, X.H., Yan, J.M., Zhang, M.M. and Hong, H.S. (2010). Diurnal Variations of Particle-bound PAHs at a Traffic Site in Xiamen, China. Aerosol Air Qual.
Res. 10: 497–506.
Wu, T.S., Hsieh, L.T., Lin, S.L., Chang, Y.C., Chen, C.B. and Hung, C.H. (2010). Emissions from Using Viscous Agent-Treated Fishing Boat Fuel Oil: Tests with a Heavy-Duty Diesel Engine (HDDE) Dynamometer. Aerosol Air
Qual. Res. 10: 76–85.
Yao, Y.C., Tsai, J.H. and Chou, H.H. (2011). Air Pollutant Emission Abatement Using Application of Various Ethanol-gasoline Blends in High-mileage Vehicles.
Aerosol Air Qual. Res. 11: 547–559.
Yoshimoto, Y., Onodera, M. and Tamaki, H. (1999). Reduction of NOx, Smoke, and BSFC in a Diesel Engine
Fueled by Biodiesel Emulsion with Used Frying Oil.
SAE Paper No. 1999-01-3598.
Yuan, C.S., Lin, H.Y., Lee, W.J., Lin, Y.C., Wu, T.S. and Chen, K.F. (2007). A New Alternative Fuel for Reduction of Polycyclic Aromatic Hydrocarbon and Particulate Matter Emissions from Engines. J. Air Waste Manage.
Assoc. 57: 465–471.
Yuan, C.S., Lin, Y.C., Tsai, C.H., Wu, C.C. and Lin, Y.S. (2009). Reducing Carbonyl Emissions from a Heavy-duty Diesel Engine at US Transient Cycle Test by Use of Paraffinic/Biodiesel Blends. Atmos. Environ. 43: 6175– 6181.
Zhang, Y., Dube, M.A., McLean, D.D. and Kates, M. (2003). Biodiesel Production from Waste Cooking Oil: 2. Economic Assessment and Sensitivity Analysis. Bioresour. Technol. 90: 229–240.
Received for review, September 13, 2011 Accepted, November 16, 2011