Improved performance of polymer/TiO
2nanorod bulk heterojunction
photovoltaic devices by interface modification
Yun-Yue Lin, Tsung-Hung Chu, Chun-Wei Chen,a兲 and Wei-Fang Sub兲
Department of Materials Science and Engineering, National Taiwan University, Taipei 106, Taiwan 共Received 25 November 2007; accepted 5 January 2008; published online 8 February 2008兲 In this article, the polymer photovoltaic devices based on the poly共3-hexylthiophene兲/TiO2nanorods hybrid material is present. An enhancement in the device performance can be achieved by removing or replacing the insulating surfactant on the TiO2 nanorod surface with a more conductive ligand, which can play the role to assist charge separation efficiency or also to prevent from back recombination, giving a large improvement in the short circuit current and fill factor. The relatively high power conversion efficiency of 1.7% under simulated AM 1.5 illumination共100 mW/cm2兲 can be achieved, providing a route for fabricating low-cost, environmentally friendly polymer photovoltaic devices by all-solution processes. © 2008 American Institute of Physics.
关DOI:10.1063/1.2839405兴
Recently, polymer solar cells have attracted a great in-terest in developing the low-cost, large-area, mechanically flexible photovoltaic devices.1–3Due to the short exciton dif-fusion length in the semiconducting polymer共⬍20 nm兲,4–6 the electron acceptors must be intermixed with polymer at a nanometer length scale to achieve efficient charge separation before recombination. The most commonly used structure is the polymer-based bulk heterojunction 共BHJ兲 solar cell, which consists of the electron accepting network formed ran-domly within the polymer matrix. In the last decade, research has been focused on the development of polymer BHJ pho-tovoltaic devices, using fullerene or fullerene derivatives as acceptors in combination of polymer as donors, and the high-est power conversion efficiency is about⬃5%.2,3An alterna-tive type of hybrid polymer solar cells, based on conjugated polymers combined with n-type inorganic nanocrystals, such as CdSe共Refs.1 and7兲 or TiO2,共Refs.8 and9兲 or ZnO,10 have been proposed due to the advantage of high electron mobility and excellent chemical and physical stability of in-organic semiconductors. Because of the short exciton diffu-sion length and the relatively low carrier mobility in poly-mer, the interfaces between the donors 共polymer兲 and acceptors共nanocrystals兲 play the crucial role in determining the photovoltaic performance.11 The environmentally friendly and low-cost TiO2 nanocrystal is a promising mate-rial for the hybrid organic:inorganic photovoltaic device applications8,9,12–14 since its surface can be easily modified with many organic molecules,15 which may influence the charge transfer efficiency at the interface. In this article, we report the improved efficiency of the photovoltaic device based on the poly共3-hexylthiophene兲共P3HT兲/TiO2 nanorod hybrid material through interface engineering between poly-mer and TiO2nanorods.
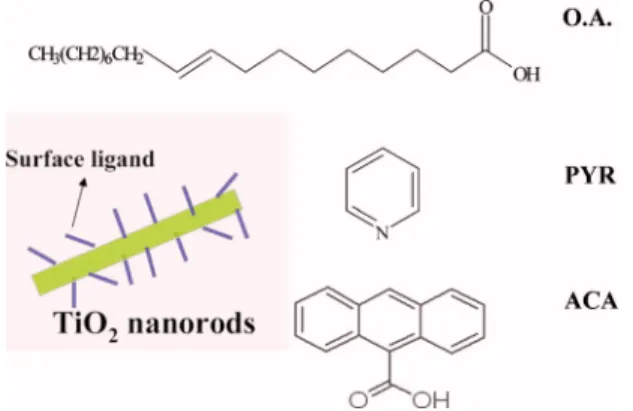
The growth of high aspect ratio anatase titanium dioxide nanorods was synthesized by the hydrolysis of titanium tet-raisopropoxide according to literature with modification.16 Details and results have been described in an earlier work.9 The dimensions of TiO2 nanorods are 20– 40 nm in length and 4 – 5 nm in diameter. Typically, the as-synthesized TiO2
nanorods are capped with insulating surfactant of oleic acid 共OA兲 consisting of long alkyl chain, which may act as a potential barrier for charge transfer. Therefore, we carried out the ligand exchange treatment to replace the original oleic acid ligand using two different kinds of ligand mol-ecules of pyridine 共PYR兲 and anthracene-9-carboxylic acid 共ACA兲. Firstly, the as synthesized OA end-capped TiO2 na-norods were washed with ethanol three times to remove the oleic acid. Then, the TiO2 nanorods were dispersed in pyri-dine and left under stirring at 70 ° C until the solution turned clear. Through these procedures, the OA 共original surface ligand兲 was removed and the pyridine of a weak binding ligand was on the surface of TiO2 nanorods, which can be removed through heating. Removal of the residual surfactant on the TiO2 nanorod surface in the hybrid material will lead to direct contact between polymer and TiO2 nanorods. To obtain the ACA end-capped TiO2 nanorods, we mixed the ethanol washed bare TiO2nanorods in combination with the ACA at 6:1 weight ratio, which were then dispersed in pyri-dine, and left under stirring at 75 ° C until the solution turned into clear and yellow color. The ACA capped nanocrystals were then precipitated by hexane and isolated by centrifug-ing, and redispersed in mixed solvent which contains pyri-dine, chloroform, and dichloromethane共1:2:2 by volume ra-tio兲. The ligand molecule of ACA, which consists of three benzenelike rings and has a large binding energy between carboxylic acid group and TiO2 nanorods, represents a con-ductive interface between TiO2 nanorods and polymer. The TiO2 nanorods connected with three different kinds of sur-face ligands are shown schematically in Fig.1.
The hybrid materials were prepared by adding appropri-ate amount of TiO2nanorods into P3HT共Mw⬃58 000, PDI 1.62, RR 96%兲 polymer solution to make P3HT/TiO2 nanorod composite samples. For the photovoltaic device fabrication, a 40 nm thick layer of poly共3,4-ethylene-dioxythiophene兲 poly 共styrenesulfonate兲 共Baytron P 4083兲 was spin cast onto the indium-tin-oxide substrate, followed by baking at 120 ° C for 30 min. The films were moved into a nitrogen-purged glovebox for subsequent depositions. The thin active P3HT: TiO2 nanorod hybrid layer about 120 nm, obtained from a 10 mg/ml solution at 1:1 weight ratio of P3HT to TiO2 in the mixed solvent, was then deposited by a兲Electronic mail: chunwei@ntu.edu.tw.
b兲Electronic mail: suwf@ntu.edu.tw.
APPLIED PHYSICS LETTERS 92, 053312共2008兲
0003-6951/2008/92共5兲/053312/3/$23.00 92, 053312-1 © 2008 American Institute of Physics
using spin coating. An additional layer of TiO2 nanorods sandwiched between the active layer and the aluminum elec-trode was included to act as a hole blocking layer9and also as an optical spacer.17 Typical device area was about 0.1 cm2. The Al electrode was then deposited onto the TiO
2 nanorod layer by thermal evaporation in vacuum at pressure around 2⫻10−6 Torr.
UV-visible absorption spectra were obtained using Jasco V-570 UV/visible/near-infrared spectrophotometer. The steady state PL spectra were taken by the FluoroLog®-3 spectrofluorometer 共Jobin-Yvon兲. Time-resolved photolumi-nescence共TRPL兲 spectroscopy was performed with a time-correlated single photon counting spectrometer 共Picoquant, Inc.兲. A pulse laser 共470 nm兲 with an average power of 1 mW operating at 40 MHz with duration of 70 ps was used for excitation. Current-voltage measurements共Keithley 2410 source meter兲 were obtained by using a solar simulator 共Newport, Inc.兲 with the AM1.5 filter under irradiation inten-sity of 100 mW/cm2. The film thickness was measured by means of the Veeco M6 surface profiler.
The inset in Fig. 2共a兲 shows absorption spectra of
as-synthesized and ACA-capped TiO2 nanorods, and the two sharp peaks between 350 and 400 nm are related to the fact that the ACA molecules are bonded on the TiO2 nanorods with the carboxylic acid group. Figure2共a兲shows the UV-visible absorption of the hybrid materials with different sur-face ligand molecules on the TiO2nanorod surface. The pris-tine P3HT exhibits a broad absorption spectrum ranged from 400 to 650 nm and TiO2 nanorods have an absorption edge at about 350 nm. The optical density of the absorption spec-tra in the hybrids is simply the sum of the absorption specspec-tra of constituent parts. For the three samples with different sur-face treatments, no significant change in the absorption spec-tra has been found, indicating that the interface layer does not contribute significantly to light harvesting. In contrast, the yield of the PL emission in the three hybrids decreases with different quantities in comparison with the pristine P3HT, as shown in Fig. 2共b兲, suggesting the occurrence of PL quenching from charge separation. The PL quenching efficiency Q for the three samples is QACA⬎QPYR⬎QOA, indicating that more efficient charge separation can be achieved at the P3HT/TiO2 nanorod interfaces by either removing the insulating surfactant or replacing with a more conductive ligand. The improved charge separation effi-ciency at the P3HT/TiO2 nanorod interfaces can also be inferred from TRPL spectroscopy. Figure2共c兲shows the PL decay curves for the pristine P3HT and the hybrid films with different surface modifications, respectively. The addition of TiO2 nanorods in polymer results in a new relaxation process, which provides a further non radiative process to the donor and leads to shortening of the measured lifetime. The measured PL lifetimes for the pristine P3HT and P3HT/TiO2 nanorod hybrids with OA, PYR, and ACA sur-factant are P3HT= 676 ps, OA= 480 ps, PYR= 310 ps, and ACA= 291 ps, respectively, indicating that more efficient charge separation takes place at the polymer/TiO2 nanorod interfaces by removing the insulating ligand OA or by re-FIG. 1. 共Color online兲 Schematic representation of three different kinds of
surface ligands on the TiO2nanorod surface.
FIG. 2. 共Color online兲 共a兲 UV-visible absorption, 共b兲 PL intensity, and 共c兲 time-resolved PL spectroscopy of the hybrid materials with different surface ligand molecules. The inset in 共a兲 shows absorption spectra of as-synthesized 共dash line兲 and ACA capped TiO2nanorods共solid line兲. 共d兲 Schematic representation of the photo-voltaic device based on the hybrid material.
053312-2 Lin et al. Appl. Phys. Lett. 92, 053312共2008兲
placing with a more conductive ligand of ACA, consistent with the PL quenching result.
We have further fabricated the photovoltaic devices us-ing the above three hybrid materials, as shown schematically in Fig. 2共d兲. All the devices consisting of different surface modified TiO2 nanorods were fabricated using the same P3HT: TiO2 nanorod ratio of 50: 50 wt %. The current-voltage characteristics of the devices with different configu-rations under simulated AM 1.5 illumination are shown in Fig.3. The device based on the P3HT: TiO2 nanorod共OA兲 hybrid material exhibits a short circuit current density 共Jsc兲 of 1.67 mA/cm2, an open circuit voltage 共V
oc兲 of 0.65 V, and a fill factor共FF兲 of 0.35, resulting in a power conversion efficiency共兲 of 0.38%. For the device based on the hybrid with TiO2nanorods by pyridine treatment, a large increase in the fill factor indicates that removal of insulating surfactant on the TiO2nanorods results in a significant improvement in the serial resistance of the device. The performance of the device based on the P3HT: TiO2nanorod共PYR兲 hybrid ma-terial exhibits a short circuit current density 共Jsc兲 of 2.62 mA/cm2, an open circuit voltage共V
oc兲 of 0.69 V, and a FF of 0.63, resulting in a power conversion efficiency共兲 of 1.14%. For the device consisting of TiO2nanorods modified by the conductive ligand of ACA, a further improvement in the device performance is found, giving a short circuit cur-rent density共Jsc兲 of 3.49 mA/cm2, an open circuit voltage 共Voc兲 of 0.75 V, and a FF of 0.65, resulting in a power con-version efficiency共兲 of 1.7%. Table I summarizes the de-vice performance for different configurations. The role of the conductive surface ligand molecule ACA may act as follows: 共i兲 it can mediate charge transfer from the polymer to the TiO2 nanorods for electron accepting and result in a more efficient charge separation, similar to the observation in Ref. 15for the polymer/TiO2 interface. However, the exact
nature of charge transfer efficiency could differ in the hybrid compared to a monolayer on a flat surface.共ii兲 The molecular structure of ACA consisting of anthracene may also interact with the thiophene rings of P3HT, which may further im-prove the compatibility between polymer and TiO2nanorods. 共iii兲 The surface modifier may also act as a barrier from back recombination, which can reduce the shunt losses and im-prove the device performance.18,19 共iv兲 The surface traps or defects of TiO2 nanorods can also be modified by the inter-face modifier, which can assist the charge transfer efficiency. The detailed origin is now under investigation. The relatively high power conversion efficiency of the P3HT/TiO2nanorod hybrid solar cells provides a route for future application of fabricating low-cost, environmentally friendly photovoltaic devices by all-solution processes.
In summary, we have demonstrated the polymer photo-voltaic devices based on P3HT/TiO2 nanorod hybrid mate-rial. The performance of the solar cell can be significantly improved through interface modification. Further optimiza-tion in the device performance can be accomplished by vary-ing the size of nanorods, improvvary-ing the polymer/nanorod in-terface, or aligning the nanorods to improve the carrier transport.
This work is supported by the National Science Council of Taiwan 共Project No. NSC95-3114-P-002-003-MY3兲 and the U.S. Airforce Project No.共AOARD 074-014兲.
1W. U. Huynh, J. J. Dittmer, and A. P. Alivisatos, Science 295, 2425 共2002兲.
2W. Ma, C. Yang, X. Gong, K. Lee, and A. J. Heeger, Adv. Funct. Mater. 15, 1617共2005兲.
3G. Li, V. Shrotriya, J. Huang, Y. Yao, T. Moriarty, K. Emery, and Y. Yang, Nat. Mater. 4, 864共2005兲.
4R. H. Friend, G. J. Denton, J. J. M. Halls, N. T. Harrison, A. B. Holmes, A. Kohler, A. Lux, S. C. Moratti, K. Pichler, N. Tessler, K. Towns, and H. F. Wittmann, Solid State Commun. 102, 249共1997兲.
5T. J. Savenije, J. M. Warman, and A. Goossens, Chem. Phys. Lett. 287, 148共1998兲.
6A. C. Arango, L. R. Johnson, V. N. Bliznyuk, Z. Schlesinger, S. A. Carter, and H. H. Horhold, Adv. Mater.共Weinheim, Ger.兲 12, 1689 共2000兲. 7N. C. Greenham, X. Peng, and A. P. Alivisatos, Phys. Rev. B 54, 17628
共1996兲.
8C. Y. Kwong, W. C. H. Choy, A. B. Djurisic, P. C. Chui, K. W. Cheng, and W. K. Chan, Nanotechnology 15, 1156共2004兲.
9T. W. Zeng, Y. Y. Lin, H.-H. Lo, C. W. Chen, C.-H. Chen, S.-C. Liou, H.-Y. Hunag, and W.-F. Su, Nanotechnology 15, 5387共2006兲.
10W. J. E. Beek, M. M. Wienk, and R. A. J. Janssen, Adv. Mater.共Weinheim, Ger.兲 16, 1009 共2004兲.
11K. M. Coakley and M. D. McGehee, Appl. Phys. Lett. 83, 3380共2003兲. 12P. Ravirajan, S. A. Haque, J. R. Durrant, D. D. C. Bradley, and J. Nelson,
Adv. Funct. Mater. 15, 609共2005兲.
13H. Wang, C. C. Oey, A. B. Djurisic, M. H. Xie, Y. H. Leung, K. K. Y. Man, W. K. Chan, A. Pandey, J. M. Nunzi, and P. C. Chui, Appl. Phys. Lett. 87, 023507共2005兲.
14Q. Wei, K. Hirota, K. Tajima, and K. Hashimoto, Chem. Mater. 18, 5080 共2006兲.
15C. Goh, S. R. Scully, and M. D. McGehee, J. Appl. Phys. 101, 114503 共2007兲.
16P. D. Cozzoli, A. Kornowski, and H. Weller, J. Am. Chem. Soc. 125, 14539,共2003兲.
17J. Y. Kim, S. H. Kim, H. H. Lee, K. Lee, W. Ma, X. Gong, and A. Heeger, Adv. Mater.共Weinheim, Ger.兲 18, 572 共2006兲.
18P. Ravirajan, A. M. Peiró, M. K. Nazeeruddin, M. Graetzel, D. D. C. Bradley, J. R. Durrant, and J. Nelson, J. Phys. Chem. B 110, 7635共2006兲. 19H. J. Snaith, A. J. Moule, C. Klein, K. Meerholz, R. H. Friend, and M.
Graetzel, Nano Lett. 7, 3372共2007兲. FIG. 3. 共Color online兲 Current-voltage characteristics of the photovoltaic
devices based on different surface ligand molecules under AM 15 共100 mW/cm2兲 irradiation.
TABLE I. Summary of the device performance based on P3HT/TiO2 nano-rod hybrid materials with different surface modifications.
Jsc共mA/cm2兲 Voc共V兲 FF 共%兲
P3HT/TiO2共OA兲 1.67 0.65 0.35 0.38
P3HT/TiO2共PYR兲 2.62 0.69 0.63 1.14
P3HT/TiO2共ACA兲 3.49 0.75 0.65 1.70
053312-3 Lin et al. Appl. Phys. Lett. 92, 053312共2008兲