PHYSICAL REVIEW B VOLUME 40, NUMBER 13 1NOVEMBER 1989
Correlations
among
clb,
T„and
Madelung
potentials
in
the
system
of RSa2Cu30
supereonductors
Shiow-Fon Tsay and Shou-Yih WangDepartment
of
Physics, National Tsing Hua University, Hsinchu, Taiwan 30042,Republicof
China Lance Horng andT.
J.
Watson YangElectrophysics Department, National Chiao Tung-Universt'ty, Hsinchu, Taiwan 30042,Republic
of
China (Received 6 March 1989;revised manuscript received 30May 1989)We have observed a correlation between the lattice-constant ratio c/b and T, in R-13a-Cu-O superconductors (where R is arare-earth element). Madelung potentials and the bond length of Cu(1)
—
O(3) are calculated and correlated with the distribution of T, over the variousRBa2Cu307 superconductors. Further consideration of YBa2Cu30„with variable
x
ofsuitableox-ygen content leads to an explanation ofthe correlation ofdecreasing c/b ratio with increasing T,
.
A Coulomb-type ionic mechanism is therefore suggested sothat shortening of thec
axis andin-creased chain length provides
(1)
achannel ofcharge-transfer excitation from chain to Cu-Olay-ers and (2)a long-range Coulomb interaction between the two Cu-O layers, both ofwhich result in stronger coupling between the two Cu-O layers and higher T,
.
Since Chu and Wu ' and their collaborators Grst
discovered in 1987the superconductor YBa2Cu307 with high transition temperature
T„many
studiesof
theY-Ba-Cu-0
quaternary system have been performed inorder toattempt tounderstand the underlying mechanismof
su-perconductivity. Research on YBa2Cu30„concerning the correlation betweenT,
and crystal structure have pro-ceeded along two routes: one by varying the oxygen con-tent(6
&x
&7),
and the other by replacingY
with other rare-earth elements, viz.RBa2Cu30„.
For example, theT,
dependenceof
the lattice constantsof
the unit cell(a,
b, andc),
3upon the distortion —,'c
—
a,
4 upon the unit-cell volume, 3 the orthorhombic splitting, (b—
a)/a
etc.
, has shown that small structural changes and the critical temperatureT, of
the superconducting phaseof
YBa2-Cu30» are related. We report now the c/b ratio for vari-ous casesof
RBa2Cu30 and YBa2Cu30(6
&x
&7).
To
explore why the ratio c/b isso strongly correlated withT„we
have calculated the Madelung potentialof
RBa2Cu307.It
is found that the variationof
Madelung potentials among superconductors with differentR
is simi-lar to thatof
the bond lengthof
Cu(1)
-0(3).
The calcu-lation and description are given in detail. Finally, we dis-cuss why the Coulomb interaction plays so important a role and draw conclusions from this.We follow the notation
of Ref.
5 for various ion posi-tions in the structureof YBa2Cu30„and
use the valuesof
their lattice parameters.Cu(1)
and0(1)
are in the chain direction (baxis); with0(2)
the vacant position(a
axis);0(3)
in thec
axis (apexof
the pyramidof
the Cu site); andCu(2)-0(4)-0(5)
as the copper oxide layer. Wenote that samplesof
the same composition can beprepared, via different processes, to exhibit differentT,
values. Thuswe make comparisons and analyses
of
data from the labo-ratory that provides the most complete dataof
the RBa2Cu 307 system. We 6nd that for the samplesRBa2Cu30„
from eachof
the laboratories investigat-ed, ' the ratio c/b always tends to decrease asT,
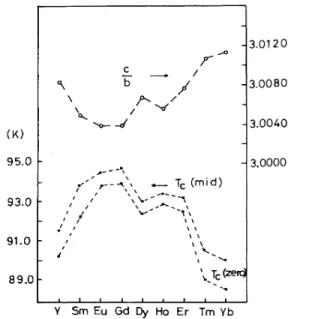
in-—3.0120 3.0080 CL st' -3.0040 95.
0-93. 0-91. 0-t,fTlIC!) I I I I I '\ —3,000089.
0— $c(zeal I I I I I I I I Y Sm Eu Gd Dy Ho Er Tm YbFIG.
l.
The c/b ratio and T,in the RBa2Cu307 system, Rbe-ing the rare-earth element.
creases. As shown in Fig. 1, the variations in the c/b ratio are
of
the orderof
magnitudeof
0.001.
Consequently, the validityof
the correlation between c/b andT,
depends mainly onthe accuracyof
measurementof
the lattice con-stants but also on the qualityof
the sample. As displayed in-TableI,
which was obtained from the data from sevenpapers by Takita and co-workers, with quite accurate measurements, we analyzed these different RBa2Cu3G7 samples on the relation between c/b and
T,
.
The result,shown in Fig. 1, correlates the
T,
at midpoint and atzero resistivity with the c/b ratio, via the seriesof
samplesof
substitutional replacementof
the rare-earth(R)
element. The relation isclear thatT,
increases with decreasing c/bCORRELATIONS AMONG c/b,
T„AND
MADELUNG.. .
9409 TABLEI.
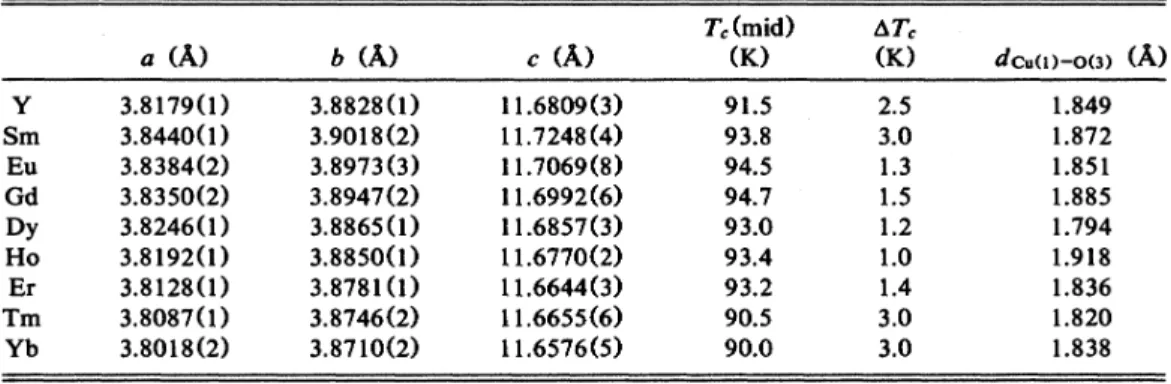
The lattice parameters of RBa2Cu30„and theirT„T,
width, AT„and the bond length ofCu(1)
—
O(3)(see Ref.5).
Y Sm Eu Gd Dy Ho Er Tm Yb a (A) 3.
8179(1)
3.8440(1) 3.8384(2) 3.8350(2) 3.8246(1) 3.8192(1) 3.8128(1) 3.8087(1)
3.8018(2) b (A) 3.8828(1)
3.9018(2) 3.8973(3)3.
8947(2) 3.8865(1)
3.
8850(1) 3.8781(1)
3.8746(2)3.
8710(2) c (A) 11.6809(3) 11.7248(4) 11.7069(8) 11.6992(6) 11.6857(3)11.
6770(2)11.
6644(3)11.
6655(6) 11.6576(5) T,(mid) (K.)
91.
5 93.8 94.5 94.7 93.0 93.4 93.2 90.5 90.0 2.5 3.0 1.3 1.5 1.2 1.0 1.4 3.0 3.0 dcu())-o(3) (&) 1.849 1.872 1.851 1.885 1.794 1.918 1.836 1.820 1.838 C b 3.0120 30080 R~30„
3,020 YBa2Cu3 x 3.0040 3.010 3. 0000-I I I I I I 90.0 92.0 94.0 3000 {b) 25 50 75FIG. 2. (a) The c/b ratio dependence of T, in the RBa2-Cu30 system. (b) The c/b ratio dependence of T, in
YBa2Cu30, with variable oxygen contents (6
(
x&7).
and viceversa. The relation between c/b andT,
is accord-ingly depicted in Fig.2(a),
where theT,
s at each mid-pointT,
(mid) and their widths AT, areshown.A similar relation is also found in the
YBa2Cu30,
su-perconductors with variable oxygen content. Basedon ex-perimental 6ndings, 'o higher oxygen content gives higherT„a
gradual decrease in the lattice constant c, and a slight increase inthe lattice constant b. Thenet result isa decreasing c/b with increasingT„as
displayed in Fig.2(b).
The widths /),T,
are also indicated similarly, as in Fig.2(a).
Taking into consideration both the resultsof
Figs.2(a)
and2(b),
we 6nd in both cases an increasingT,
with decreasing c/b. In the comparison we further found the correlation is more pronounced when the lattice con-stants are known more accurately. We also tried to relate the c/a ratio to
T„but
did not 6nd such a good correla-tion as c/b withT,.
In order tounderstand the close rela-tion between c/b andT„we
proceeded to calculate the Madelung potential for the systemof
the RBa2Cu307 su-per conductors.The valences
of Y
+ andBa
+ in the YBa2Cu30„sys-tem(x
6-7)
have been generally accepted.It
is alsoknown that
YBa2Cu30„
is an ionic insulator whenx
6, but is metallic whenx
&6.
5.
However, its charge-carrier concentration(-10
' cm)
in the metallic phase is solow that one can assume a high ionicity in
YBa2Cu30„
and deal with an ionic model. In such a crystal, the long-range Coulombic interactions (Madelung potential ener-gy) contribute dominantly tothe total potentialof
the ions(over 80%
of
the total potential, although they vary withthe ionicity).
"'2
The mechanically repulsive interactions between nearest ions also contribute to some extent, but are found to be small compared with Coulombic interac-tions. We neglect contributions from the repulsive in-teractions and also van der Waal's attractive interactionin this work. We remark further that our Madelung-potential calculation, treating only the relative effect aris-ing from substitutional replacement
of
theR
element in RBa2Cu307 rather than treating a general Madelung problem, is expectedto
yield more accurate and meaning-fulpredictions.Next, we calculated the Madelung potential per YBa2-Cu307 "molecule"
(i.e.
, formula unit) according to the well-known Ewald's method. '3 The desired total potentialof
the reference ion iinthe 6eldof
all the other iona inthe crystal is )ir(i)4x
QS(G)G
exp Q2 4 ' 1/2+g
"F«»",
),
(1)
x
J rJwhere 6, isthe volume
of
the unit cell,G
isthe reciprocal lattice vector and ri is a control parameter which allowsboth sums
of
Eq.(1)
to converge rapidly. The functionsF(x)
andS(G)
are de6ned, respectively, as2 g2
F(x)
J
es ds,
S(G)
-
gq/exp(iG
rj),
Jin which
S(G)
is the structure factorof
the crystal. Based on the conditionof
electric neutrality and some ex-perimental results, ' we assume that there are one Cu + at theCu(1)
site, two Cu + at the two equivalentCu(2)
sites, and vacancies attheO(2)
sites. InTableII
we showthe Madelung energies for various ions in the RBa2Cu307 molecule
(R
Y,
Sm, Eu, Gd, Dy, Ho,Er,
Tm, andYb).
The total Madelung potential energy which isthe sum
of
all the iona inthe molecule is shown inFig.
3(a).
Now wecompare the total Madelung potentials with the sum
of
the Madelung potentialsof
Cu(1)
andO(3)
as shown in Fig.3(b).
The variationof
the total Madelung potentialTSAY, WANG, HORNG, AND YANG
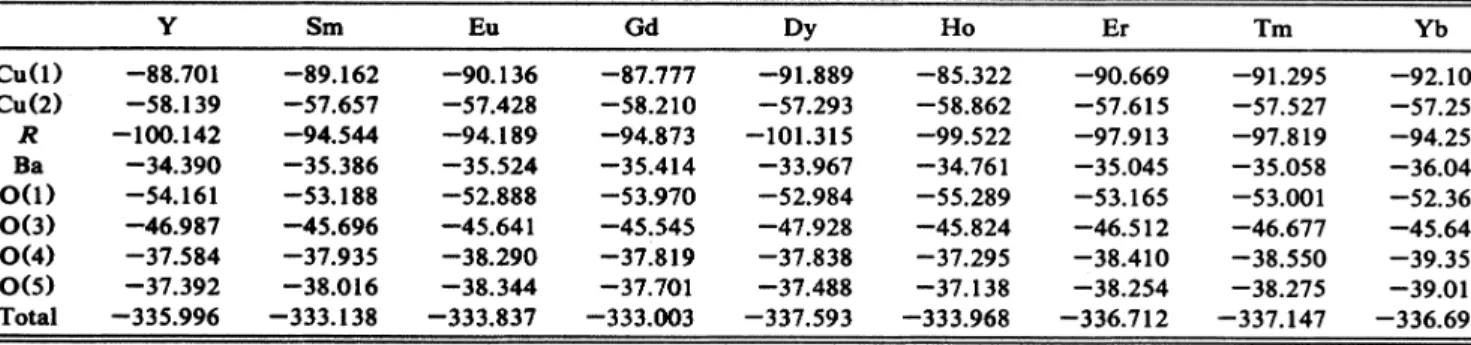
TABLEIL Madclung potential ofeach Ion and the total Madelung potential ofaRBaqCu307 unit cell (units in eV). In obtaining the total Madelung potential, the values of Ba,Cu(2),
O(3),
O(4),and O(S)are each doubled due tothe symmetry ofthe unit cellofRBa2Cu307.
Cu(l)
CU(2) R BaO(l)
O(3)o(4)
o(s)
Total Y-88.
701-58.
139-100.
142-34.
390-S4.
161-46.
987—
37.584-37.
392-335.
996-89.
162-57.
657—
94.544—
35.386—
53.188-45.
696-37.
935-38.
016-333.
138 Eu-90.
136—
57.428—
94.189-35.
524—
52.888-45.
641-38.
290—
38.344-333.
837—
87.777—
58.210—
94.873—
35.414—
53.970—
45.545—
37.819—
37.701-333.
003—
91.
889-57.
293—
101.315—
33.967—
52.984-47.
928-37.
838-37.
488-337.
593 Ho—
85.322-58.
862-99.
522—
34.761—
55.289—
45.824-37.
295-37.
138—
333.968 Er—
90.669—
57.615—
97.913—
35.045—
53.165—
46.512—
38.410—
38.254—
336.712 Tm—
91.
295—
57.527-97.
819—
35.058—
53.001—
46.677—
38.550—
38.275—
337.147 Yb—
92.100—
57.258—
94.251—
36.048—
52.367—
45.649—
39.359—
39.015—
336.691 --333.0 I L(a)
I 1 II I 1 I I 1 I 1 I I I {eV) t -&3&.0— I ) 1 I 'L I' I I L) I I I A I -135.0— I d' 1 I I '1 I ll , I LI(b)
f l50 -~ Ig o Ig 4y I -&33.0— —&37. 0-1.900~ -&3S. 0---334.0 --335.0 --336.0 ~ —-337.0--338.
0 (eV} Y Sm EQ Gd Dy Ho Er Tfn YbFIG.
3.
(a) The total Madelung potential (eV) of RBa2-Cu307. (b)The sum ofthe Madelung potentials (eV)ofCu(l)
and O(3)ofRBaqCu3O7. The inset represents thebond length of
Cu(l)-O(3)
inthe RBaqCu3O~. The abscissa isthe same asinthe main part ofthe Sgure.
with various
R
elements has very clearly the same tenden-cy as the sumof
the Madelung potentialsof
Cu(l)
andO(3).
This implies that the sumof
the potentialof
Cu(1)
andO(3)
mainly reflects the variationof
the total Madelung energy. The dominance suggestsa
correlation between the Madelung potential and the bond lengthof
Cu(1)-O(3)
becauseof
the natureof
Coulombic interac-tion. In fact, DyBa2Cu307 has the smallest total Madelung potential, the smallest potential sumof
Cu(1)
andO(3),
and also the smallest bond lengthof
Cu(1)-O(3).
On the other hand, HoBaqCu30q has relativellarger total Madelung potential, largest
Cu(l)
andO(3
Madelung potential sum, and largest bond lengthof
Cu(l)-O(3).
These are manifested in Figs.3(a)
and3(b),
and in the insetof
the flgure, respectively. Thus, the total Madelung potential energyof
RBa2Cu307 is quanti-tatively correlated tothe bond lengthof
Cu(1)
—
O(3).
Now we turn back to
T,
of
Fig. 1 and compareT,
withthe total Madelung potential
of
Fig.3(a).
There exists the same tendency in the variations over the superconduc-tors with differentR
elements except Sm and Tm forwhich the cause, while not known for certain, might be due to some individual feature
of
the ions themselves. Aside from this small discrepancy we have observed a consistent correlation among these quantities. A decreasein c/b is correlated to an increase in
T„Madelung
total potential, Madelung potential sumof
Cu(l)
andO(3),
and the bond lengthof
Cu(l)
-O(3).
Having obtained the correlation among those quantities from substitutional replacement
of
theR
element in RBa2Cu307, we reconsider the situationof YBa2Cu30„
with variable oxygen content. The experimental result' is that greater oxygen content will cause longer bondlength
of
Cu(1)-O(3),
and thus shorter bond lengthof
Cu(2)-O(3).
This can be explained qualitatively fromour concept
of
the ionic model due to Coulomb interac-tion. As oxygen content isincreased, the occupation prob-abilityof
O(1)
sites would be increased. This would in-crease the lengthof
the b axis. The bond lengthof
Cu(l)-O(3)
would be increased due to the Coulombrepulsive force on
O(3)
byO(1)
and possibly the presenceof
O(2).
The reaction force fromO(3)
would furtherre-pel
O(l)
and lengthen the b axis. AsO(3)
is pushedto-ward
Cu(2)
the increase in the Coulomb attraction be-tweenO(3)
andCu(2)
would makeCu(2)
come closer toO(3)
such that the overallc
axis isshrunk. Thus the ionic Coulomb interaction as displayed by using increasingoxy-gen content tends to yield a decreasing c/b, increasing bond length
Cu(1)
-O(3),
increasing Madelung potential, and increasingT,
.
During the processof
decreasing inc/b,
etc.
, there could be charge transfer excitation andsimultaneously long-range Coulomb interaction between the two
Cu-0
layers. This therefore suggests a Coulom-bic mechanism that, increasing chain length and decreas-ing thec
axis provides:(i)
a channelof
charge transfer''
from the chain to theCu-0
layers and(ii)
a long-range Coulomb interaction between the twoCu-0
layers, bothof
which result in stronger coupling betweenCORRELATIONS AMONG
clb, T„AND
MADELUNG.. .
9411 the twoCu-0
layers and higherT,.
The partof
thesuper-conductivity mechanism occurring within the Cu-O lay-ers, which still exists there, is not addressed. To summa-rize, we Grst observed the correlation between the ratio c/b and
T,
.
Then we calculated the total Madelung po-tentials, the sumof
Madelung potentialsof
Cu(1)
andO(3),
and also the bond lengthof
Cu(l)
—
O(3)
for the various RBaqCu 307 superconductors.
Comparisonof
these calculated results with the distribution
of
T,
over these superconductors shows a correlation between the bond lengthof
Cu(1)-O(3)
andT,
.
On the other hand, still using the ionic Coulomb interaction(i.
e.
, the same physicsof
Madelung potentials) but considering the su-perconductorsYBaqCu30„with
variablex,
we explain the experimental resultof
decreasing c/b ratio with increasing oxygen content and increasingT,
.
To
conclude, acorrelation between c/b andT,
has been observed and explained. Consequently, a Coulomb-type charge-transfer excitation mechanism is suggested that, as a resultof
decreasingc
and increasing b, charge transfer from chain to copper-oxide layers could happen and simultaneously along-range Coulomb interaction between the two Cu-O layers could prevail, resulting in enhanced coupling between the twoCu-0
layers and higherT,
.
The authors are indebted to
Dr. W.
P.
Hsieh, Chiem-Jamg Wu, Ching-Jium Wu, and Chein-Ming Tsai for their helpful and stimulating discussions. This research was 6nancially supported by the National Science Coun-cil, Taiwan, Republicof
China, under Grants No.NSC78-0208-M007-65
and No.NSC78-0208-M009-13.
M.K.Wu,
J.
R.
Ashobor, C.J.
Torng, P.H. Hor,R.
L.Meng, L.Gao, Z.J.
Huang, Y. Q. Wang, andX.
W.Chu, Phys. Rev.Lett. 5$, 908(1987).
~M. Apostal and M.Popescu, Philos. Mag. Lett. 57, 305
(1988).
M. Matsui, K.Ohmori,T.
Shimizu, and M. Doyama, PhysicaB+C
14$, 432(1987).
4I-Wei Chen et al., Solid State Commun. 63,997
(1987).
5H. Asano,T.
Ishigaki, and K.Takita, Jpn.J.
Appl. Phys. Pt.226,L714(1987); T.Ishigaki, H. Asano, and K.Takita, ibid Pt. 2 26, L987 (1987); K.Takita, H.Akinaga, H. Katoh,
T.
Ishigaki, and H. Asano, ibid Pt. 2 2.6, L1023 (1987);H. Asano,T.
Ishigaki, and K. Takita, ibid. Pt. 2 26, L1064 (1987); T.Ishigaki, H. Asano, and K.Takita, ibid Pt. 226.,L1226 (1987);Pt. 226, L1323(1987);H.Asano, K.Takita, H. Katoh, H.Akinaga,
T.
Ishigaki, M.Nishino, M.Imai, andK.Masuda, ibid Pt.226,L141.0
(1987).
sP. M.Grant etal.,Phys. Rev.B 35, 7242(1987).
7G.V.Subba Rao,Physica
B+
C 14$, 237(1987).
SS.C.Leeetal. , Phys. Rev.B37, 2285(1988).
9Y. Nakaza~a, M. Ishika~a, and
T.
Takabatake, Physica 88cC 14$, 404(1987).
~OR.
J.
Cava etal., Phys. Rev.B 36, 5719(1987).
"H.
Aiza~a andE.
Iguchi,J.
Phys. Chem. Solids 43, 1147 (1982).' K.Aizawa, E.Iguchi, and R.
J.
D.Tilley, Proc.R.
Soc.Lon-don, Ser. A 394, 299(1984).
t3C.Kittel, Introduction toSolid State Physics, 6th ed (Wile.y, Singapore, 1986),p.606.
'"S.
X.
Dou et al., Solid State Commun. 6$,221(1988).
~5Hajime Asano and Koki Tabita, Physica
B+C
148, 302(1987).
' R.
J.
Cava,B.
Batlogg, K.R.
Rabe,E.
A.Riztman,P.
K.Gal-lagher, and L.W.Rupp,
Jr.
, Physica C 156, 523(1988).
' L.