Industrial & Engineering Chemistry Research is published by the American Chemical Society. 1155 Sixteenth Street N.W., Washington, DC 20036
Article
Design Alternatives for the Amyl Acetate Process:
Coupled Reactor/Column and Reactive Distillation
Sheng-Feng Chiang, Chien-Lin Kuo, Cheng-Ching Yu, and David S. H. Wong
Ind. Eng. Chem. Res., 2002, 41 (13), 3233-3246 • DOI: 10.1021/ie010358jDownloaded from http://pubs.acs.org on November 28, 2008
More About This Article
Additional resources and features associated with this article are available within the HTML version: • Supporting Information
• Links to the 6 articles that cite this article, as of the time of this article download • Access to high resolution figures
• Links to articles and content related to this article
SEPARATIONS
Design Alternatives for the Amyl Acetate Process: Coupled Reactor/
Column and Reactive Distillation
Sheng-Feng Chiang,†Chien-Lin Kuo,†Cheng-Ching Yu,*,†and David S. H. Wong‡
Department of Chemical Engineering, National Taiwan University of Science and Technology, Taipei 106-07, Taiwan, and Department of Chemical Engineering, National Tsing Hua University, Hsinchu 300-13, Taiwan
Recent advances in reactive distillation have led to renewed interest in the design/redesign of many esterification processes. For the esterification of amyl acetate, design alternatives exist that offer a gradual transition from the conventional recycle structure to reactive distillation. The phase behavior of the quaternary mixture immediately excludes the possibility of the conventional recycle structure, and in this work, we explore the designs of two different configurations, a coupled reactor/column and reactive distillation column , for the amyl acetate process. The total annual cost (TAC) is used to evaluate the economic advantages of different designs. Systematic design procedures were devised for the coupled reactor/column system, as well as for the reactive distillation process. The results clearly indicate the economic advantage of reactive distillation for the amyl acetate process. Unique characteristics of amyl acetate process include a significant two-liquid zone and high-purity water from the aqueous phase of a decanter. This leads us to a single-end-composition control problem. However, limit cycles can occur if the net reaction rate and production rate are not properly balanced or if the feeds are not stoichiometrically balanced. Therefore, a second composition loop is used to infer possible imbalances. Dynamic simulations indicate that reasonable control can be achieved for very complex reactive distillation dynamics.
1. Introduction
Reactive distillation has been employed in industry for decades, but it has received renewed attention in recent years.1,3,6,12,19,21,22Reactive distillation can reduce
capital and energy costs in some systems, particularly when the reactions are reversible or when the presence of azeotropes makes conventional separation expensive. As pointed out by Malone and Doherty,19 combining
reaction and separation is not always advantageous, and in some cases, it might not even be feasible. Most process design begins with the conventional reactor/ separator recycle structure7,18,25 where the reactor is
designed first, followed by the separators, with the design typically being done in a hierarchical order.7
From the process operation perspective, reaction and separation are carried out sequentially. For reversible reactions, the coupled reactor/column might be an attractive alternative. It features a reactor with a rectifier and/or a stripper.27The principle behind the
coupled reactor/column, similar to reactive distillation, is that it removes the products continuously from the reaction mixture by distillation, which reduces the effect of the backward reaction. It also has the advantages of
easy catalyst replacement, large reactor holdup, and reuse of existing equipment.27 The full-blown version
of the coupled reactor/column is, certainly, reactive distillation.3,17,19,22Classical success stories include the
methyl acetate process and the production of methyl
tert-butyl ether (MTBE).
Amyl acetate has been used in industry as a solvent, an extractant, and a polishing agent, for example. It can be synthesized from acetic acid and amyl alcohol via an esterification. However, ternary azeotropes were found
* Corresponding author, currently with Department of
Chemical Engineering, National Taiwan University, Taipei 106-17, Taiwan. Fax: +886-2-2362-3040. Tel.: +886-2-3365-1759. E-mail: ccyu@ccms.ntu.edu.tw.
†National Taiwan University of Science and Technology. ‡National Tsing Hua University.
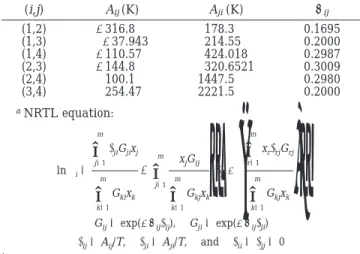
Table 1. Binary Parameters of the NRTL Modelafor
Acetic Acid (1) + Amyl Alcohol (2) + Amyl Acetate (3) + Water (4) Systems4,14 (i,j) Aij(K) Aji(K) Rij (1,2) -316.8 178.3 0.1695 (1,3) -37.943 214.55 0.2000 (1,4) -110.57 424.018 0.2987 (2,3) -144.8 320.6521 0.3009 (2,4) 100.1 1447.5 0.2980 (3,4) 254.47 2221.5 0.2000 aNRTL equation: ln γi)
∑
j)1m τjiGjixj∑
k)1m Gkixk +∑
j)1 m x jGij∑
k)1m Gkjxk[
τij-(
∑
r)1m xrτrjGrj∑
k)1m Gkjxk)
]
Gij) exp(-Rijτij), Gji) exp(-Rijτji) τij) Aij/T, τji) Aji/T, and τii) τjj) 0 . 3233Ind. Eng. Chem. Res. 2002, 41, 3233-3246
10.1021/ie010358j CCC: $22.00 © 2002 American Chemical Society Published on Web 06/04/2002
in the mixture of 1-pentanol-amyl acetate-water.4,14,15
This might lead to difficulties in downstream separation when the conventional recycle structure is employed. The coupled reactor/column and reactive distillation could be effective in overcoming these difficulties. To our knowledge, this is the first thorough study of the amyl acetate process in the open literature.
The objective of this work is to evaluate design alternatives for the amyl acetate esterification process. Rather than going directly to reactive distillation, a gradual transition is considered. Different process de-signs are evaluated quantitatively on the basis of their economic potentials. Finally, the dynamics and control of reactive distillation are studied.
2. Amyl Acetate Process
2.1. Reaction Kinetics. The esterification of acetic
acid with amyl alcohol (1-pentanol) follows the
elemen-tary reaction
The reaction is catalyzed by acidic cation-exchange resin (Amberlyst 15 from Aldrich Chemical Co.15). From the
experimental data of Lee et al.,15for a given catalyst
density (0.8 g/cm3) and porosity of 30%, the reaction rate
can be expressed according to a quasi-homogeneous model26
where r is the reaction rate per unit volume [kg mol/ (m3 s)] and C represents the molar density of the
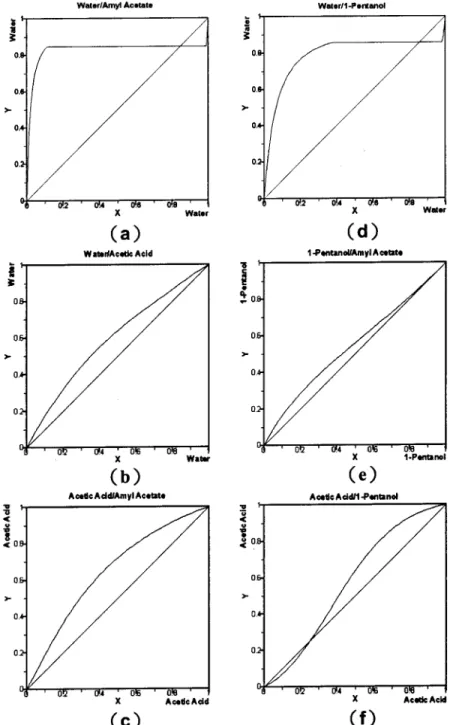
Figure 1. Binary VLE for (a) amyl acetate-water, (b) acetic acid-water, (c) acetic acid-amyl acetate, (d) water, (e)
1-pentanol-amyl acetate, and (f) acetic acid-1-pentanol.
CH3COOH + C5H11OH S kb kf C5H11COOCH3+ H2O (1) r ) kf
(
CHOAcCAmOH -CAmOAcCH 2O Keq)
(2)corresponding component (kg mol/m3). k
fis the forward
reaction rate constant given by
with T in kelvin, and Keqis the equilibrium constant
From the kinetics, it is observed that this is a slightly endothermic reaction with an almost-negligible heat effect. This implies that an increase in the reaction temperature will increase the equilibrium conversion but by only a very small amount.15 Note that the
parameters of the concentration-based rate expression (eqs 2-4) were obtained directly from the experimental data via regression and that good agreement between the model predictions and the experimental data were also reported.15The equilibrium constant was found to
be about 2 for the temperatures of interest (100-150 °C). The catalyst price is around 14 $/lb, and a catalyst life of 1 year is assumed in this study.
2.2. Phase Equilibria. For the amyl acetate process,
the normal boiling points are ranked, in ascending order, as follows:
The two reactants, acetic acid and 1-pentanol, are intermediate boilers, whereas the products, water and amyl acetate, are the low and high boilers, respectively. Following Lee and Liang,13Chang,4and Lee et al.,14,15
the nonrandom two-liquid (NRTL) activity coefficient model20was used for the vapor-liquid-liquid
equilib-rium (VLLE) for the quaternary system containing acetic acid, 1-pentanol, amyl acetate, and water. Vapor-liquid equilibrium (VLE) data are available for all of the binary pairs, and liquid-liquid equilibrium (LLE) data for two ternary systems (acetic acid-1-pentanol-water and acetic acid-amyl acetate-acid-1-pentanol-water) and the quaternary system can also be found in the litera-ture.14,16,18Eight very different sets of binary
param-eters for the NRTL model were presented in Lee et al.,14
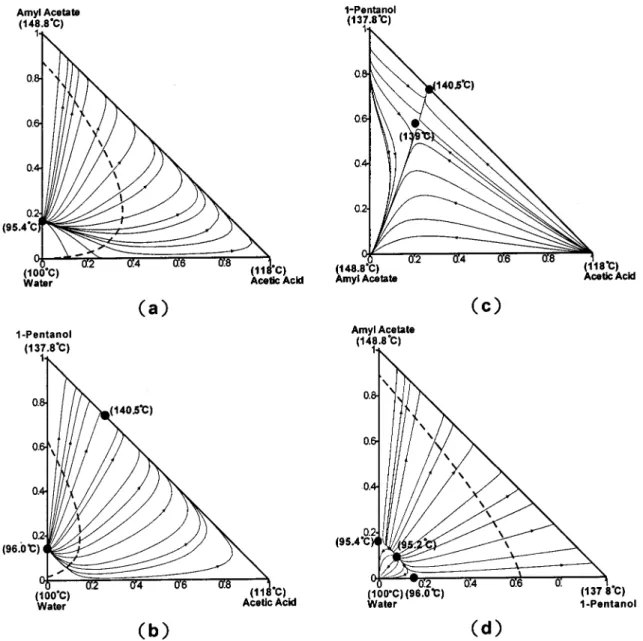
Figure 2. Residue curve maps (solid lines) and liquid-liquid envelopes (dashed lines) for the ternary mixtures (a) water-acetic
acid-amyl acetate, (b) water-acetic acid-1-pentanol, (c) acetic acid-1-pentanol-acid-amyl acetate, and (d) water-1-pentanol-acid-amyl acetate.
kf) 17 500e -6223.2/T (3) Keq)kf kb ) 13.9e-777/T (4) H2O 100 °C < CH3OOH 118 °C < C5H11OH 137.8 °C < C5H11COOCH3 148.8 °C (5)
and even though they gave a good description of the VLE, they were regressed from different combinations of data sets. As will become clear later, that the ternary LLE plays an important role in describing product purity, so the binary parameters from two ternary LLE data sets (set 1 of ref 14 and the original set of ref 4) are employed in this work. Table 1 gives the binary parameters of the NRTL model. The Hayden-O’Conell second virial coefficient model10 with association
pa-rameters was used to account for the dimerization of acetic acid. The Aspen Plus2built-in association
param-eters were employed to compute fugacity coefficients. The quaternary system has two minimum-boiling binary azeotropes (1-pentanol-water and amyl acetate-water) and one maximum-boiling binary azeotrope (acetic acid-1-pentanol). Whereas the azeotrope be-tween acetic acid and 1-pentanol is homogeneous, the other two are heterogeneous azeotropes with rather high-purity water in the aqueous phase. Figure 1 shows the VLE for all six binary pairs. There are two ternary azeotropes for acetic acid-1-pentanol-amyl acetate and water-1-pentanol-amyl acetate, and they are an un-stable node and a saddle point, respectively, as shown
in Figure 2. The wa1-pentanol-amyl acetate ter-nary azeotrope temperature is 95.2 °C, which is in good agreement with the experimental result, 94.9 °C.11Note
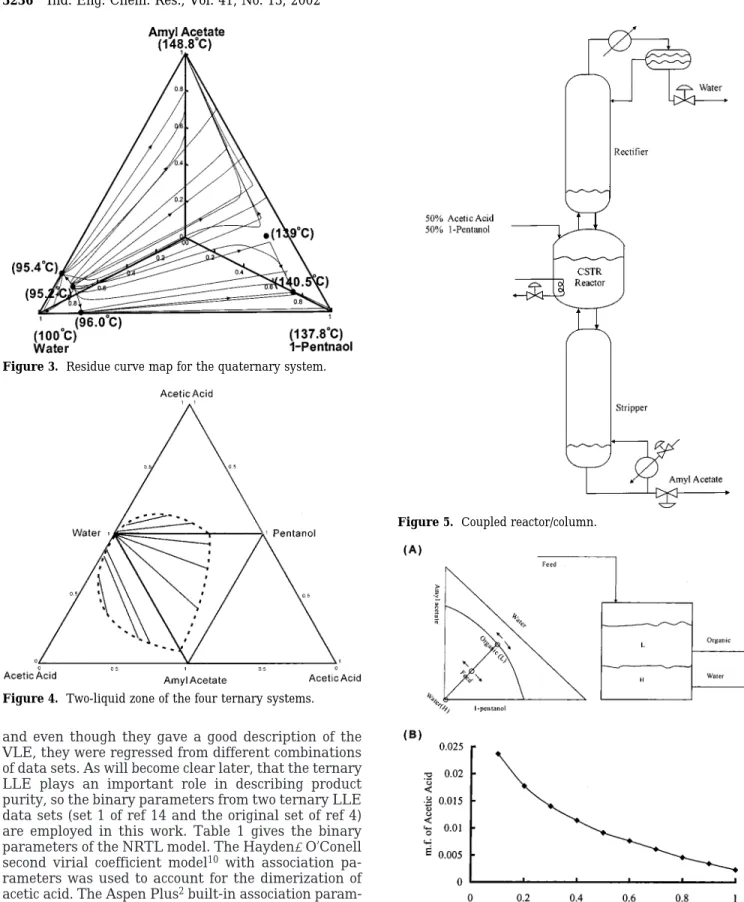
that a very large liquid-liquid (LL) envelope is observed for the water-1-pentanol-amyl acetate system (Figure Figure 3. Residue curve map for the quaternary system.
Figure 4. Two-liquid zone of the four ternary systems.
Figure 5. Coupled reactor/column.
Figure 6. (A) Schematic description of compositions in the organic
phase and aqueous phase of the decanter and (B) largest allowable acetic acid composition in the feed to achieve 99% water in the aqueous phase for a typical feed water/1-pentanol/amyl acetate ratio of 0.385/0.385/0.230.
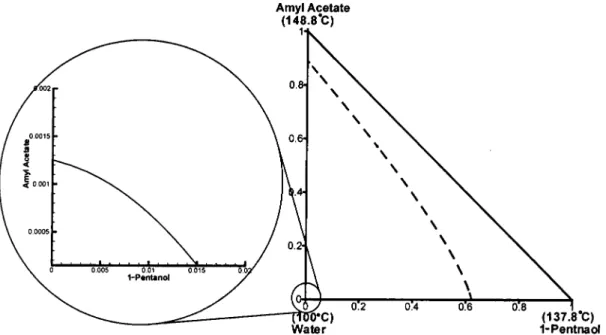
2d), with one end of the tie lines connected to very high-purity water. Moreover, more than 50% of the composi-tion space in Figure 2d is in the LL envelope. Figure 3 shows the residue curve map3,9,24 for the quaternary
mixture. The residue curves start from the minimum-boiling ternary azeotrope (95.2 °C in Figure 3) and move toward three binary azeotropes (95.4, 96.0, and 140.5 °C) and two pure-component vortexes (100 and 148.8 °C), as shown in Figure 3. Two branches are of particu-lar interest, one is the residue curves moving toward the amyl acetate vortex (148.8 °C) and the other is the residue curves converging to the maximum-boiling binary azeotrope (140.5 °C) near the 1-pentanol corner. A distillation boundary is clearly observed between these two branches; it is a surface that starts from the
minimum-boiling ternary azeotrope (95.2 °C), moves toward the amyl acetate-1-pentanol-acetic acid sur-face, and then curves down and ends at 1-pentanol vortex. Note that the products of the esterification process are amyl acetate and water, which lie on the top and the lower left corner, respectively, of Figure 3. Because three of the four ternary systems show an LL envelope (Figure 2), a two-phase zone persists toward the acid-rich portion, as can be seen in Figure 4, where a large portion of the ternary composition space lies inside the two-phase zones. More importantly, moving toward the acid-lean surface, all of the tie lines point to pure water.
The phase behavior of the amyl acetate process is quite different from that of the methyl acetate or ethyl Figure 7. The water corner of the LL envelope for 1-pentanol-amyl acetate-water.
Table 2. Steady-State Design Parameters and Total Annual Cost (TAC) for the Coupled Reactor/Column
case 1 2 3 4 5 6
reactor temperature (°F) 176 176 176 176 176 176
VR(ft3) 7593.0 8122.0 8476.0 8829.0 9182.0 10594.4
DR(ft) 16.91 17.29 17.54 17.78 18.01 18.89
stripping section
amyl acetate at the bottom (mf) 0.99 0.99 0.99 0.99 0.99 0.99
no. of trays (Ns) 14 14 14 14 14 14
BR 2.48 2.46 2.44 2.43 2.41 2.38
DC(ft) 10.29 10.26 10.16 10.17 10.14 10.06
QR(107Btu/h) 2.25 2.23 2.20 2.20 2.19 2.15
rectifying section
water at the top (mf) 0.99 0.99 0.99 0.99 0.99 0.99
no. of trays (NR) 60 47 43 40 38 33
RR 2.34 2.33 2.33 2.36 2.37 2.42
DC(ft) 13.63 13.58 13.64 13.87 14.03 14.52
QC(107Btu/h) 3.92 3.91 3.91 3.94 3.96 4.01
heat exchanger areas (ft2)
reboiler 1998.2 1985.1 1959.9 1951.2 1942.7 1911.8 condenser 11672.6 11650.5 11640.8 11725.9 11783.2 11946.2 heater 1138.2 1138.2 1138.2 1138.2 1138.2 1138.2 capital costs ($1000) reactor 2,509.4 2,616.8 2,687.3 2,756.4 2,824.5 3,087.7 stripper 490.2 488.4 483.4 483.8 482.6 478.3 stripper trays 53.7 53.4 52.6 52.7 52.5 51.8 rectifier 2,124.2 1,740.5 1,627.9 1,563.3 1,519.0 1,407.2 rectifier trays 355.4 277.0 255.1 243.4 235.5 215.7 heat exchangers 1,797.9 1,794.8 1,791.2 1,795.6 1,798.3 1,805.1 operating costs ($1000) catalyst cost 1,881.3 2,012.4 2,100.1 2,187.6 2,275.0 2,625.0 energy cost 1,544.7 1,542.5 1,347.9 1,354.5 1,357.6 1,586.4
total annual cost ($1000) 5,869.5 5,878.6 5,747.1 5,840.5 5,936.8 6,560.1
acetate process in the order of the boiling points and in the shape of the two-liquid-phase zone. However, it is quite similar to the butyl acetate process23where a large
LL envelope is connected to the almost pure water region.
3. Steady-State Design
The production rate of amyl acetate is 551 lb mol/h (about the annual demand in Taiwan) with a product purity of 99 mol % and with the other stream having a specification of 99% water. Because a distillation bound-ary exists for the quaternbound-ary system, the conventional recycle structure, with either a direct or an indirect sequence,7is immediately excluded from further
inves-tigation.5
The phase behavior (Figures 3 and 4) of the quater-nary system gives a good indication of the configuration of the reaction/separation process. First, the light product (water) is enclosed by a two-liquid zone, and therefore, the top product is withdrawn from the aque-ous phase of a decanter. Amyl acetate, the heaviest component, should be taken from the bottom of the process unit (i.e., top section of the coupled reactor/ distillation column in Figure 5) and the reaction takes place in the middle section. In both designs, the coupled reactor/distillation column and the reactive distillation column, a decanter is installed, and the aqueous phase is taken as the top product (without recycle), while the organic phase is totally recycled back to the column.
Because the product specification on the top is 99% water, it is helpful to locate the likely composition space of the overhead vapor flow (the feed to the decanter). Equation 5 indicates that the boiling point of acetic acid is next to that of water and that the two-liquid zone shrinks gradually toward the acetic-acid-rich region. Therefore, a large acetic acid concentration prevents us from achieving high-purity water. Figure 6 shows the maximum allowable acid mole fraction as a function of column pressure, and at atmospheric pressure, only 0.23% acid is allowed in the overhead vapor flow. This implies that most of the acid must be consumed in the reaction zone or that an extremely large number of trays is needed in the rectifying section. When the column pressure is reduced to 0.1 atm, the allowable acid concentration becomes 2.4%. With a small amount of
acid in the system, we are practically looking at the 1-pentanol-amyl acetate-water phase diagram (Figure 2d). A closer look at the water corner of the LL envelope (Figure 7) reveals that a high water purity can be obtained if the overhead vapor is located toward the amyl acetate-water side.
3.1. Coupled Reactor/Column. Yi and Luyben27
studied the design and control of various coupled reactor/column systems with different levels of complex-ity. A similar approach was taken for the design of the amyl acetate process, but the design is complicated by the phase-split behavior in the decanter. With a given decanter and a given pressure (0.1 atm), the design procedure for the coupled reactor/column (Figure 5) consists of the following steps: (1) Fix the reactor volume (VR) and guess the tray numbers in the stripping
and rectifying sections (NS and NR, respectively). (2)
Find the minimum number of trays in the stripping section (NS,min) from the short-cut design7with a given
specification of amyl acetate (99%), and set NS) 2NS,min.
(3) Change the number of trays in the rectifying section (NR) such that the top product specification (99% water)
is met. (4) Repeat steps 2 and 3 until NS converges
(usually NS does not change much for different NR
values). (5) Compute the total annual cost (TAC;7,8see
Appendix A). (6) Change the reactor volume (VR) such
that the TAC is minimized.
Table 2 shows the steady-state design for different reactor volumes. Case 3, giving the lowest TAC, is the optimal design and corresponds to NR) 43, NS) 14,
BR ) 2.44 (boilup ratio), RR (reflux ratio, which is the organic-phase flow rate divided by the aqueous-phase flow rate) ) 2.33, and 85% of the equilibrium conversion in the reactor. Because the conversion in the reactor is close to the equilibrium conversion, the assumption of equilibrium conversion can be used as the initial start-ing point for the design. As mentioned earlier, a very small amount of acid can be tolerated in the column overhead (2.4% at 0.1 atm), and even with such a low pressure, a large number of trays in the rectifier is still required to separate acetic acid from water (which is known to be a difficult separation). Also, note that NR
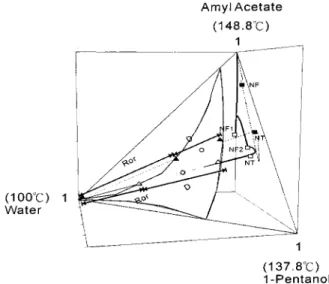
increases dramatically as the reactor volume drops below a certain value (e.g., case 1) and that the catalyst cost play a significant role in the TAC. Figure 8 shows the distillation line of the coupled reactor/distillation column. It starts from the almost-pure (99%) amyl acetate and moves toward the 1-pentanol-amyl acetate-water plane. The liquid composition on the top tray (labeled NT on the dashed line of Figure 8) is outside
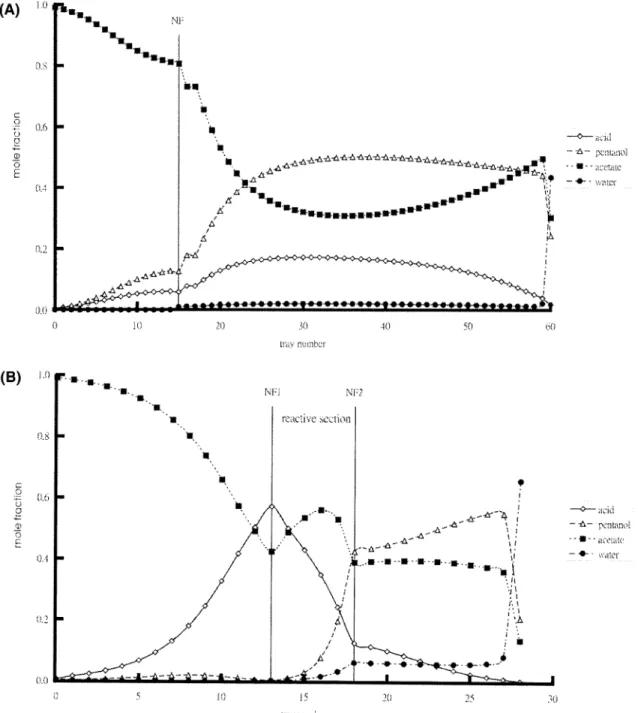
the two-phase zone, whereas the overhead vapor (feed to the decanter; identified by the solid triangle in Figure 8) is clearly inside the LL envelope. The tie line points to 99% water in the aqueous phase as the top product, and the other end corresponds to the composition of the organic reflux. Figure 9 shows the composition profile of the coupled reactor/distillation column. The result reveals a sharp increase in the amyl acetate composition at the feed location (around tray 15) and that it takes 43 trays in the rectifier to bring the acid composition down to an acceptable level (Figure 9A). It is interesting to note that the composition profiles of the two heavy components (1-pentanol and amyl acetate) in the rec-tifying section behave very differently from the profiles in typical distillation systems, as they stay relatively constant in the rectifier (Figure 9A). The reason for this is that the organic reflux (mostly amyl acetate and Figure 8. Distillation lines and corresponding tie lines for the
coupled reactor/distillation column (dashed lines) and reactive distillation column (solid lines).
1-pentanol) brings these heavy components back to the rectifying section as opposed to bringing back only light components as in conventional distillation.
3.2. Reactive Distillation. Unlike conventional
dis-tillation, detailed design of reactive distillation (e.g., minimum number trays, minimum number of reactive trays, feed locations, etc.) is less obvious. Sneesby et al.21
presented a design procedure for the ETBE reactive distillation system, Subawalla and Fair22 proposed a
general procedure for the design of reactive distillation, and Luyben17compared design alternatives (neat versus
excess reactant) and studied several design parameters for ideal reactive distillations. The reactive distillation apparatus for the amyl acetate process (Figure 10) differs from the above-mentioned designs in the phase-split behavior in the decanter. With a given decanter, the design procedure is quite similar to that of the coupled reactor/distillation column, but one can choose from several feed locations for the acetic acid and
1-pentanol. From the reaction kinetics point of view (keeping the reactant concentrations as high as pos-sible), the heavy reactant (1-pentanol) is fed to the column from the top section of the reactive zone, and the light reactant (acetic acid) comes in from the lower section of the reactive zone (Figure 10).1In the
simula-tion, a 15-s residence time was assumed for the reactive trays. Once the feed locations are determined and the column pressure is fixed at 1 atm, the design procedure becomes: (1) Fix the number of reactive trays (NRxn),
and guess the numbers of trays in the stripping and rectifying sections (NSand NR, respectively). (2) Find
the minimum number of trays of the stripping section (NS,min ) from the short-cut design with a given
speci-fication of amyl acetate (99%), and set NS) 2NS,min .
(3) Increase the number of trays in the rectifying section (NR) until the top product specification (99% water) is
met. (4) Repeat steps 2 and 3 until NSconverges (usually
NSdoes not change much for different NR values). (5)
Figure 9. Composition profiles for (A) coupled reactor/column and (B) reactive distillation column.
Compute the total annual cost (TAC; see Appendix A). (6) Change the number of reactive trays (NRxn) so that
the TAC is minimized.
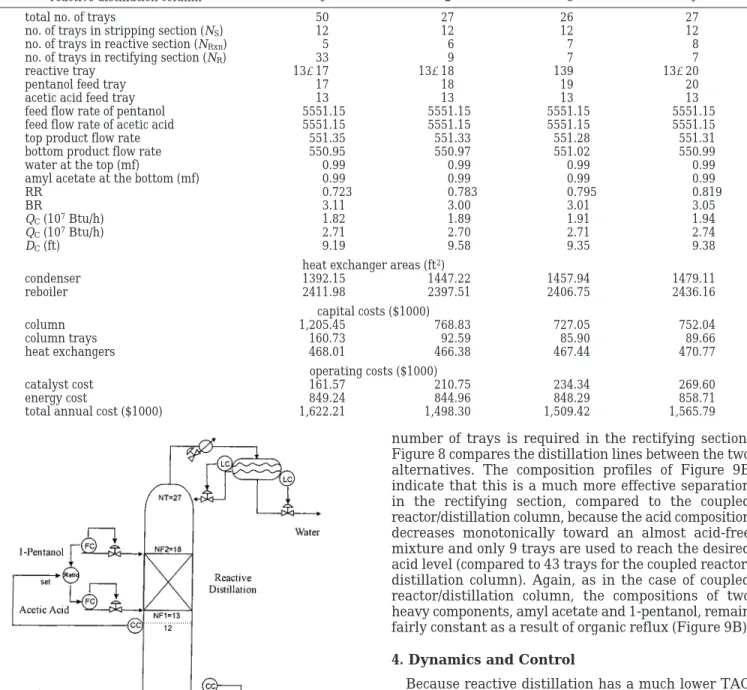
Table 3 gives the steady-state design for different numbers of reactive trays (NRxn). Case 2 (Table 3) is the
optimal design, which corresponds to NRxn) 6, NR) 9,
NS ) 12, BR ) 3.0, and RR ) 0.78. It is important to
note that the TAC is approximately one-fourth (26%) of that for the coupled reactor/column (case 3 of Table 2). In other words, reactive distillation is 4 times more efficient (in terms of TAC) than the coupled reactor/ column for the amyl acetate process.
As in the case of coupled reactor/column, if the number of reactive trays drops below 6, a significant
number of trays is required in the rectifying section. Figure 8 compares the distillation lines between the two alternatives. The composition profiles of Figure 9B indicate that this is a much more effective separation in the rectifying section, compared to the coupled reactor/distillation column, because the acid composition decreases monotonically toward an almost acid-free mixture and only 9 trays are used to reach the desired acid level (compared to 43 trays for the coupled reactor/ distillation column). Again, as in the case of coupled reactor/distillation column, the compositions of two heavy components, amyl acetate and 1-pentanol, remain fairly constant as a result of organic reflux (Figure 9B).
4. Dynamics and Control
Because reactive distillation has a much lower TAC than the coupled reactor/distillation column, the reac-tive distillation process was subjected to further study. Despite the economic potential of reactive distillation, only a few papers studying the dynamics and control of reactive distillation have been published. Al-Arfaj and Luyben1 gives a review on the closed-loop control of
reactive distillation. For reactive distillation with two products, all control structures of Al-Arfaj and Luyben1
share a common feature: To maintain stoichiometric amounts of the two fresh feeds, the composition of one reactant in the reactive section is controlled by adjusting the feed ratio. For the amyl acetate process (Figure 10), the major product is amyl acetate, and water is with-drawn from the decanter with the purity set by the tie lines (Figure 7) with reasonable purity levels. Therefore, only the bottom composition is controlled. Actually, if all of the aqueous condensate is removed (because of its relatively high purity) and all of the organic conden-sate is refluxed, then only one degree of freedom remains (instead of two in a typical column). The control structure becomes: (1) Control the column pressure with cooling water flow rate. (2) Maintain the
aqueous-Table 3. Steady-State Design Parameters and Total Annual Cost (TAC) for Reactive Distillation
reactive distillation column 1 2 3 4
total no. of trays 50 27 26 27
no. of trays in stripping section (NS) 12 12 12 12
no. of trays in reactive section (NRxn) 5 6 7 8
no. of trays in rectifying section (NR) 33 9 7 7
reactive tray 13-17 13-18 139 13-20
pentanol feed tray 17 18 19 20
acetic acid feed tray 13 13 13 13
feed flow rate of pentanol 5551.15 5551.15 5551.15 5551.15
feed flow rate of acetic acid 5551.15 5551.15 5551.15 5551.15
top product flow rate 551.35 551.33 551.28 551.31
bottom product flow rate 550.95 550.97 551.02 550.99
water at the top (mf) 0.99 0.99 0.99 0.99
amyl acetate at the bottom (mf) 0.99 0.99 0.99 0.99
RR 0.723 0.783 0.795 0.819
BR 3.11 3.00 3.01 3.05
QC(107Btu/h) 1.82 1.89 1.91 1.94
QC(107Btu/h) 2.71 2.70 2.71 2.74
DC(ft) 9.19 9.58 9.35 9.38
heat exchanger areas (ft2)
condenser 1392.15 1447.22 1457.94 1479.11 reboiler 2411.98 2397.51 2406.75 2436.16 capital costs ($1000) column 1,205.45 768.83 727.05 752.04 column trays 160.73 92.59 85.90 89.66 heat exchangers 468.01 466.38 467.44 470.77 operating costs ($1000) catalyst cost 161.57 210.75 234.34 269.60 energy cost 849.24 844.96 848.29 858.71
total annual cost ($1000) 1,622.21 1,498.30 1,509.42 1,565.79
Figure 10. Reactive distillation with light feed on the bottom and
phase level in the decanter by changing the top product flow rate. (3) Control the organic-phase level in the decanter by manipulating the reflux flow rate. (4) Control the base level by manipulating the bottoms flow rate. (5) Control the bottoms composition by changing the vapor boilup. (6) Control the two feeds according to the ratio between them.
Figure 10 shows the basic control structure; note that, at the present stage, the feed ratio is fixed at 1 (i.e., the feed ratio is not controlled). This control configura-tion is far simpler than alternatives that can be imag-ined. It is a single-input-single-output system, and a PI controller is used. From a step increase in the heat input, the transfer function between the amyl acetate composition and the heat input becomes
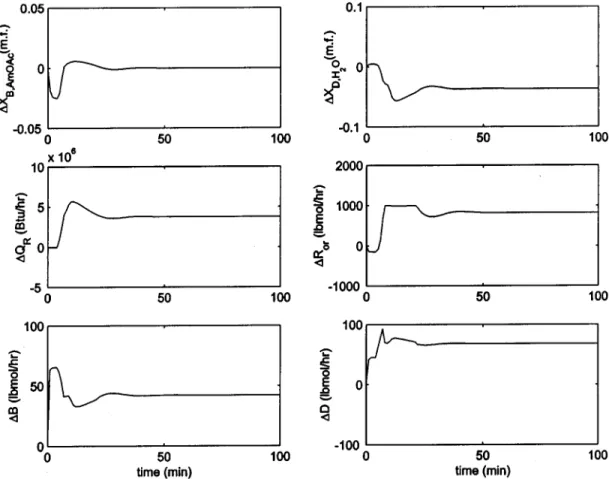
Four minutes of analyzer dead time was assumed for the composition measurements, a transmitter span of 0.1 mole fraction was used, and the valve gain was set to twice the nominal steady-state value. The composi-tion loop was tuned using Tyreus-Luyben settings. This gave values of Kc) 0.3 and τI) 17 min. Figure 11 shows
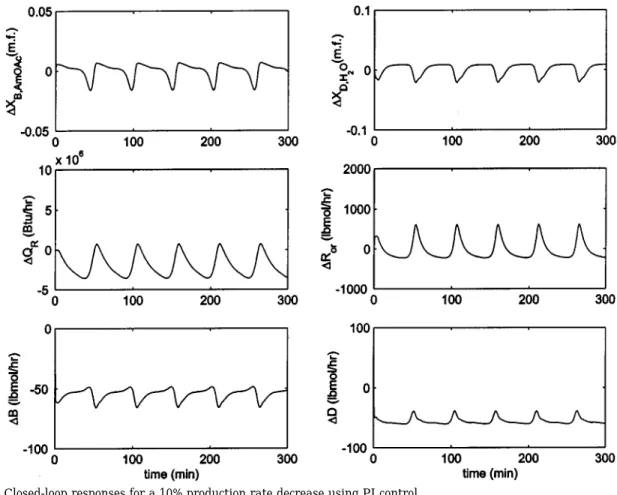
the response for a 10% increase in the feed flow rate. It takes 30 min to reach the new steady state, and reasonable control can be obtained using simple PI control. However, for a 10% step decrease in the feed flows, a limit-cycle-like behavior is observed with a period close to 80 min (Figure 12). The reason for this behavior is that the reactive section produces more acetate than desired, which results in the accumulation of products (acetate and water), which subsequently
slows the net reaction (producing less acetate). However, after most of the products are removed from the system, the reactive section again produces more acetate than desired. The process repeats itself, which leads to a sustained oscillation, as shown in Figure 12. One approach to overcoming the continuous cycling is to increase product purity when the production rate is decreased. Figure 13 shows the response for the same disturbance (a 10% decrease in the feed rate) when the set point of the acetate is changed to 99.4%. The results clearly indicate that oscillation disappears and the speed of response is close to that of a positive production rate change. Another alternative is to use P-only control for the bottoms composition. Very fast control is obtained using the P-only composition control, as shown in Figure 13. This leads to a PI-P controller for the same disturbance (in different directions). In practice, such a configuration can be accomplished by gain scheduling (reset time scheduling to be exact). This should be of little problem, because the production rate of reactive distillation is set by the operators.
The proposed single-loop control seems to be viable for reactive distillation. However, as pointed out by Al-Arfaj and Luyben,1there is always some inaccuracy in
flow measurements, and even the slightest imbalance in the reactants would lead to a gradual buildup of the component. Because there is no way for the excess reactant to leave the system except from the product stream, one of the product specifications cannot be met unless this imbalance is handled via feedback control. Figure 14 shows how the single-loop PI control responds to flow imbalances. For a 1% acid excess, the bottoms composition can be controlled quite well, but for a 1% alcohol excess, again, a limit cycle is observed (dashed Figure 11. Closed-loop responses for a 10% production rate increase using PI control.
(
xB,AmOAc QR)
)1.09e-4s
(0.27)2s2+ 2(0.27)(0.52)s + 1
line in Figure 14). The excess heavy reactant, alcohol, results in a less pure bottoms product composition
initially, and the composition controller counteracts with the increase in the vapor boilup. This restores the Figure 12. Closed-loop responses for a 10% production rate decrease using PI control.
Figure 13. Closed-loop responses for a 10% production rate decrease using PI control with a set-point change of xB,AmOAcset ) 0.994 (solid lines) and P-only control (dashed lines).
bottoms composition temporally and allows the excess alcohol to boil up toward the top. Because the decanter is a natural composition controller, most of the excess alcohol is recycled back to the column via organic reflux. The recycled alcohol cannot increase the net reaction rate further (acid is the limiting reactant), and a gradual buildup of alcohol in the bottom again results in a decrease in the bottoms composition. Again, the whole process repeats itself, and a limit cycle is clearly shown
in Figure 14. Therefore, a second composition loop is, indeed, necessary to prevent the accumulation of excess reactants.1
Because acetate and acid (instead of alcohol) are the major components in the stripping section, either one can be used to control the feed ratio (Figure 10). In this work, we chose acetate to infer the imbalance of the feeds. Figure 15 shows the acetate composition profiles for 1% changes in the feed ratio (FHAc/FAmOH). It is
Figure 14. Closed-loop responses using PI control for errors in feed ratios (FHAc/FAmOH) of 1.01/1 (solid lines) and 0.99/1 (dashed lines).
Figure 15. Composition profile of amyl acetate for a 1% change in feed ratios (FHAc/FAmOH).
Figure 17. Closed-loop responses for errors in feed ratios of FHAc/FAmOH) 1.01/1 (solid lines) and 0.99/1 (dashed lines) using PI control for the bottoms composition and P-only control for the tray 12 amyl acetate composition.
Figure 16. Closed-loop responses for 10% production rate changes using PI control for the bottoms composition and P-only control for
interesting to note that changes in either direction (acid excess or alcohol excess) give less pure product composi-tions. In other words, most of the excess reactant (acid or alcohol) ends up in the bottom as a result of the VLLE characteristics (only very pure water is allowed to leave the column top, as seen in Figure 7). Only three trays (trays 11-13) in the stripping section can be used to infer the feed imbalance, and the amyl acetate composi-tion on tray 12 was chosen to adjust the feed ratio. Note that the selection of the right tray location to infer feed imbalance is very important, as seen in Figure 13. If a wrong location (e.g., tray 8) is chosen, a very difficult nonlinear control problem or an uncontrollable process might result. Following Al-Arfaj and Luyben,1a P-only
controller was employed. From a step test, the transfer function between the tray 12 acetate composition and feed ratio becomes
Again, 4 min of analyzer dead time was assumed, and the transmitter spans for the composition and ratio were 1 and 2, respectively. This gave Kc) 0.008. Figure 16
demonstrates that this control structure works well, and that it requires a slightly longer time, 50 min, to settle 10% production rate changes. The inaccuracy in the flow measurements can also be handled easily as shown in Figure 17.
5. Conclusion
In this work, the acidic cation-exchange resin cata-lyzed amyl acetate process was studied thoroughly. Two design alternatives, coupled reactor/distillation column and reactive distillation column, were evaluated. The phase equilibria of the quaternary system revealed that a significant two-liquid region exists, and more impor-tantly, that the tie lines are all pointed to high-purity water for the aqueous phase. Therefore, a decanter was placed on top of the column, where high-purity water was withdrawn from the aqueous phase, and the organic phase was totally refluxed. Systematic design proce-dures were proposed for these two alternatives. Results, in terms of the total annual cost, indicated that reactive distillation is 4 times more efficient than the coupled reactor/separator for the amyl acetate process. The dynamics of reactive distillation with a decanter is much more complicated than expected. Limit cycles can occur if the control structure is not properly designed. The imbalance in feed must be taken into account in any realistic process operation.
This often leads to at least two composition loops (or two inferential variables). Despite the complex dynam-ics, simulation results show that reasonable control can be achieved with the proposed control system.
Appendix A. Formula for the Computation of the Total Annual Cost
The equipment cost estimation follows the procedure of Douglas,7 and the specific equations of Elliott and
Luyben8 were used. The annual equipment cost was
assumed to be one-third of the capital cost (3-year payback). The energy costs were calculated as
The cooling water cost was computed from
For the catalyst, a 1-year catalyst lifetime was assumed, giving the following expression for the cost
Acknowledgment
This work was supported by the China Petroleum Corporation of Taiwan under Grant NSC88-CPC-E011-017. Insightful discussions with professors M. J. Lee, H. M. Lin, and Y. S. Chou are gratefully acknowledged.
Nomenclature
BR ) boilup ratio (vapor boilup/bottoms flow)
CAmOH ) molar concentration of 1-pentanol (amyl
alcohol, kg mol/m3)
CAmOAc) molar concentration of amyl acetate (kg mol/
m3)
CHOAc ) molar concentration of acetic acid (kg mol/
m3)
CH2O) molar concentration of water (kg mol/m3) DC) column diameter
FAmOH) 1-pentanol feed flow rate (lb mol/h)
FHAc) acetic acid feed flow rate (lb mol/h)
NF) feed tray for coupled reactor/column
NF1) lower feed tray for reactive distillation
NF2) upper feed tray for reactive distillation
NR) number of trays in the rectifying section
NRxn) number of trays in the reactive section
NS) number of trays in the stripping section
NS,min) minimum number of trays in the stripping
section
NT) total number of trays
kb ) backward rate constant for the esterification
reaction
Keq ) equilibrium constant for the esterification
reaction
kf ) forward rate constant for the esterification
reaction
QC) heat removed from the condenser (Btu/h)
QR) heat input to the reboiler (Btu/h)
r ) reaction rate per unit volume [kg mol/(m3s)]
Ri) reaction rate on the ith tray (kg mol/s)
Ror) organic reflux flow rate
Rtotal) total reaction rate in the column (kg mol/s)
RR ) reflux ratio (organic reflux/top product flow) TAC ) total annual cost
VR) reactor volume
xB,AmOAc ) amyl acetate mole fraction at the bottom
xB,AmOAcset ) set point of amyl acetate composition at
the bottom
xD,H2O) water mole fraction at the top Literature Cited
(1) Al-Arfaj, M.; Luyben, W. L. Comparison of Alternative Control Structures for an Ideal Two-Product Reactive Distillation Column. Ind. Eng. Chem. Res. 2000, 39, 3298.
(
x12,AmOAc FHAc/FAmOH)
) 38e-4s (0.12)2s2+ 2(0.12)(0.72)s + 1 steam cost(
$ year)
) $0.0306QRcooling water cost
[
$year
]
) $0.000977QCcatalyst cost ($) ) catalyst weight (lb)×
14.1875
(
$ lb)
(2) Aspen Technology, Inc. Aspen Plus User’s Manual 10.2; Aspen Technology, Inc.: Cambridge, MA, 2000.
(3) Barbosa, D.; Doherty, M. F. The Simple Distillation of Homogeneous Reactive Mixtures. Chem. Eng. Sci. 1988, 43, 541. (4) Chang, Y. C. Feasibility Study on the Preparation of Amyl Acetate via Reactive Distillation. M.S. Thesis, National Tsing Hua University, Hsinchu, Taiwan, 1997 (in Chinese).
(5) Chiang, S. F. Design Alternatives for the Amyl Acetate Process. M.S. Thesis, National Taiwan University of Science and Technology, Taipei, Taiwan, 2000 (in Chinese).
(6) Doherty, M. F.; Buzad, G. Reactive Distillation by Design. Trans. Inst. Chem. Eng. 1992, 70, 448.
(7) Douglas, J. M. Conceptual Process Design; McGraw-Hill: New York, 1988.
(8) Elliott, T. R.; Luyben, W. L. Quantitative Assessment of Controllability during the Design of a Ternary System with Two Recycle Streams. Ind. Eng. Chem. Res. 1996, 35, 3470.
(9) Fien, G. J. A. F.; Liu, Y. A. Heuristic Synthesis and Shortcut Design of Separation Processes Using Residue Curve Maps: A Review. Ind. Eng. Chem. Res. 1994, 33, 2505.
(10) Hayden, J. G.; O’Connell, J. P. A Generalized Method for Predicting Second Virial Coefficients. Ind. Eng. Chem. Process Des. Dev. 1975, 14, 209.
(11) Horsley, L. H. Azeotropic Data III; Advances in Chemistry Series No. 116; American Chemical Society: Washington, DC, 1973.
(12) Lee, J. W.; Hauan, S.; Westerberg, A. W. Graphical Methods for Reaction Distribution in a Reactive Distillation Column. AIChE J. 2000, 46, 1218.
(13) Lee, L. S.; Liang, S. C. Phase and Reaction Equilibria of Acetic Acid-1-Pentanol-Water-n-Amyl Acetate System at 760 mmHg. Fluid Phase Equilib. 1998, 149, 57.
(14) Lee, M. J.; Chen, S. L.; Kang, C. H.; Lin, H. M. Simulta-neous Chemical and Phase Equilibria for Mixture of Acetic Acid, Amyl Alcohol, Amyl Acetate, and Water. Ind. Eng. Chem. Res. 2000, 39, 11.
(15) Lee, M. J.; Wu, H. T.; Kang, C. H.; Lin, H. M. Kinetic Behavior of Amyl Acetate Synthesis Catalyzed by Acidic Cation Exchange Resin. J. Chin. Inst. Chem. Eng. 1999, 30, 2.
(16) Lee, M. J.; Wu, H. T.; Lin, H. M. Kinetics of Catalytic
Esterification of Acetic Acid and Amyl Alcohol over Dowex. Ind. Eng. Chem. Res. 2000, 39, 4094.
(17) Luyben, W. L. Economic and Dynamic Impact of the Use of Excess Reactant in Reactive Distillation Systems. Ind. Eng. Chem. Res. 2000, 39, 2935.
(18) Luyben, W. L.; Tyreus, B. D.; Luyben, M. L. Plantwide Process Control; McGraw-Hill: New York, 1999.
(19) Malone, M. F.; Doherty, M. F. Reactive Distillation. Ind. Eng. Chem. Res. 2000, 39, 3953.
(20) Renon, H.; Prausnitz, J. M. Local Compositions in Ther-modynamics Excess Functions for Liquid Mixtures. AIChE J. 1968, 14, 135.
(21) Sneesby, M. G.; Tade, M. O.; Datta, R.; Smith, T. N. ETBE Synthesis via Reactive Distillation. 1. Steady-State Simulation and Design Aspects. Ind. Eng. Chem. Res. 1997, 36, 1855.
(22) Subawalla, H.; Fair, J. R. Design Guidelines for Solid-Catalyzed Reactive Distillation Systems. Ind. Eng. Chem. Res. 1999, 38, 3696.
(23) Venimadhavan, G.; Malone, M. F.; Doherty, M. F. A Novel Distillation Policy for Batch Reactive Distillation with Application to the Production of Butyl Acetate. Ind. Eng. Chem. Res. 1999, 38, 714.
(24) Widagdo, S.; Seider, W. D. Azeotropic Distillation. AIChE J. 1996, 42, 96.
(25) Wu, K. L.; Yu, C. C. Reactor Separator Processes with Recycle: 1. Candidate Control Structure for Operability. Comput. Chem. Eng. 1996, 20, 1291.
(26) Xu, Z. P.; Chuang, K. T. Kinetics of Acetic Acid Esterifi-cation over Ion Exchange Catalyst. Can. J. Chem. Eng. 1996, 74, 493.
(27) Yi, C. K.; Luyben, W. L. Design and Control of Coupled Reactor/Column System Part 2. More Complex Coupled Reactor/ Column Systems. Comput. Chem. Eng. 1997, 21, 47.
Received for review April 24, 2001 Revised manuscript received March 13, 2002 Accepted April 9, 2002