國立臺灣大學生物資源暨農學院昆蟲學系 博士論文
Department of Entomology
College of Bioresources and Agriculture National Taiwan University
Doctoral Dissertation
臺灣鐵線蟲生物多樣性及寄生對於寄主形態發育的操控 Biodiversity of the Taiwanese horsehair worms and the
host morphological development manipulated by infection
邱名鍾
Ming-Chung Chiu 指導教授:吳文哲 博士
蕭旭峰 博士 Advisors: Wen-Jer Wu, Ph.D.
Shiuh-Feng Shiao, Ph.D.
中華民國 106 年 1 月
January 2017
宣告
根據國際動物命名規約 (International Code of Zoological Nomenclature (ICZN, 2004)) 第 8 條,本論文中對新種鐵線蟲的命名非遵循正式的命名原則。因此本 文中所提及的新種名稱 (Acutogordius formosanus n. sp.) 將在發表之後始正式生 效。
Disclaimer
According to article 8 of International Code of Zoological Nomenclature (ICZN, 2004), the name of horsehair worms (Acutogordius formosanus n. sp.) in this dissertation will only become available by the referring publications.
誌謝
從初步認識鐵線蟲到本文完成的這十年間,研究的重心都環繞在鐵線蟲這個 類群身上,但鐵線蟲其實始終都不是我真正關心的對象。真正令我感到幸運與自 豪的是能與不同領域的科學家一同在研究活動的過程中探索人類理解自然的方 式。當然這個過程並不順利,特別是在當代的主流認知中尋求突破,無可避免去 衝撞主流價值觀,並無止盡的懷疑與批判自己曾經相信的一切事物。這十年間有 賴吳文哲老師的指導與容忍,特別是在我思考上過度理想化時不時引導我在現實 與理想中尋求平衡。吳老師的支持也是這十年間研究得以持續的原因,特別是目 前臺灣的環境幾乎不可能支持這類長期且基礎的研究。而這段期間內昆蟲生態室 的成員,從一開始進入實驗室時指導我進行實驗的周倖瑜學姐,到黃旌集學長,
鄧雅文學姐,與許雅均學姐,王瑞中學長,均讓我在昆蟲生態室的研究工作得以 順利進行。博士班後期的研究則有賴昆蟲系統分類研究室的支援,特別是蕭旭峰 老師的指導與簡維君小姐在電顯上的技術支援,簡小姐至今仍然是我所遇過對掃 描式電子顯微鏡最為熟悉的技術人員。在博士班研究的最後一年藉由千里馬計畫 的支持到神戶大學溪流生態實驗室參與一年的合作計畫,讓我經歷了一個完全不 同於以往的實驗室氣氛,並在佐藤拓哉老師與佐倉綠老師的支持下與木村文,佐 田恭子,相樂理嘉等學生初步建立了鐵線蟲的研究系統,為將來的研究打下基礎,
並在這一年中認識了許多有趣的朋友。
除了這些主要經歷的實驗室之外,臺灣大學的臨床醫學研究所的王弘毅老師,
昆蟲族群研究室的奧山利規老師,生命科學系的施秀惠老師,長庚醫學大學寄生 蟲研究所的陳維鈞老師,與屏東科技大學植物醫學系的鄭光哲老師,均在研究的 過程中給予極大的支援與鼓勵。樣本的採及有賴大野康邦,土岐和多瑠,蔡緯毅,
劉人豪,方華德學長,臺北市立動物園的黃龍椿學長,蕭忠義學長的協助。實驗 的技巧和分析有賴謝佳宏學長,許弘瑋,張倫賢學長的協助。並特別感謝博士班 後期生病期間協助我度過的朋友,楊世綵學姐,何俊頤學長,林彥成學長,王庭 碩,呂易澤,廖朝盛,吳宗學,于品馨,陳柏諺,王沿竣等壘球隊的朋友。
最後感謝在求學階段始終在背後支援我的父母與家人,持續不斷的恩澤往往 讓人理所當然的忽略,然而它卻是工作與學業上最重要的基石。我有幸能夠通過 這段時間的訓練,並有機會延續研究生涯。在失去學校的庇蔭後未來將面臨更大 的挑戰。但和許多的科學家一樣,我們始終對於走在一條崎嶇的道路上而感到幸 運與自豪,幸運的是我們仍然有選擇的權利,自豪的是我們能經由這條路來實踐 各自的理念。希望在下一個十年後回頭看到這段文字,仍然能夠為自己的選擇而 感到驕傲,並不辜負曾經接受過的幫助。
中文摘要
鐵線蟲在生態上的獨特性使其在寄生蟲的研究中佔有一定的地位。儘管牠在 應用上的價值不高,長久以來的傳說以及對鐵線蟲的誤解推動了大部分的相關研 究。鐵線蟲的生活史橫跨水陸兩域,幼蟲在水中孵化並寄生在水生的保幼寄主體 內形成包囊。受感染的保幼寄主被陸生最終寄主攝食後胞囊得以進入最終寄主體 內發育,並在成熟後鑽出回到水中繁殖。在我們的採集中,臺灣目前發現三種鐵 線 蟲 , 分 別 是 臺 灣 索 鐵 線 蟲 (Chordodes formosanus) , 臺 灣 尖 尾 鐵 線 蟲 (Acutogordius formosanus n. sp.),原鐵線蟲 (Gordius sp.),並在水生保幼寄主與 淡水魚體內各別發現一種形態與上述種類均不相符的鐵線蟲。三種已知的鐵線蟲 除了原鐵線蟲的最終寄主尚不明瞭之外,臺灣索鐵線蟲的主要寄主為斧螳 (Hierodula spp.),而少部分個體也曾在花螳螂科 (Hymenopodidae) 及螽斯科 (Tettigoniidae) 的體內 發現; 臺灣尖 尾鐵 線蟲則廣 泛的被 發現 寄 生在直 翅目 (Orthoptera) 的不同科之中。在臺灣索鐵線蟲的發育過程中,牠會影響最終寄主 的形態發育並造成形態上的異速生長與間性。這兩個現象可能有利於寄生蟲提高 對寄主資源掠奪的效率。間性現象也廣泛的被發現存在不同的寄生關係中,但至 今其機制仍然不明瞭。昆蟲的性徵分化一直以來被認為不會受到細胞外的因子所 調控,這包含寄生蟲的影響在內。因此藉由幼年化的方式阻斷寄主的性徵發育,
使其停留在分化之前被認為是造成昆蟲間性的原因。然而在受到臺灣索鐵線蟲感 染的寬腹螳螂 (H. patellifera) 身上,寄生蟲造成的影響卻不支持幼年化的假說,
反而偏向寄主的雌雄二型性性分化過程受到干擾。儘管目前我們尚未收集到足夠 多的樣本來支持這個假說,鐵線蟲的研究卻很有可能在當代的昆蟲性徵發育領域 中帶來新的觀點。
關鍵詞:線形動物們,臺灣索鐵線蟲,臺灣尖尾鐵線蟲,斧螳屬,間性,幼年化,
昆蟲性徵分化
Abstract
The horsehair worm is the parasite which is famous for its unique properties instead of its applicability in human lives. Its life cycle typically goes through aquatic and terrestrial environment, with two parasitic phases in aquatic paratenic host and terrestrial definitive host. There are three known horsehair worm species in Taiwan (Chordodes formosanus, Acutogordius formosanus n. sp., Gordius sp.) and two undetermined species, respectively, detected from aquatic paratenic hosts and a fresh water fish. Except Gordius sp. whose definitive host is still unknown, the host range of C. formosanus, which is specific to the Hierodula mantids (despite it can be found to parasitize Hymenopodidae mantids and katydids), is much narrower than that of the
Acutogordius formosanus n. sp. which is frequently found in different family of the
orthopteran hosts. During the definitive host phase, C. formosana manipulates the morphological development of its mantid host for increasing the efficiency of resource exploitation, and consequently cause the morphological allometry and intersexuality on the infected host. Mechanism of the parasitic intersexuality is unclear. Since the insect sexual differentiation has long been believed to be the cell-autonomous process which lacks regulation outside a cell, it is generally believed that the parasitic intersexuality is caused by the inhibition of ontogeny, which is known as juvenilization. However, our study of the horsehair worm effect on H.patellifera is likely to conflict to the hypothesis of juvenilization and suggests the
alternative hypothesis of the interference in insect sexual differentiation. Despite the sample size is still less to confidently support this conclusion, studies of the horsehair worms might provide the new light in the understanding of the developmental process of the insect sexual differentiation.Key words: Nematomorpha, Chordodes formosanus, Acutogordius formosanus n. sp.
Hierodula, intersexuality, juvenilization, insect sexual differentiation.
CONTENTS
誌謝 ... i
中文摘要 ... ii
Abstract ... iii
Contents ... iv
List of figures ... vii
List of tables ... x
1 General introduction: the parasitic property of horsehair worms ... 1
1.1 Horsehair worms in the myths ... 1
1.2 The definition of a parasite ... 3
1.2.1 Parasite: a special consumer ... 3
1.2.2 Macroparasite and microparastie ... 6
1.3 The parasitic properties of the horsehair worm ... 10
1.3.1 Paratenic host phase ... 10
1.3.2 Definitive host phase ... 13
1.4 The studies in this dissertation ... 19
2 Materials and methods ... 21
2.1 Description of the horsehair worm's morphology (adult) ... 21
2.1.1 Sample preservation ... 21
2.1.2 Morphological examination of adult horsehair worms ... 21
2.2 Description of the horsehair worm's morphology (Egg and larva)... 23
2.3 DNA amplification and phylogenetic analysis ... 24
2.4 Examination of the cysts in the paratenic hosts ... 25
2.5 Artificial infection ... 26
2.5.2 Infection of paratenic hosts ... 26
2.5.3 Infection of definitive hosts ... 28
2.6 Host morphological examination... 29
2.6.1 Measurement of wing, leg and pronotum characteristics ... 29
2.6.2 Measurement of antennal characteristics ... 30
3 Biodiversity of the horsehair worm in Taiwan ... 33
3.1 Phylogenetic relationships of phylum Nematomorpha ... 33
3.2 Five known species of the horsehair worm in Taiwan ... 37
3.3 Key to the three known horsehair worm species in Taiwan ... 65
3.3.1 Adult stage ... 65
3.3.2 Cyst stage in paratenic hosts ... 66
3.4 Seasonality and host specificity of Chordodes formosanus ... 67
4 Horsehair worm-induced host developmental manipulation ... 74
4.1 The extended phenotype: Morphological alternation in the infected host ... 75
4.2 The symptoms resulted from reallocation in host energy budget ... 83
4.3 The mechanism of parasitic intersexuality ... 87
4.3.1 Sexual differentiation in Hierodula patellifera ... 87
4.3.2 Interference in insect sexual differentiation or juvenilization ... 104
5 Conclusion ... 112
6 References ... 114
7 Appendixes ... 132
Appendix 1. (Chiu et al., 2011) A new horsehair worm, Chordodes formosanus sp. n.
(Nematomorpha, Gordiida) from Hierodula mantids of Taiwan and Japan with redescription of a closely related species, Chordodes japonensis
horsehair-worm-infected mantids, Hierodula formosana (Mantodea: Mantidae) Appendix 3. (Chiu et al., 2016) Annual survey of horsehair worm cysts in northern Taiwan,
with notes on a single seasonal infection peak in chironomid larvae (Diptera:
Chironomidae)
Appendix 4. (Chiu et al., manuscript) A new horsehair worm Acutogordius formosanus sp. n.
(Nematomorpha, Gordiida) parasitizing orthopteran hosts, with the redescription of Chordodes formosanus and novel host recordings from Taiwan
Appendix 5. (Chiu et al., project report) Mechanism in host morphological manipulation:
Mimetic Wnt protein alters host sexual differentiation
LIST OF FIGURES
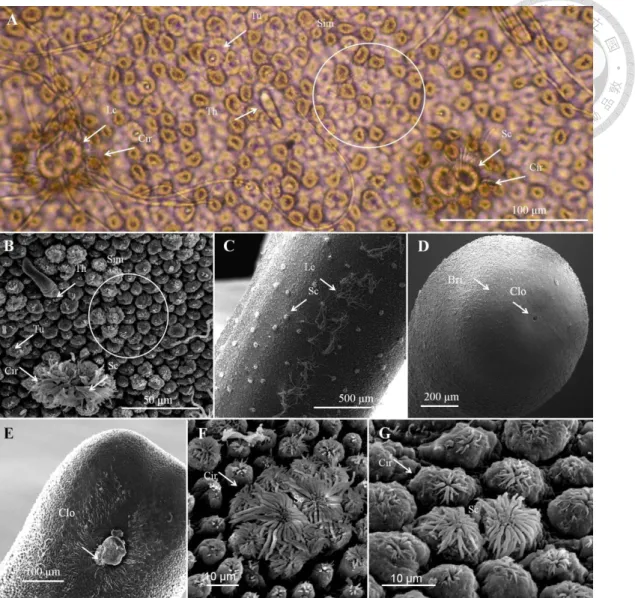
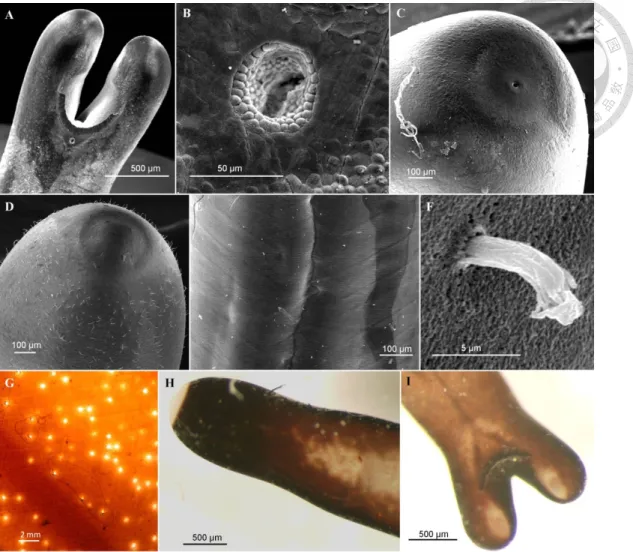
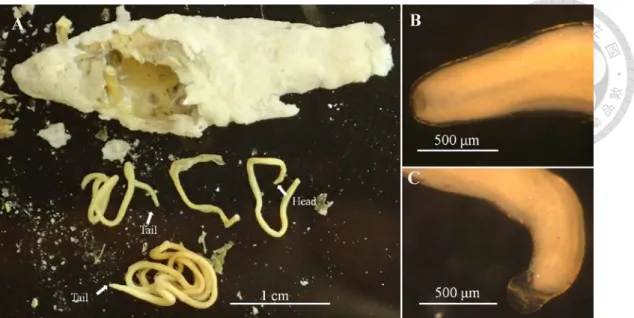
Fig. 1. Adult cuticle of Chordodes formosanus ... 44
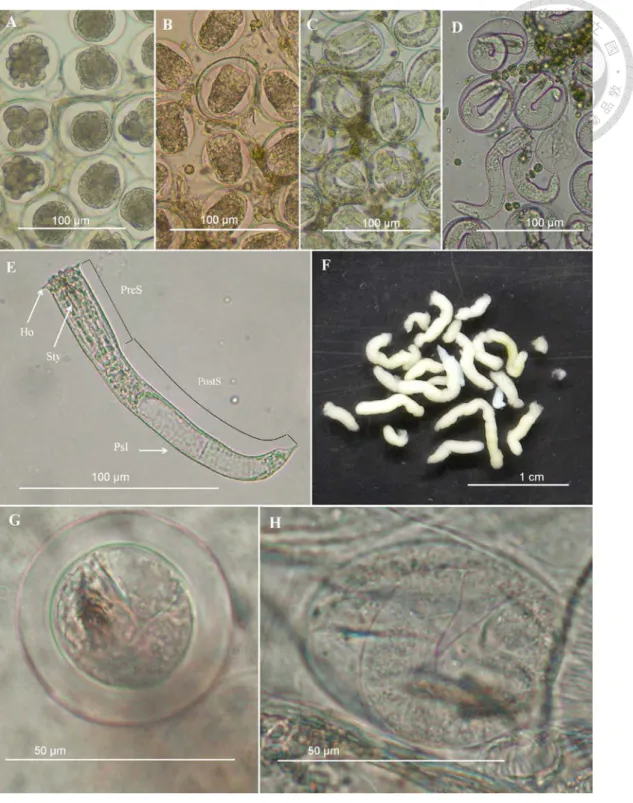
Fig. 2. Larvae and cysts of Chordodes formosanus ... 45
Fig. 3. Chordodes formosanus in the field ... 46
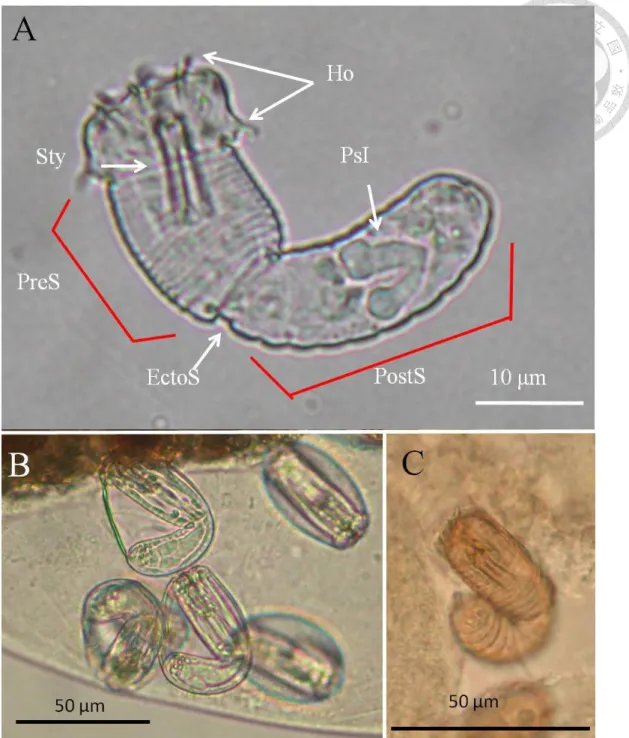
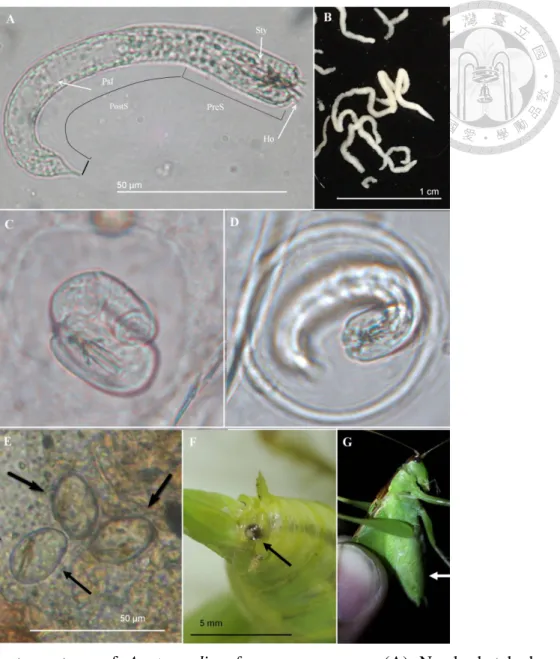
Fig. 4. Adult of Acutogordius formosanus n. sp. ... 52
Fig. 5. Immature stage of Acutogordius formosanus n. sp. ... 53
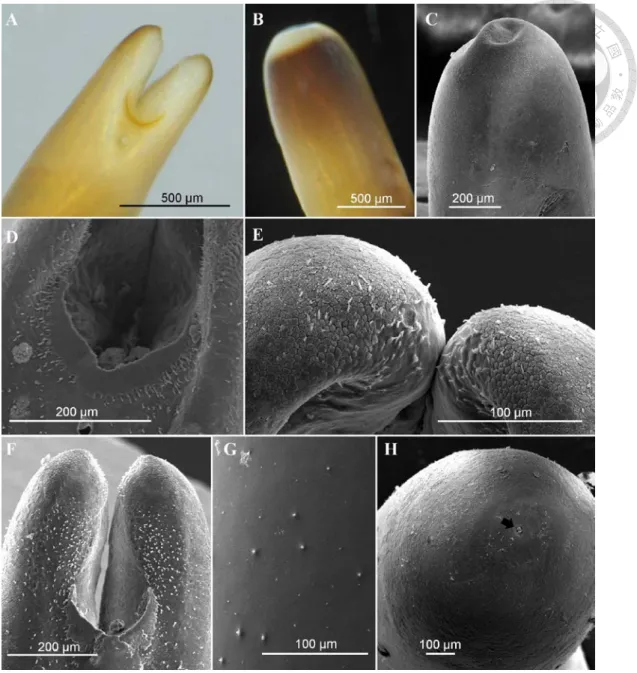
Fig. 6. Adult of Gordius sp. ... 58
Fig. 7. Immature stage of Gordius sp. ... 59
Fig. 8. Gordius sp. in the field ... 60
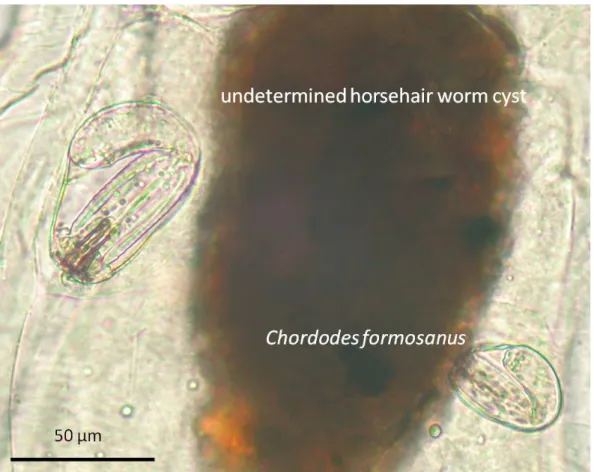
Fig. 9. Cysts of Chordodes formosanus and an unknown nematomorph with larger size in the paratenic host (larval chironomid). ... 62
Fig. 10. Two horsehair worms in a fried sailfin molly (Poecilia latipinna).. ... 64
Fig. 11. Mortality rate and infection rate of the 20 newly hatched mosquitoes, Aedes
albopictus, infected by the egg of Chordodes formosanus developing for
different days ... 71Fig. 12. Sexual dimorphism and parasitic effects of the horsehair worm (Chordodes
formosanus) on the legs and wing lengths of infected mantids (Hierodula formosana) ... 76
Fig. 13. Parasitic effects of the horsehair worm (Chordodes formosanus) on the forewing shapes of infected mantids (Hierodula formosana) ... 78
Fig. 14. Parasitic effect of the horsehair worm (Chordodes formosanus) on the mean (± standard deviation) number of grooved basiconic sensilla per 25 μm2 on 10 selected segments of antennal flagella of infected and uninfected mantid adults (Hierodula formosana) ... 80 Fig. 15. Parasitic effect of the horsehair worm (Chordodes formosanus) on the
antennal characteristics of the first flagellum segment bearing the grooved basiconic sensilla and the total numbers of flagellum segments of infected and uninfected mantids, Hierodula formosana ... 81 Fig. 16. Parasitic effect of the horsehair worm (Chordodes formosanus) on the size
and number of internal sex organs of infected and uninfected mantid adults (Hierodula formosana) ... 85 Fig. 17. Ventral view of sterna on the 1st to 3rd instar nymphs of Hierodula patellifera
by SEM ... 89 Fig. 18. Ventral view of sterna on the 4th, 5th, and 9th (last) instar nymphs of Hierodula
patellifera ... 90
Fig. 19. Detailed comparison of the 6th-8th Sternum in the female Hierodulapatellifera of the last instar nymph and the adult ... 91
Fig. 20. Red pigments on 5th-7th tergum of the mantid, Hierodula patellifera ... 94 Fig. 21. Nymphal male mantids, Hierodula patellifera, infected by Chordodesformosanus and Chordodes sp. ... 95
Fig. 22. Comparison of sensillum amounts between the male and the femaleHierodula patellifera ... 101
Fig. 23. Comparison of antennal size (surface area) between the male and the femaleHierodula patellifera ... 102
Fig. 24. Developmental trajectories of the sensillum distribution on mantid antennae,Hierodula petallifera ... 103
Fig. 25. Sensillum distributions in the adult male mantid, Hierodula patellifera, of thecontrol mantids and that infected by the horsehair worm, Chordodes sp. .... 107 Fig. 26. The begin time when the horsehair worm, Chordodes sp., start to manipulate
the development of the mantid, Hierodula patellifera ... 108
Fig. 27. Sensillum distributions in the last instar male mantid, Hierodula patellifera, of the control mantids and that infected by the horsehair worm, Chordodes sp.
... 110 Fig. 28. Developmental trajectorie of the sensillum distribution (the antennal segment
first bearing the grooved basiconic sensilla) on female mantid antennae,
Hierodula petallifera and the female individuals infected by the horsehair
worm, Chordodes sp. or C. formosanus ... 111LIST OF TABLES
Table 1. Comparisons of sensillum amounts and antennal characteristics between male and female mantids, Hierodula patellifera ... 99 Table 2. Difference of the the grooved basiconic sensilla in the three sexually
dimorphic zones of adult Hierodula patellifera ... 100
1 General introduction : the parasitic property of horsehair worms
1.1 Horsehair worms in the myths
The horsehair worm is the parasite which is famous for its unique properties instead of the relationship with human activities. Instead of the applicability, people's misunderstanding or scares promote scientific studies of the horsehair worm. One early study conducted by Leidy (1850) is to clarify the "origin" of the horsehair worm.
The "horsehair worm", as the most frequently used common name, is not only named after its long, dark, and slender body shape, but also implied how it is created: the fallen horse hair in the water. Since the adult worm was often found in water filling a wagon rut or the drinking trough of horses, it is believed to be the horse hair which has become "vivified" in the water (Leidy, 1850). Leidy (1850) argued this
"abiogenetical myth" by putting horse hair in the water and introduced the reproductive behavior of horsehair worms by describing its egg developmental process. Before the end of the 19th century, the horsehair worm's life history with both a parasitic and a free-living phase has basically been established (Schmidt-Rhaesa,
2012). And in the early 20
th century, the adoption of pseudoparasitism to describe the existence of horsehair worms in human also reflects the well-understanding of the parasitic property of horsehair worms (Hall, 1912; Schmidt-Rhaesa, 2012). However, people's negative impression of horsehair worms did not stop after their life history was revealed. Horsehair worms has long been thought to be poisonous and lethal to the livestock (Baylis, 1944 reviewed in Schmidt-Rhaesa, 2012) but they are likely to cause only mechanical damages to animal's intestine(Hanelt et al., 2005). The fear
and importance of the horsehair worms in veterinary is now approximately erased since the real livestock parasites were found, but the adult horsehair worms in thetrout recently inspire the bright new hypothesis of the parasite-mediated allochthonous input from the terrestrial environment to the water (Sato et al., 2008,
2011).
To date, most studies surrounding horsehair worms still comes from the old myth:
host suicide induced by its parasites
(Heinze, 1941 reviewed in Schmidt-Rhaesa and Ehrmann, 2001). And other than the host behavior manipulation, our recent studies in
the horsehair worm's effect on host developmental manipulation might give a new light in the "myth" of the cell-autonomous insect sexual differentiation (Chiu et al.,2015). In contrast to most of the parasitological studies, studies surrounding the
horsehair worm are generally not that develops for practical applications, but improves and challenges the current understanding of natural or other phenomena.Horsehair worms are grouped as the micro-parasite. This category involves how as scientists consider the parasite, the micro-parasite, and the parasitic properties of the horsehair worm. As the part less mentioned in the study of parasite, it might be worth to begin from the definition of a parasite.
1.2 The definition of a parasite
1.2.1 Parasite: a special consumer
All species of horsehair worms are generally acknowledged to be parasites
(Hanelt et al., 2005), included the species found emerging from their hosts and nearly
half of the species which are "believed to be" parasites since their hosts have not yet been known (Poinar and Chandler, 2004; Schmidt-Rhaesa, 2012). The term "parasite"is widely used and it is commonly accepted that a parasite adopts one of the most unique strategy to be a consumer. Nevertheless, parasitism, despite it has been suggested for more than one century from 1879
(Zelmer, 1998), is not a constant
concept in each assay. As the opposite concept of the free-living organism, parasites are generally considered to be the organisms live in their hosts. To date, using the host as both source of nourishment and habitat is the most common definition of parasitism(Zelmer, 1998; Poulin, 2007). However, this rule groups various parasitic
organisms which is much more than people image. As almost all organisms have other organisms living in it (including parasites infected by other parasites, termed hyperparasitism), the free-living strategy may be more unique than parasitism(Matthews, 1998). Thus, parasite discussed is always further defined by the limited
taxa or cases (Matthews, 1998), despite parasitism is more likely to be an ecological concept instead a systematic group (Eggleton and Belshaw, 1992; Zelmer, 1998).The predator is always considered to be most closely related to the parasite. The most significant difference between parasite and predators is that if the prey are alive during being consuming. This property makes parasitoids, which consume the live hosts but finally kill them, become the special parasitic organisms. There are two properties mainly applied in characterizing a parasitoid: 1) the free-living stage in the life cycle (Eggleton and Gaston, 1990; Zelmer, 1998) and killing the host in the end of
the parasitic stage (Eggleton and Gaston, 1990; Zelmer, 1998; Kuris and Lafferty,
2000). The former property emphasizes the habitat role of the host to a parasite and
makes the parasitoid more likely to be a predator which, comparing with the normal predators, spend longer time on exploiting the food resource from its prey (Zelmer,1998). This concept also rules out the "insidious and consumptive predators" usually
has been considered ecto-parasites like mosquitos, which do not really live on their hosts. Since the emphasis on the habitat role of the host, whether the host is killed is not the essential property to characterize a parasitoid. This point of view is not much of the problem in the parasitoid wasps, but is debatable in the parasitoid flies. In the early concept, the parasitoid is suggested to specially describe the insect parasite especially the parasitoid wasps (Eggleton and Gaston, 1990). In many literatures to date, the parasitoid is still usually equal to the "parasitic" Hymenoptera (Eggleton andBelshaw, 1992). With the highly host specific, the parasitoid wasps generally
parasitized with insect hosts and kill it in the end of the parasitic stage (Eggleton andBelshaw, 1992). However, the flies, as also common "parasitic" insects, highlights the
non-essential property of killing host by its diverse host taxa. As the different way in evolving the parasitic life style, host range of the "parasitic flies" is much wider than the parasitoid wasp which can be invertebrates or vertebrates (Eggleton and Belshaw,1992). In the fly species parasitizing insect hosts, the "parasitic flies" usually kill their
hosts before pupation whose behavior is similar to the parasitoid wasps. Whereas the obligatory myiasis (e.g., screw worms, bot flies and warbles) which lay eggs on mammal hosts is usually less harmful to cause the lethal damage(Eggleton and
Belshaw, 1992). Whether the host die or not among the parasitism of flies and wasps
might be mainly due to the host relative size (Zelmer, 1998). Thus, death of the hosts is more likely to be an extended property but not a defining character. Sinceconsidering the host as a prey instead of habitat, the parasitoids frequently kill their hosts by the extreme virulence or damage, which tends to be reduced in the parasites
(Alizon et al., 2009).
The high virulence in the parasitoids, than the parasites, indicates their diverged way in exploiting their hosts. In contrast to the definition emphasizing on the habitat role of hosts, the host death as the character makes the parasitism more close to be a foraging strategy. The foraging model causes one special dichotomy for dividing the parasite from other consumers: the number of host/prey attacked during the particular life-history phase (Kuris and Lafferty, 2000). The functional response, which is the important component of the most widely known Lotka-Volterra predator prey model, describe how predators eat "many" prey (Kuris and Lafferty, 2000). This model is obviously not applicable in describing the parasite-host system as a parasite generally attacks one host in a particular life-history phase. Under this rule, typical parasites, the parasite contains typical parasites, parasitoids, and micro-predators (e.g., ecto-parasites, insect herbivores) whose size are smaller than their victims. However, comparing with the disease model mainly based on epidemiology, parasitoid models are derived more from predator-prey theory (Kuris and Lafferty, 2000). One of the example is the Nicholson-Bailey model. This model describes how the competition of female parasitoids search and lay eggs in the hosts, which is also known as the competition model
(Royama, 1971). This model emphasizes the predator property of
parasitoids in the free-living stage and implies the parasitic larval stage as the mean of handling the prey. Thus, the key difference between parasitoids and parasites, killing the host, could be considered as the predator property of the mother parasitoids. The separation of the free-living adult phase and the parasitic larval phase makes it more clear to characterize the foraging strategies in different developmental stages.Although it is less mentioned in the parasitoid model, during the parasitic larval phase, parasitoids still show the high similarity with typical parasite.
During evolutionary processes, the parasitic strategy independently evolved in wide phylogenetic taxa. It can be fixed in the population under different environmental conditions. Thus, it might be difficult and impractical to group all the parasitism evolved with different adaptations by a signal or few properties. Zelmer
(1998) summarized different definitions of parasitism into a property that all parasitic
organisms have the ability to evade the immune response of the host. However, this definition is rarely applied and actually does not highlight the significance of parasitism from other foraging strategies or ecological relationship. In the contrast, many characters of the parasitic organisms including the smaller size, feeding alive organisms, evading the host immune response, are all related to the habitat role of hosts. Thus, it is not surprisingly that "the habitat and nourishment role" become two critical parasite's properties most commonly applied, despite there are still a gray area between the parasitic organism and others.1.2.2 Macroparasite and microparastie
The epidemiological model for parasitism not only suggests the fundamental difference from predators, but also leads to the classical dichotomy of microparasite/macroparasite
(Kuris and Lafferty, 2000). One role to separate micro-
and macro-parasite is if the parasite multiply in the host (Begon, 2009). For most of the macroparasites, they grow in the host without increasing their number. Whereas the microparasites often increase rapidly after establishing in the hosts (Begon, 2009;Rosà et al., 2006). Multiplying of the microparasites makes the infected hosts go into
dichotomized conditions: "infectious hosts" which contain enough parasites to infect other hosts, and "non-infectious hosts" with few or no parasites, which are suppressedby immune response. In this situation, prevalence, the probability of infected host (infectious hosts) in the host population, become the most and only considerable parameter in modeling the transmission of microparasites. This concept is applied in the original epidemiological model known as SIR model which characterize the host individuals into susceptible (S), infectious (I), and recovered (R). To date, the SIR or its extended models are still applied in estimating the outbreak of some human disease
(e.g. Jansen and Stollenwerk, 2005). Considering the host as transmission unit implies
the reproductive potential of microparasites dependents on the ratio of the infectious hosts. However, for the macroparaites, especially that reproduce outside of the hosts, the reproductive potential is highly related to the total parasite number. Moreover, the unequal distribution of parasite individual in each host (Kuris and Lafferty, 2000;Rosà et al., 2006) makes the total parasite number unable to be estimated by the
infected hosts. Anderson and May (1978) suggested the model which adds intensity (number of parasite in an infected host) as a parameter known as intensity-dependent model(Kuris and Lafferty, 2000; Rosà et al., 2006). In contrast to the SIR model
which takes only host as variables, variables in the intensity-dependent model contains "the density of hosts" as well as "the parasite burden" (distribution of adult macroparasites in the hosts) and " the density of free-living larvae of parasite"(Rosà et al., 2006). Both of these models (SIR model/microparasite model and
intensity-dependent model/macroparasite model) are now widely applied in the description of parasite-host dynamics and makes the three indicators (prevalence, mean intensity, mean abundance (prevalence times mean intensity)) become the most important parameters in description the parasite's aggregation in their hosts (Poulin,
2007).
The dichotomy of micro- and macro-parasite also implies the parasite size (or
relative size to the host) as one important property in their infection. As it is showed literally, "small parasites" (protozoa and smaller microbes) generally fit the microparasite model whereas the "large parasites" (helminths and arthropods) generally fit the macroparasite model (Lafferty and Kuris, 2002). However, some exceptions (e.g. ciliates or coccidians is better to be modeled as macroparasites; and larval digeneans in molluscs and rhizocephalan barnacles are better to be modeled as microparasites) makes the parasite size is originally argued to be associated with the trophic strategy
(Lafferty and Kuris, 2002). It is understandable that some
macroparasite (e.g. cercaria of Pleurogonius malaclemys in the snail hosts) asexually reproduce in their hosts (Hunter, 1967) while the host infectious level is not always constant in the parasitic bacteria, Pasteuria ramosa, due to the various spore number in a host (Vale and Little, 2012). However, Lafferty and Kuris (2002) reviewed the current known host-parasite systems and suggest the inconsiderable relationship in parasite size and the trophic strategy. Especially in the parasitoids and castrators, their extreme large body size (mass), which approach the same mass with the hosts, is hard to be excluded as a possible factor in promoting the evolution of their specific trophic strategies (Lafferty and Kuris, 2002).As the argument in separating "the parasite" from other consumer, it is hard to define a parasite as the micro- or macro-parasite by one or few properties. In fact, a parasitic life history is the combination of several characters multiply evolved in adapting to diverse environment. Each model or role is applied to describe a combination of properties instead of a real parasite. Horsehair worms, as parasites with large size and long-term development inside the host, show the specific living strategy in exploiting hosts. They leave their hosts at the end of the infection and finally kill the host. From any aspect, they match the definition of the parasitoid, but it
is undoubtedly that the living style of horsehair worms are much different from other insect parasitoids. It might be hard and not necessary to characterize them as any category created, especially in some definition, the horsehair worm might be excluded from a parasite. Thus, instead of defining the horsehair worm as a parasite type, it might be better to focus on the adaptation of its trophic strategy and host effect, and compare that with the organisms with similar properties.
1.3 The parasitic properties of the horsehair worm
Horsehair worms adopt parasitic life strategy, but its parasitic properties change drastically in each developmental stages. The parasitic life history of a horsehair worm is typically composed of two parasitic phases: paratenic host phase and definitive host phase. Eggs of the horsehair worm hatch in the aquatic environment.
Larvae stay on the water bottom and encyst in the aquatic animals after being swallowed. The worm cysts in these aquatic animals, which are known as paratenic hosts, are carried to the terrestrial environment and enter terrestrial definitive hosts after the infected paratenic host is consumed as prey. The worm larvae inside the definitive host excyst and enter host hemocoel to develop into wormlike juveniles.
The juvenile worms grow and become adult inside the haemocoel of the definitive host. Nearly the end of parasitic phase, the adult worms might manipulate the host behavior to enter aquatic environments and finally emerge to reproduce in the water
(Hanelt et al., 2005; Bolek et al., 2015).
1.3.1 Paratenic host phase
The paratenic host, which is not required for the parasite's proper development
(Baer, 1951 reviewed in Hanelt and Janovy, 2004a), carries the worm cysts to the
terrestrial environment. Serving as vehicles in horsehair worm's life history, they are believed to interact less with the horsehair worm cysts. Few studies showed the negative effects of the cyst to hosts including inducing internal defense reaction(Inoue, 1962; Hanelt and Janovy, 2003, 2004a), reducing the wing pad size and
inhibiting pupation in some aquatic insects (White, 1969). In our experiments, we also found the mortality of the snails and larval chironomids tended to increase with exploring high density of the worm larvae (data not showed).The host range of the horsehair worm's paratenic host is extreme wide. The
worm cysts have been found in phylogenetically far taxa of hosts from invertebrates (aquatic insects, crustaceans, water snails, trematodea) to vertebrates (fish) (Cort,
1915; Schmidt-Rhaesa and Ehrmann, 2001; Hanelt and Janovy, 2003). Hanelt and Janovy (2003)
suggested the foraging behavior might be the most important factor affecting the infection of paratenic hosts since larval horsehair worms are able to infect almost all animals swallowed them. This suggestion is also supported by the unequal infection in different functional groups of larval chiromids (Chiu et al.,2016a). This extreme wide host range is rare among parasites which might be caused
by the costs incurred from the ability to exploit multiple hosts or decreased abilities to exploit hosts rarely encountered (Arbiv et al., 2012). In this view, the true generalists indirectly support the larval horsehair worms do not evolve tight interaction with their hosts. It makes the relationship in horsehair worms and paratenic hosts is more likely to be passengers to carriers instead of parasites to hosts. Such passengers-carriers relationship is commonly seen in the phoretic mite attaching on the body surface of larger, flying insects for dispersal (Schwarz and Müller, 1992; Palevsky et al., 2001).Nevertheless, it is worth to note that the paratenic hosts not only bring the larval worms to the definitive hosts, but also increase their infectiousness (Inoue, 1962;
Schmidt-Rhaesa and Ehrmann, 2001; Hanelt et al., 2005), longevity (Hanelt and
Janovy, 2004b), and ability to survival the freezing (Bolek et al., 2013b). These
phenomena were found in the rearing experiment since Inoue (1962) found the mantids fed with larval worms had lower infection rate than that fed with infected insects contains worm cysts and Hanelt and Janovy (2004b) found the larval worms in the water are infectious for two days after being hatched but the cysts in the paratenic hosts can be infectious for more than six months. The mechanism to change the physiological properties from larvae to cysts are still unknown, but it might makes theparatenic hosts are not only ecologically but also physiologically essential in completing a horsehair worm's life history. In some literatures, the use of parasitic host indicates a host which facilitates the transmission but does not always appear in a parasite's life history (e.g. Jezewski et al., 2013). This concept might be different from that of the paratenic host in horsehair worm's life history. In some cases, the term
"intermediate host" is also seen in describing these "aquatic carriers" of horsehair worms (e.g. Schmidt-Rhaesa and Ehrmann, 2001).
The wide host range insures the larval horsehair worms to be encysted in the aquatic animals ingesting them, but not all species of the paratenic hosts take the worm cysts to their definitive hosts. Water snails are common paratenic hosts in nature which were frequently found to harbor cysts of horsehair worms (Hanelt et al.,
2001; Bolek et al., 2013a; Harkins et al., 2016), but they are rarely preyed by the
crickets, which is the definitive hosts of horsehair worms in Northern America(Hanelt and Janovy, 2004b). These paratenic hosts servering as carriers instead of
transmitters are called spurious paratenic hosts(Hanelt and Janovy, 2004a). Cysts in
the spurious paratenic hosts is unable to directly finish their life histories, but can be transmitted to the definitive hosts via the horizontal transmission to the (true) paratenic hosts. Such horizontal transmission is called paratenesis. A horsehair worm cyst proceeds paratenesis to be encysted again in the new paratenic host which ingests the previous paratenic host (Hanelt and Janovy, 2004a). Paratenesis enable the horsehair worm cysts to be transmitted in through the food web, and theoretically make the cyst density to be concentrated in the predator hosts (Moravec andS kor´ıkova´, 1998; Brown et al., 2001)
. However, the predator concentration was not found in the infection of different functional groups of larval chironomids (Chiu et al.,2016a).
Now the wide host range makes the cyst stage of the horsehair worms has recently been attended by the potential in the investigations of horsehair worm's geographic distribution, species composition, and seasonality
(Hanelt et al., 2001;
Bolek et al., 2013a; Chiu et al., 2016a; Harkins et al., 2016). The hosts used in these
studies (snails or larval chironomids) might be not the main host bring the cysts to the definitive hosts, but they might well reflect the present and relative amount of the horsehair worm's larvae in the environment. In this dissertation, we also develop the technique to increase the efficiency in examine the infection of cysts in aquatic insects and hope the accumulation of field data will promote the understanding of the epidemiological properties of cyst in horsehair worm's life history.1.3.2 Definitive host phase
The main post-embryonic development of a horsehair worm is launched from the cyst is ingested by the definitive host and finishes when the adult worm leave the host.
During the time, a horsehair worm proceeds metamorphosis from a cyst to become the wormlike juveniles
(Hanelt and Janovy, 2005), rapid increase of the body size (Schmidt-Rhaesa, 2005), and finally become a mature worm (Schmidt-Rhaesa, 2005).
These three stages are respectively associated with three interactions with the host:
determination of the host specificity, parasite's trophic strategy with altering host development, and host behavior manipulation.
The metamorphosis of the cyst is the first step to determine a host to be the definitive host or another paratenic host. Unfortunately, it has never been mentioned about the factors triggering the excysted larvae to proceed metamorphosis. In fact, definitive host data are still lacked in many horsehair worm species since they were frequently described only by the free-living adult collected in the environment (Poinar
and Chandler, 2004) or were artificially infected to the laboratory-reared insects by
field-collected cysts (Bolek et al., 2013a). Unlikely the wide host range in the paratenic host, the definitive hosts of horsehair worms are limited to arthropods mainly including millipedes, orthopterans (crickets, grasshoppers, etc.), beetles, cockroaches, and mantids in freshwater environments (Schmidt-Rhaesa, 2012; Bolek
et al., 2015) and crustaceans in the oceans (McDermott et al., 2010; Schmidt-Rhaesa,
2012). In each species, despite only a few surveys mentioned about the host
specificity (Schmidt-Rhaesa, 2012; Chiu et al., 2011), a horsehair worm seems to only able to develop in one or few species of definitive hosts.After the metamorphosis to become wormlike juvenile, a horsehair worm go through the rapid growth in host's body cavity. This is the only known trophic stage of a horsehair worm to exploit resource from it host. It is believed that a horsehair worm consumes host non-essential fat and reproductive organs to support its growth
(Lafferty and Kuris, 2012) since these two tissues in the infected hosts are commonly
atrophied (Lafferty and Kuris, 2012; Chiu et al., 2015; but in our observation, the fatbody might be not always reduced in the infected mantids). Nevertheless, there is not
yet an evidence confirmed how a horsehair worm gains nourishment from these tissues. By comparing the morphology of the horsehair worm developing for different time inside the definitive hosts, Schmidt-Rhaesa (2005) suggested the intestine is present and occupies half of the body cavity in the early development of the juvenile.The intestine maintains its size in the first third of its developmental period and then become smaller (Schmidt-Rhaesa, 2005). In the adult worms, the intestine is almost degenerated
(Schmidt-Rhaesa, 2005). The degeneration of the intestine not only
support the hypothesis that the horsehair worms emerges from the hosts as non-trophic adult (Schmidt-Rhaesa, 2005), but also suggests the juvenile might digest the nutrition through intestine. However,Schmidt-Rhaesa (2005) in the same report
also suggested the alternative possibility that the horsehair worm directly absorb host nutrition from the "larval cuticle", which covers the juvenile and is much thinner than the fibrous adult cuticle. If the juvenile holding up the resource in the host body flow, the reduction of fat and reproductive organ of host might be not caused by the parasite consumes it but due to the loss of energy or altered development.
No matter how a horsehair worm intake the resource from the host, several reports suggest the infected host suffers the destruction of gonad tissues (Wülker,
1964; Lafferty and Kuris, 2009; Chiu et al., 2015). This effect on the host also makes
the horsehair worm to be categorized as the parasitoids with "prior castration"(Lafferty and Kuris, 2009). This trophic strategy castrating the host is frequently seen
in the parasitic helminths and insects (Wülker, 1964;Baudoin, 1975; Hurd, 2001;
Lafferty and Kuris, 2009) whose body size is larger than the usual parasites (Lafferty and Kuris, 2009). It is believed that the rapid growth of these parasites until almost
occupied all the host body cavities is supported by the intensive exploitation of host resource, and thus promotes them consumes the energy invested in host reproductive system to insure their survival, both hosts and parasites (Obrebski, 1975; Lafferty andKuris, 2009). The effort to maintain the host survival is the common adaptation of
every parasite, but castrating a semelparous host will deprive it of the heredity contribution to its own population, which is equal to evolutionarily kill it. It is the reason why many of the parasitic castrators finally switch to be a parasitoid and kill the "snatched body". In the parasitism of the horsehair worm, the parasite adopts a unique way to kill and leave the host: forcing the suicide behavior.Adult horsehair worms reproduce in the water but they are unable to move themselves on the ground. The only way is emerging from the terrestrial host fallen in the water. Parasite-manipulated host behavior is the hot spot of the horsehair worm's
study. It has been noted for more than one century
(Heinze, 1941 reviewed in Schmidt-Rhaesa and Ehrmann, 2001) but has been systematically researched in the
recent 10 years. The "suicide behavior" in the horsehair worm-infected host has been frequently reported as field observation until the first control experiment was conducted by Thomas et al. (2002). It has long been believed that the horsehair worms cause the hosts thirsty and promote them to approach water (Schmidt-Rhaesaand Ehrmann, 2001), but the behavior of "searching the water" was not supported in
the Y-maze (Thomas et al., 2002).Thomas et al. (2002)
found both infected and non-infected crickets were randomly choosing one direction of the Y-maze whatever if the arm contained the trough filled with water or not. The similar behavior was also found in the infected crickets which were brought from their forest habitat to the open area near a pool, while only half of individuals moved toward the pool (Thomas et al.,2002). These two experiments suggested the infected host just moving around instead
of searching and heading the water, but this behavior is still abnormal for a cricket since it always quickly hides itself back to the dark area or forest habitat (Thomas etal., 2002). This "erratic behavior" is the reason why the infected crickets are always
found in the non-habitat area, like parking area or ground in the house (Thomas et al.,
2002), and further suggested as the first step of the host behavior manipulation caused
by the horsehair worm (Sánchez et al., 2008b). The second step is to make the infected host to "suicide" when it encounters the water. Thomas et al. (2002) in the same Y maze experiment found that all the infected crickets, although they showed no tendency to the direction with water, jumped into the water if they randomly go into the arm with water. Whereas most of the non-infected crickets (11/12) just stopped near the edge of the trough. This phenomenon confirms the "suicide behavior" long spread as a legend. After ten years, Ponton et al. (2011) found the tendency ofapproaching reflection of light in the infected crickets, which suggests the "suicide behavior" is likely to be the attraction of light reflected by the water.
The two-step behavior manipulation composed of "erratic behavior", which causes the infected host leaves its habitat, and "suicide behavior", which promotes the host jump into the water, has been generally established. Now scientists are still striving for realizing the physiological mechanism behind these behavior changes.
Several physiological changes have been noted. Thomas et al. (2003) found the infected crickets displayed abnormal concentration of several amino acids and increasing mitosis in mushroom bodies. Biron et al. (2005a) and
Biron et al. (2006)
noted the infected katydids and crickets showed the similar proteomic changes when performing the erratic behavior. These proteins are related to insect's neurogenesis, circadian rhythm and neurotransmitter activities. To date, expression of at least six protein families whose function are related to the physiological processes of insect's central nervous system have been known to change during the behavior manipulation(Libersat et al., 2009). Two of the proteins belonged to Wnt family is likely to be the
special case of molecular mimicry since the protein sequences in the horsehair worm is similar to that in insects instead of the phylogenetically close nematodes (Biron etal., 2005a, 2006; Biron and Loxdale, 2013). It is once believed we are nearly to the
answer of the molecular cross-talk between horsehair worms and their hosts, but the linkage of these physiological changes to the host behavior is still unclear (Biron and
Loxdale, 2013). In addition, more parasitic effects induced by the horsehair worms
found in the recent years including the nymphal appearance on the infected female adult crickets (Biron et al., 2005b), morphological allometry and intersexuality in the infected adult mantids (Chiu et al., 2015). The coevolution of the horsehair worms with their definitive hosts has proceeded since early Early Cretaceous(Poinar and
Buckley, 2006; Bolek et al., 2015) and creates the complex interaction to each other.
Many of the old myths have been understood now, but more will be created with the future studies.
1.4 The studies in this dissertation
There are two main topics discussed in this dissertation: 1) Biodiversity of the horsehair worm in Taiwan and 2) Horsehair worm-induced host developmental manipulation.
Biodiversity is one of the most basic understanding of living organisms.
Horsehair worms attract scientists' attention since 19th century, but the related study is lacked in Taiwan. Although they are frequently witnessed to emerge from manitds, the horsehair worm in Taiwan has never been scientifically described until Chiu et al.
(2011). To date, Chordodes formosanus Chiu et al., 2011 is still the first and only one
horsehair worm species ever had been reported, despite many evidences suggest several species are still existing in Taiwan. In the following context, five species of the horsehair worms collected from Taiwan are introduced, including the advanced researches of the seasonality and host specificity of the most common species, C.formosanus.
Horsehair worm-induced host developmental manipulation is another issue about the interaction of Chordodes and its mantid host. The altered developmental process in creating the abnormal morphology on the infected mantid. The abnormal morphology induced by the horsehair worm has ever been reported in early 20th century in the infected katydid (Ebner, 1940 reviewed in Wülker, 1964) and recently reported in an infected mantid (Roy, 2003). In the both cases, the infected host showed the abnormal characters resemble that in the opposite sex which is known as intersexuality
(Wülker, 1964). These abnormal characteristics represent the special
adaptation evolved in the co-evolution but have not yet been advanced discussed. In the following context, this issue contains the abnormal characteristics found in theHierodula mantids infected by the Chordodes horsehair worms, the adaptation
promoting the evolution of this manipulation, the possible physiological mechanism, and the new evidence to challenge the current understanding of insect sexual differentiation.
2 Materials and methods
2.1 Description of the horsehair worm's morphology (adult)
Description of the adult horsehair worms was applied for the species identification and the examination of inter- and intra-species morphological variation.
2.1.1 Sample preservation
Adult worms collected directly from the environment (free-living) or induced by water from definitive hosts were killed by hot water (80–90°C). The dead worms were fixed in a 75% alcohol solution for several days and then kept in a 95% alcohol solution to preserve the DNA. The worms in the 95% alcohol solution were preserved under room temperature for future examination.
2.1.2 Morphological examination of adult horsehair worms
The anterior and posterior ends of worms were cut and examined directly under a stereomicroscope. Cuticle of the mid-body was removed for about 1 cm long and cut longitudinally from the lateral side. Soft tissue under the cuticle was removed by dipping the fragment into a 1% KOH solution for 30–180 min under room temperature until the soft tissue becomes semitransparent. The cuticles were washed with a 5% alcohol solution and then placed on a microslide for the observation under a light microscope at a magnification of 40–200×. The cuticle of the mid-body persevered as permanent slides were further dehydrated with a series of 75%, 95%, 100% ethanol solutions, and xylene for 15 min respectively and then fixed in Canada balsam (Entellan®).
The preparation protocol for the observation under SEM followed that of
Schmidt-Rhaesa (2002b). Fragments of the anterior end, mid-body, and posterior end
of preserved adult and larval specimens were dehydrated with a series of 75%, 95%,alcohol/acetone mixtures of 2:1, 1:1, 1:2, and 0:1. Samples were then critical-point-dried, gold-sputter-coated, and examined under an SEM (JEOL JSM-5600, Tokyo, Japan) at a magnification of 100–15,000×.
2.2 Description of the horsehair worm's morphology (Egg and larva)
Descriptions of the eggs and larval horsehair worms were applied for the advanced description of the immature stages of the horsehair worms from Taiwan and as the reference for identifying the cysts from field-collected paratenic hosts.
Each couple of adult horsehair worms for laying eggs were reared with 800 ml aerated tap water and under 28–30°C for Acutogordius and Chordodes, and 16°C for
Gordius. Males were removed one days after they stick sperm drops on females' tails.
Females were kept in the water for laying eggs for 2–4 weeks and then removed. Egg strings (Chordodes, Acutogordius and Gordius) found on the water bottom or stuck on the substances were reared under the same condition with the adults and change the water for every 4-5 days until hatched.
The live eggs and larvae were examined under a light microscope. Larvae for measurement were first killed by the heat water (90–100°C) and photographed. For SEM, egg strings were first crashed in a microtube fixed by a 75% alcohol solution for 15 min. The series of dehydration for SEM is the same with that of the adult worms by exchanging upper phase of the liquid with the crashed eggs after the centrifugation for few seconds. Samples were then critical-point-dried, gold-sputter-coated, and examined under an SEM (JEOL JSM-5600, Tokyo, Japan) at a magnification of 100–15,000×.
2.3 DNA amplification and phylogenetic analysis
DNA amplification and phylogenetic analysis is the main evidence applied to justify the conspecific status of the horsehair worms collected from the field in Taiwan.
Genomic DNA of adult and larval horsehair worms was extracted using an ALS Tissue Genomic DNA Extraction Kit (Kaohsiung, Taiwan). A set of universal primers (LCO1490 and HC02198) (Folmer et al., 1994) were applied to amplify and sequence the partial COI sequence. For some of the Acutogordius and Gordius samples which are not able to be amplified by the universal primer set, new primers were designed from the conserve region of the sequences of Acutogordius and Gordius, respectively.
The new sets of primers are AcCOiF (TGAGCTGCCTTTTTAG) and AcCOiR (TGTATTAATGTTTCGGTC) for amplifying sequences of Acutogordius, and GoCOiF-1 (TTAGGAACTGCTTTAAG) and GoCOiR-1 (ATAGGGTCAAAGAAGGAGG) for that of Gordius. The PCR with the primer sets were initiated at 95°C for 5 min, and the amplification was conducted for 40 cycles, at 95°C for 1 min, 50 °C for 1 min, and 72°C for 1 min, with a final extension at 72°C for 10 min.
For the phylogenetic analysis, the pairwise genetic distances and a phylogenic tree reconstructed by the Neighbor-joining (NJ) method based on the Kimura 2-parameter model were used. The COI sequences with high sequencing quality were first aligned using CLUSTALX 2.0.10 (Thompson et al., 1997) and the analysis was conducted by using MEGA 6.0 (Tamura et al., 2013). The support for the topology of the NJ tree was estimated by bootstrapping using 1000 replicates.
2.4 Examination of the cysts in the paratenic hosts
Examination of the cysts in the paratenic hosts was applied for the investigation of the distribution, the species components, and the seasonality of the horsehair worms in Taiwan.
Horsehair worm cysts inside paratenic hosts were examined under a light microscope. The examination protocol for checking snail hosts was followed that of
Hanelt et al. (2001). The snail shells were removed first and the soft tissue was flatted
by two glass slides, then the slides were examined under a light microscope at 200×magnification.
Aquatic insect hosts examined were first cleared tissues by heating the samples in a lactophenol solution (75 ml of lactic acid, 14 ml of acetic acid, 7 ml of phenol, and 4 ml of distilled water; Winterbourn, 2005) or Nesbitt’s fluid (40 g of chloral hydrate, 25 ml of distilled water and 2.5 ml of concentrated hydrochloric acid; Walter
and Krantz, 2009) for 15–20 min at 60°C or 40°C, respectively. The treated aquatic
insect host was flattened between a glass slide and a coverslip and examined under a light microscope. In the observation of the folded larvae in Acutogordius and Gordius cysts, additional treatment by 1–5% KOH solution for 3–6 hr under room temperature was performed to release the folding.2.5 Artificial infection
Artificial infections of the horsehair worms, Chordodes spp., to their paratenic hosts, physid snail (Physa sp.), larval chironomids (Chironomus sp.), and larval mosquitoes (Aedes albopictus), and definitive host, mantid (Hierodula patellifera) were applied for estimating the egg developmental time of C. formosanus and further examination of sexual differentiation and parasitic effect on the mantid hosts.
2.5.1 Mantid rearing
The mantids were reared from the egg. The eggs were collected from the field (Kobe University, Kobe, Japan) or laid by the reared mantids (Miaoli District Agricultural Research and Extension Station, Council of Agricultural, Executive Yuan, Taiwan). Each newly hatched nymph was reared alone in a box under 29–30°C, 50–70% RH. Each mantid was fed by Drosophila sp. (1st–5th instar) and Tenebrio
molitor (6
th instar to adult) 3 times a week.Each mantids were recorded the time of hatching, molting, and eclosion.
2.5.2 Infection of paratenic hosts
The worm larvae for infection were hatched from the egg strings collected from Yamaguchi prefecture, Japan (Chordodes sp.) or laid by the female horsehair worms (C. formosanus) in Taiwan. The egg strings were reared in aerated tap water under the temperature of 26–28°C. The eggs nearly hatching turn dark in color and were cut into a 2-3 mm piece for the infection.
The infection of physid snail and larval chironomids were used in the further infection of the definitive mantid host. In the infection of the snail, each snail was exposed to an egg piece (Chordodes sp.) for 4 nights in a well of the 24–well culture plate. Then all the snails were reared together in aerated tap water and fed with the cabbage. The snails survived for 15–20 days (71 of the 236 infected snails) were
randomly sampled 23 individuals for examining the infection. Their shells were removed and the soft tissue was flatted by two glass slides before counting the cyst number under light microscope. All these 23 snails were all infected with 153 ± 136 (19–616) cysts. The examined snails were all preserved under –80°C until fed to the mantid host (Bolek et al., 2013b). In the infection of the larval chironomid, each larval chironomid (3rd instar) was exposed to an egg piece (C. formosanus) in a well of the 24–well culture plate for two nights. In the three times of the infection, 24 larval chironomids exposed were randomly chosen for checking the infection by
Method 2.4. Twenty-three of them (infection rate: 95.83%) were infected with 43 ±
33 (0, 12–141) cysts. The remaining larval chironomids were then used for the infection of the mantids.The infection of the larval mosquitoes were used for estimating the egg developmental time of the horsehair worm (C. formosanus). The mosquito eggs were laid by female mosquitoes reared in the Department of Public Health and Parasitology, Chang Gung University and preserved under 4°C until used in the experiment. The larval mosquitoes were hatched one night after the eggs were dipped in the water under room temperature. In each infection, 24 newly hatched larval mosquitoes were isolated individually in each well of the 24–well culture plate and exposed to an egg piece of horsehair worm contained 623.94 ± 102.12 (413–833) eggs. The infection were conducted for 12 times with the horsehair worm eggs developing for different days respectively (18, 21, 24, 27, 30, 33, 36, 39, 42, 45, 48, and 59 days) from being laid and replicated for twice. The larval mosquitoes (both dead and live individuals) were flatted by a glass slide and a coverslip and count the cysts under a light microscope. The motility rate and infection rate of the hosts at 24 hours post exposure were recorded.
2.5.3 Infection of definitive hosts
Infection of definitive hosts was applied for examining parasitic effects of the horsehair worms.
The infected snails preserved under –80°C harboring cysts of Chordodes sp.
were used to infect the mantid host (hatched from the ootheca collected from Yamaguchi prefecture, Japan). The infected snails were separated into 2–3 pieces which contained 65 ± 60 (6–308) cysts in a piece. Each piece of the infected snail was mixed with the fat body of T. molitor and touched the mouth part of a mantid until the mantid consumed all the snail part. The infected larval chironomids with the cysts of
C. formosanus inside (two chironomids in each infection contained 85 ± 65 (0,
24–282)) were directly mixed with the fat body of T. molitor and fed to the mantids.The manitds (4th to 8th instar) fed with infected snails or chironomids were all reared to adult and collect the molting skins and the adult antennae for further examination. The infection of the mantids were determined by the presence of juvenile and adult horsehair worms in the mantids dissected.
2.6 Host morphological examination
Measurements of the host morphologies were applied for comparing the sexual dimorphism, the developmental trajectory in both sexes of the mantids, and the parasitic effect on the mantids hosts, H. formosana (field-collected) and H. patellifera (reared).
2.6.1 Measurement of wing, leg and pronotum characteristics
Wings, legs and pronota of the field-collected H. formosana were removed from specimens and preserved in 75% ethanol solution. The wings were unfolded, flattened and covered by a transparent plastic slide. All dissected parts were photographed using a camera with a scale of 1 mm.
Pronotum and leg lengths were measured according to the description provided by Prete et al. (1990, 2002). The pronotum was measured from the rostral-most to the caudal-most midpoint of the prothoracic tergum. The leg was measured from the coxa to the end of the tarsus (the length of the tarsus was not included in the analysis of raptorial legs). The wings were measured according to sclerotized wing venation, as described by Roy (2005). The length of the forewing was measured from the base of the costal vein to the end of the second radial vein, which is the visually longest wing length. The length of the hindwing was measured from the base of the costal vein to the end of the radial posterior vein. The measurements were performed using the segmented line function in ImageJ 1.47 (Rasband, 1997-2016), and calibrated spatially to the scale included in each picture.
The forewing shape index was computed using the following formula:
(BR−AR)/(BR+AR), where AR is the area above the radial vein and BR is the area below the radial vein. A high shape index indicates the relatively large area below the radial vein. The area of each part was measured using the polygon selection function
in ImageJ 1.47.
Detailed methods is described in Chiu et al. (2015).
2.6.2 Measurement of antennal characteristics
The antennae were collected and preserved in a 75% alcohol solution before being examined. Each molting skin was preserved in a small tube and rinsed by 75%
ethanol solution before being examined.
The comparison of the field-collected H. formosana is to examine the sexual dimorphism and parasitic effect on the field-collected mantids. Three antennal characteristics including 1) density of the grooved basiconic sensillum on antennae, 2) the first segment bearing grooved basiconic sensilla, and 3) the total number of antennal segments were examined using SEM (same method in the sample preparation with Method 2.1–2.2) or a light microscope. The density of grooved basiconic sensilla was calculated using the average number of sensilla per 25 × 25μm2 on each selected antennal flagella. One antennal flagellum was selected for every 10 segments from the 10th to 100th segment; thus, 10 antennal segments were selected from each sample. The narrow median strip of each antennal segment was first divided into 16–96 cells of 25 × 25μm2. The total number of grooved basiconic sensilla in this narrow median strip was counted and then divided by the number of square areas. In addition to the sensillum density, the first segment bearing grooved basiconic sensilla and the total number of flagellum segments were also recorded (samples with incomplete or broken antennae were not included in the analysis of the total number of segments). Detailed methods is described in Chiu et al., (2015).
The comparisons of antennae in different instars of H. patellifera which were free from horsehair worm's infection is to examine the developmental trajectories and sexual differentiation in both sexes of the mantids. All the antennae (adults) and the
antennal part of the molting skin (nymphs) were examined under SEM. For every flagellum segments, we counted number of the large trichoid sensilla, small trichoid sensilla, and groove basiconic sensilla, and measured the length and the wide of each segment. According to these measurements, the total number of large trichoid sensilla, the total number of small trichoid sensilla, the total number of groove basiconic sensilla, the total number of flagellum segment, antennal surface area of each flagellum segment, the first flagellum segment bearing the grooved basiconic sensilla, and the total number of flagellum segment bearing the grooved basiconic sensilla ("the total number of flagellum segment" minus " the first flagellum segment bearing the grooved basiconic sensilla") were compared between the sexes of the adult, the first instar nymph, the 8th instar nymph, and the 9th (last) instar nymph. The antennal surface area ("the length" times "the wide") of each flagellum segment were also compared between the sexes of the adult, the first instar nymph, and the 9th (last) instar nymph. According to the first flagellum segment bearing the grooved basiconic sensilla (BS) and the total number of flagellum segment (TS) in the different sexes, an antenna was separated into three zones: male BS to female BS (Zone1), female BS to female TS (Zone2), and female TS to male TS (Zone3) (see chapter 4.3.1, Table 2).
Total number of the grooved basiconic sensilla on each of these three zones were compared between the sexes of adult mantids. The antennal surface area of the Zone2 was also compared between the sexes of the adult, the first instar nymph, and the 9th (last) instar nymph.
The examination of the antennae on the male adult mantids artificially infected by Chordodes sp. was to investigate the relationship of the host morphological change and horsehair worm's developmental time. The antennae were examined by a light microscope and recorded the first flagellum segment bearing more than three grooved