Randomized Controlled Trial of the Use of an Educational Cancer Website to Increase Cancer
Patient’s Participation into a Research Study
MALCOLM KOO1,2,*, ALISON TUCKER2, MICHELLE COTTERCHIO2,3, NANCY
KREIGER2,3,4, JOHN McLAUGHLIN2,3 AND STEVE GALLINGER5
1Graduate Institute of Natural Healing Sciences, Nanhua University, Taiwan
2Dalla Lana School of Public Health, University of Toronto, Ontario, Canada
3Population Studies and Surveillance, Cancer Care Ontario, Toronto, Ontario, Canada
4Department of Nutritional Sciences, University of Toronto, Ontario, Canada
5Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada
ABSTRACT
The present randomized controlled trial was conducted to determine if providing access to an educational colorectal cancer Website for patients with colorectal cancer might increase their subsequent participation in a population-based cancer study. A total of 384 potential participants recruited from colorectal cancer cases identified from the Ontario Cancer Registry and the Mount Sinai Hospital in Toronto, Ontario, Canada were randomly divided into two groups. The control group was mailed an invitation package containing a brochure describing a population-based cancer registry (the Ontario Familial Colorectal Cancer Registry, OFCCR), a family history questionnaire, and a colorectal cancer educational pamphlet. The intervention group was mailed the information for access to a colorectal cancer educational Website in addition to the materials sent to the control group. Results indicated that providing access information to an educational Website about colorectal cancer did not increase the participation of colorectal cancer patients in a population-based cancer study. The participation for the intervention group (66%) was not significantly different (p=0.38) from the control group (62%). The additional provision of a Website with colorectal cancer information to patients appears not to be an effective strategy to improve subsequent participation in a population-based cancer study.
Key words: colorectal cancer, data collection, epidemiologic methods, Internet, patient participation.
1. INTRODUCTION
Colorectal cancer is the fourth most common cancer in men and the third most common cancer in women worldwide (Center et al., 2009). Effective primary and secondary preventive approaches must be developed to reduce the morbidity and mortality from colorectal cancer. The Ontario Familial Colorectal Cancer Registry (OFCCR) in Canada is a research resource designed to facilitate population-based colorectal cancer studies. It is one of six international sites participating in the Co-operative Familial Registry for Colorectal Studies established by the US National Cancer Institute. Detailed personal and family history, epidemiologic data, blood samples and tumor specimens from a population-based sample of colorectal cancer patients and their families have been collected since its inception in 1997 (Cotterchio et al., 2000).
*Corresponding author. Email:m.koo@utoronto.ca
A high participation rate is important to ensure that those participating in the registry are representative of those with colorectal cancer and thereby reduce the threat to internal validity as a result of self-selection bias. With the decline of participation in epidemiologic studies over time, particularly controls in population-based case-control studies (Morton et al., 2006), there is a need to consider and evaluate different strategies to enhance response in potential study participants. A systematic review of 98 strategies for influencing response to postal questionnaires based on 372 randomized controlled trials found that the odds of response doubled when a monetary incentive was used, when the questionnaires were sent by recorded delivery, when a teaser was put on the envelope, or when the questionnaire topic was more interesting (Nakash et al., 2006). Provision of educational material has previously been investigated as a strategy to improve participation in annual mammography (Lerman et al., 1992), influenza immunization (Moran et al., 1996), colorectal screening (Hart et al., 1997; Wardle et al., 2003), and breast cancer screening (Bonfill et al., 2001). Most of the studies concluded that educational material about the disease of interest was effective in increasing participation. In addition, a qualitative study of subject recruitment for familial cancer research on cancer patients, relatives of cancer patients, and individuals from the general population revealed that educational material about the disease and its familial nature was one of the themes emerging that would increase participants’ commitment to the study (Kreiger et al., 2001).
Use of the World Wide Web for medical information is common among patients with cancer (Chen & Siu, 2001; Lake et al., 2004). Although print products remain the most common source of information sought by patients with cancer (Basch et al., 2004) presumably because of the lack of health information quality control on the Web (Sajid et al., 2008) or the lack of trustable sites (Powell et al., 2006). It is not clear whether the provision of a Website on colorectal cancer information sponsored by a research organization, in addition to print materials on colorectal cancer, can fulfill health information needs and whether it can improve study participations. Therefore, we conducted a randomized controlled trial to determine if the participation rate to the OFCCR would increase with the provision of an educational colorectal cancer Website.
2. MATERIALS AND METHODS
2.1 Study Population
The present randomized controlled trial was embedded within the OFCCR methods of recruitment and follow-up. At the time of the study, the OFCCR was recruiting patients with colorectal cancer which included adenocarcinoma of the colon, rectum or anal canal, under the age of 52 years who resided in the province of Ontario and were diagnosed between January 1, 2003 and November 31, 2003.
Living colorectal cancer cases were identified every month using the population-based Ontario Cancer Registry, physician consent was obtained and they were then invited to join the OFCCR. In addition to recruiting all cases under
52 years of age, and to supplement the OFCCR sample, cases who were 52 years of age or older diagnosed at the Mount Sinai Hospital in Toronto, Ontario, Canada were also recruited. Ethics approval was granted from the Research Ethics Board, University of Toronto.
2.2 Study Design
Each month, all new cases with physician consent were randomized to a control or an intervention group using the SAS randomization procedure (Version 8, SAS Institute Inc., Cary, NC, USA) with stratification by two age groups, those under 52 years of age and those 52 years or older. The participants in the control group were sent the standard OFCCR invitation package containing an introductory letter inviting them to participate in the registry, a brochure describing various phases of the OFCCR, consent forms for participation, a family history questionnaire (FHQ), and an educational pamphlet “Understanding Colorectal Cancer” (Nicholson et al., 1999). The participants in the intervention group were sent in the same package, in addition to the standard OFCCR invitation package, information on the OFCCR invitation letter that allowed them to access a colorectal cancer educational Website designed specifically for the study. The information included the Uniform Resource Locator (URL) of the Website, a unique username and password for each participant, and a brief description of the Website. The unique usernames and passwords were used for keeping track of who logged on to the Website, the duration they spent on the Website, and the pages they visited.
Four weeks after the invitation packages were mailed out, reminder postcards were sent to the eligible participants who had not responded or had not indicated a refusal to participate. Follow-up telephone calls, with a maximum of six attempts, were made approximately eight weeks after the initial mailing. After the sixth unsuccessful attempt, a new invitation package was sent. A second round of telephone follow-up with a maximum of four calls was conducted after the second mailing. Once these attempts had been exhausted the eligible participant was considered as a non-respondent.
2.3 Educational Colorectal Cancer Website
An educational colorectal cancer Website was constructed specially for this study. The Website contained five main parts including information on colorectal cancer, a letter from an OFCCR participant describing her experiences with the OFCCR, frequently asked questions and answers, links to other selected colorectal cancer Websites, and “Ask the Expert”. In the “Ask the Expert” section, participants could submit their questions on the Webpage confidentially and answers would be provided by one of the investigators (S. G.) who is a physician specializing in colorectal cancer.
Throughout the text on the Website, definitions in plain English were provided for all medical terminology. Those terms were highlighted with color in the text and their plain English definitions would automatically appear in a smaller pop-up window when the mouse cursor was rolled over the highlighted terms. The
readability of the Website was kept at the Flesch-Kincaid level of grade 10 (Hendrickson et al., 2006). The Website was closed at the end of the randomized controlled study. Participants had approximately nine months to return their FHQ.
2.4 Website Questionnaire
Four months after the initial invitation, each participant in the intervention group was mailed a Website questionnaire with a covering letter. Beside basic demographic information, the questionnaire asked what information was most helpful, what other features could be added and the reasons for not logging onto the Website if they had not done so.
2.5 Data Analysis
The participation rate was defined as the number of participants who returned FHQs divided by the number of participants mailed the OFCCR invitation package.
The participation proportions for the control and the intervention group were compared using Pearson chi-squared test. The characteristics of participants who logged into the educational Website and those who did not were compared using Pearson chi-squared test or Fisher’s exact test. For age at diagnosis, which was coded as continuous variable, t-test was used. Crude and adjusted odds ratios with 95% confidence intervals for returning the FHQ were estimated using logistic regression with intervention, sex, region of residence and age at diagnosis as independent variables. Region of residence was categorized as rural or non-rural based on the postal codes of participants’ address. Statistical analyses were conducted in SAS version 8.0 (SAS Institute Inc., Cary, NC, USA).
3 RESULTS
Of the 419 eligible participants, physician consent was obtained for 403 (96%) of them. They were randomized into a control group with 202 participants and an intervention group with 201 participants. After the randomization, it was determined that 19 participants did not meet the OFCCR inclusion criteria including wrong diagnosis, wrong diagnosis date and died between physician consent and invitation to participate. Therefore, the final numbers of participants in the control and intervention groups were 193 and 191, respectively.
3.1 Randomized Controlled Trial
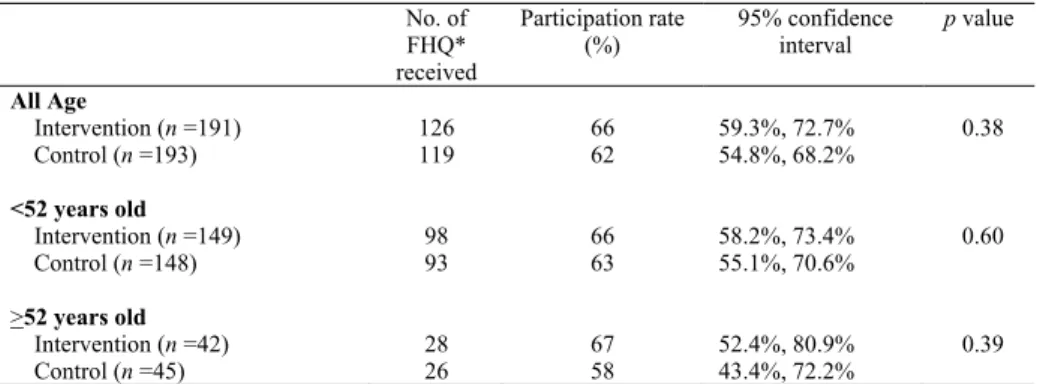
No difference was observed in the participation between participants under 52 years of age and those 52 years or older in the control and intervention groups (p = 0.60 and p = 0.39), respectively (Table 1). Therefore, the two groups were combined for all further analyses. Participation with the provision of the colorectal cancer educational Website in addition to the print material was 66% while that of
the group with only the print material was 62%. The difference in participation was not statistically significant (p = 0.38) (Table 1). Overall, the participation was 64%.
Table 1. Comparison of participation rate between the intervention and control group in the Ontario Familial Colorectal Cancer Registry study
No. of
FHQ*
received
Participation rate (%)
95% confidence interval
p value
All Age
Intervention (n =191) 126 66 59.3%, 72.7% 0.38
Control (n =193) 119 62 54.8%, 68.2%
<52 years old
Intervention (n =149) 98 66 58.2%, 73.4% 0.60
Control (n =148) 93 63 55.1%, 70.6%
>52 years old
Intervention (n =42) 28 67 52.4%, 80.9% 0.39
Control (n =45) 26 58 43.4%, 72.2%
Note. FHQ = Family history questionnaire.
A total of 139 participants did not return their FHQ. In the intervention group, 65 did not return their FHQ and among them, 39 indicated refusal to participate, six were unable to be contacted, three had died, and 17 did not respond. In the control group, 74 did not return their FHQ and among them, 41 indicated refusal to participate, five were unable to be contacted, three had died, and 25 did not respond.
Results from multiple logistic regression show that the Website intervention was not significantly associated with OFCCR participation (adjusted odds ratio (OR) = 1.19, 95% confidence interval (CI): 0.78, 1.81) (Table 2). Participation was significantly higher in female (adjusted OR = 1.72, 95% CI: 1.13, 2.62), although region of residence and age at diagnosis were not significantly associated with OFCCR participation.
Table 2. Adjusted odds ratios for participation in the Ontario Familial Colorectal Cancer Registry study
Variables Crude odds ratio
(95% confidence interval)
Adjusted odds ratio*
(95% confidence interval) Intervention
Control group Website group
1.00 1.21 (0.80, 1.83)
1.00 1.19 (0.78, 1.81) Sex
Male Female
1.00 1.74 (1.14, 2.65)
1.00 1.72 (1.13, 2.62) Region of residence
Rural Non-rural
1.00 1.16 (0.70, 1.90)
1.00 1.10 (0.65, 1.82)
Age at diagnosis 1.00 (0.98, 1.02) 1.00 (0.98, 1.02)
Note. Multiple logistic regression was adjusted for intervention, sex, and region of residence, and age (as a continuous variable).
3.2 Website Report
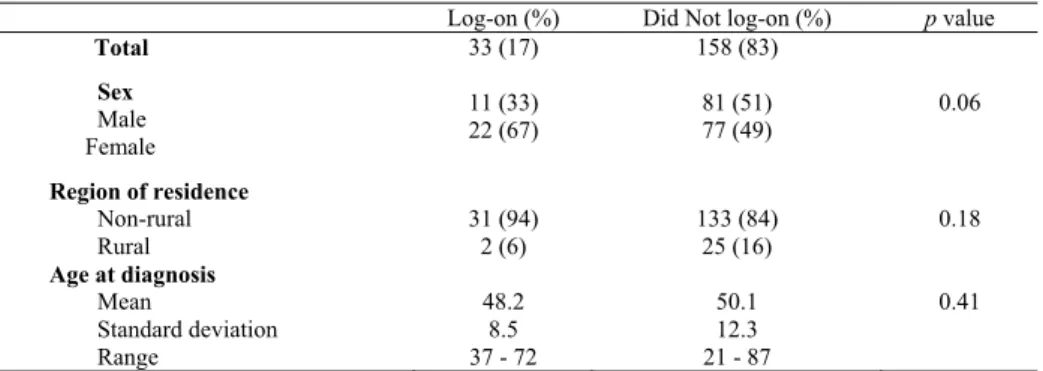
In the intervention group, only 33 (17%) of the 191 participants logged onto the educational Website (Table 3). There was no statistically significant difference between those who logged onto the Website and those who did not, with respect to sex, region of residence, and age at diagnosis.
Results provided by the Web server log file analysis showed that 33 participants logged onto the educational Website once, two logged on twice, and one logged on three times. Of the 33 cases who had logged onto the Website, 32 (97%) returned the FHQ. The time spent by each participant on the Website was estimated by the time difference between log-on to the time the last page was accessed. The median duration was 10.5 minutes with a range of one minute to 46 minutes. Besides the main welcoming page, which was accessed by the participants 61 times, the page with information on the stages of colorectal cancer (43 times) and the page on risk factors were among those most accessed (39 times).
Table 3. Comparison of the participants who log-on and those who did not log-on to the educational colorectal cancer Website
Log-on (%) Did Not log-on (%) p value
Total 33 (17) 158 (83)
Sex Male Female
11 (33)
22 (67) 81 (51)
77 (49) 0.06
Region of residence Non-rural
Rural 31 (94)
2 (6) 133 (84)
25 (16) 0.18
Age at diagnosis Mean
Standard deviation Range
48.2 8.5 37 - 72
50.1 12.3 21 - 87
0.41
3.3 Website Questionnaire
Of the 191 participants randomized to receive the intervention, 160 (79.6%) were sent the Website questionnaire. Among those who did not receive the questionnaire, 18 refused to participate in OFCCR, eight had died since the OFCCR invitation package was sent, three were unable to be contacted at the invitation stage, and two were non-English speaking. Seventy-two (45.0%) questionnaires were returned. Seven participants who returned the Website questionnaire did not return the FHQ in the randomized controlled trial. Among the 33 participants who logged on the Website, 28 (84.8%) returned the Website questionnaires.
There were no significant differences between those who logged on (n = 28) and those who did not log on (n = 44) to the educational Website in sex (p = 0.065), age (under 50 years versus 50 years or older) (p = 0.127), education level (vocational or technical school or less versus some college or university and above) (p = 0.054), and reading the print pamphlet (p = 0.422). Among those 44 individuals who returned the Website questionnaire but had not logged on the
Website, 24 (54.5%) indicated that they had no access to a computer, seven (15.9%) indicated that they were not interested, and four (9%) indicated that they forgot.
Other reasons included “too ill”, “never got around to it”, “do not use computer at home”, “used other Websites”, and “low priority to spend quality time.”
Among those who logged on the Website and also read the “Understanding Colorectal Cancer” pamphlet, 12 (52.2%) indicated they preferred both or either, seven preferred the pamphlet (30.4), three (13.0%) preferred the Website, and one (4.4%) preferred neither.
4. DISCUSSION
This population-based randomized controlled trial was a unique attempt to evaluate the use of a disease-specific educational Website to increase recruitment of patients into a population-based cancer study. We found that providing access information to an educational Website to colorectal patients during subject recruitment did not significantly (p = 0.38) increase the participation.
This result could be attributable to two reasons. First, the invitation package in the control group already included a printed educational material “Understanding Colorectal Cancer” which could have satisfied the needs for information about the disease. The addition of the Website intervention did not further add to the effect of written materials. The participation rate in the control group was already relatively high at 62% and comparable to those in trials involving cancer treatment which ranged from 21% to 69% (Ford et al., 2006; Chang et al., 2002). The first-year participation rate of the OFCCR was 61% (Cotterchio et al., 2000). Furthermore, study participation is only one of several possible outcomes. Other indicators such as the levels of understanding the OFCCR study or cancer-related knowledge might be different between the intervention and control groups and should be considered in future studies.
Second, although one of the opinions expressed by cancer patients and their relatives in a previous focus group study was that educational materials would maintain participants’ commitment in epidemiologic cancer research (Kreiger et al., 2001), there has been no evaluation about exactly what type of educational materials would be the most effective. In addition, the characteristics of the potential participants may determine the preference for the type of educational materials. Patients with prostate cancer who used the Internet for self-education were found to be younger, had a higher education level, owned a personal computer, and had prior computing experience, compared to those who did not (Pautler et al., 2001).
A very high proportion (83%) of the individuals in our intervention group did not log onto the Website. Over half of them indicated that the reason was the lack of access to a computer. Printed materials or video materials (Zapka et al., 2004) may be a more convenient educational medium for them. A possible explanation for the low access to the educational Website is the duration between diagnosis of cancer and the arrival of the invitation package. Most individuals for this study were approached three to four months after their diagnosis and they might have researched and obtained enough information about their disease already. In addition,
the mailed package might have contained sufficient information to fulfill the needs of the individuals. Logging on to the Website might be considered unnecessary by the patients. Another possible reason is the need to use access information to log on to the Website. Although we used a short URL (www.ofccr.ca) and relatively simple usernames and passwords, the need to correctly enter three pieces of information could have been a deterrent for accessing the Website.
Although the provision of an educational Website did not significantly increase the participation rate in patients who had already received printed material with information on their disease, the Website may still provide a reliable source of information and it can potentially enable patients and their families to make better-informed decisions. The total cost of the educational Website included the design and construction of the Website in the development phase and a Web hosting fee in the maintenance phase. In addition to financial costs, resources needed for responding to questions in the “Ask the Expert” section need to be considered. However, during the entire study, only three participants submitted their questions. A prostate cancer Website study reported a similar finding with their on-line help-line (Pinnock & Jones, 2003). Preliminary surveys about the Website in their study suggested that the help-line would be popular among the cancer patients, and although it was the most accessed part of the Website, only 0.8% of the patients sent questions.
5. CONCLUSIONS
In this randomized controlled trial of colorectal cancer patients, providing access information to an educational Website about colorectal cancer did not increase their participation in a population-based cancer study. Due to the study design where the Website intervention was added to but not substituted for the printed material control, the sole contributing effect of Website intervention could not be estimated. Future research on improving study recruitment may consider comparing the effect of printed materials versus Website intervention. This is particularly relevant for studies that accrue participants exclusively through email or special recruitment Websites where participation rates were generally very low (Koo & Skinner, 2005). The present study focused on the effect of Website intervention on patients with colorectal cancer. The needs of educational materials for the patients’ family members may be different and the effect of the educational Website intervention needs to be evaluated separately in future studies.
ACKNOWLEDGEMENTS
This work was supported by the Interdisciplinary Health Research Team - Canadian Institutes of Health Research Studentship Award (CRT43821 to A.T.).
REFERENCES
Center, M. M., Jemal, A., Smith, R. A., & Ward, E. (2009). Worldwide variations in colorectal cancer. CA: A Cancer Journal for Clinicians, 59(6), 366-378.
Basch, E. M., Thaler, H. T., Shi, W., Yakren, S., & Schrag, D. (2004). Use of information resources by patients with cancer and their companions. Cancer, 100(11), 2476-2483.
Bonfill, X., Marzo, M., Pladevall, M., Marti, J., & Emparanza, J. I. (2001).
Strategies for increasing women participation in community breast cancer screening. Cochrane Database Systematic Reviews, No.1, CD002943.
Chang, S. M., Barker, F. G. 2nd, Schmidt, M. H., Sloan, A. E., Kasper, R., Phillips, L., Shih, K., Hariharan, S., & Berger, M. S. (2002). Clinical trial participation among patients enrolled in the Glioma Outcomes Project. Cancer, 94(10), 2681-2687.
Chen, X., & Siu, L. L. (2001). Impact of the media and the Internet on oncology:
survey of cancer patients and oncologists in Canada. Journal of Clinical Oncology, 19(23), 4291-4297.
Cotterchio, M., McKeown-Eyssen, G., Sutherland, H., Buchan, G., Aronson, M., Easson, A. M., Macey, J., Holowaty, E., & Gallinger, S. (2000). Ontario Familial Colon Cancer Registry: methods and first-year response rates.
Chronic Diseases in Canada, 21(2), 81-86.
Ford, B. M., Evans, J. S., Stoffel, E. M., Balmana, J., Regan, M. M., & Syngal, S.
(2006). Factors associated with enrollment in cancer genetics research. Cancer Epidemiology, Biomarkers and Prevention, 15(7), 1355-1359.
Hart, A. R., Barone, T. L., & Mayberry, J. F. (1997). Increasing compliance with colorectal cancer screening: the development of effective health education.
Health Education Reseach, 12(2), 171-180.
Hendrickson, R. L., Huebner, C. E., & Riedy, C. A. (2006). Readability of pediatric health materials for preventive dental care. BMC Oral Health, 6, 14.
Koo, M., & Skinner, H. (2005). Challenges of Internet recruitment: a case study with disappointing results. Journal of Medical Internet Research, 7(1), e6.
Kreiger, N., Ashbury, F., Cotterchio, M., & Macey, J. (2001). A qualitative study of subject recruitment for familial cancer research. Annals of Epidemiology,11(4), 219-224.
Lake, J. P., Ortega, A., Vukasin, P., Kaiser, A. M., Kaufman, H. S., & Beart, R.W.
Jr. (2004). Internet use by colorectal surgery patients: a surgeon's tool for education and marketing. American Surgeon, 70(6), 553-558.
Lerman, C., Ross, E., Boyce, A., Gorchov, P. M., McLaughlin, R., Rimer, B., &
Engstrom, P. (1992). The impact of mailing psychoeducational materials to women with abnormal mammograms. American Journal of Public Health, 82(5), 729-730.
Moran, W. P., Nelson, K., Wofford, J. L., Velez, R., & Case, L. D. (1996).
Increasing influenza immunization among high-risk patients: Education or financial incentive?, American Journal of Medicine, 101(6), 612-620.
Morton, L. M., Cahill, J., & Hartge, P. (2006). Reporting participation in epidemiologic studies: a survey of practice. American Journal of Epidemiology, 163(3), 197-203.
Nakash, R. A., Hutton, J. L., Jorstad-Stein, E. C., Gates, S., & Lamb, S. E. (2006).
Maximising response to postal questionnaires--a systematic review of randomised trials in health research. BMC Medical Research Methodology, 6, 5.
Nicholson, N., Shah, A., Charbonneau, F., Gallinger, S., Couture, F., & Tremblay, F. (1999). Pharmacia Oncology Bringing Discovery to Life. Understanding Colorectal Cancer [brochure].
Pautler, S. E., Tan, J. K., Dugas, G. R., Pus, N., Ferri, M., Hardie, W. R., & Chin, J.
L. (2001). Use of the Internet for self-education by patients with prostate cancer. Urology, 57(2), 230-233.
Pinnock, C. B., & Jones, C. Education Committee of the Australian Prostate Cancer Collaboration. (2003). Meeting the information needs of Australian men with prostate cancer by way of the Internet. Urology, 61(6), 1198-1203.
Powell, S. M., McStay, R. A., Hanson, J. M., & Plusa, S. M. (2006). Use of the Internet by colorectal cancer patients. Colorectal Disease, 8(1), 62-63.
Sajid, M. S., Iftikhar, M., Monteiro, R. S., Miles, A. F., Woods, W. G., & Baig, M.
K. (2008). Internet information on colorectal cancer: commercialization and lack of quality control. Colorectal Disease, 10(4), 352-356.
Wardle, J., Williamson, S., McCaffery, K., Sutton, S., Taylor, T., Edwards, R., &
Atkin, W. (2003). Increasing attendance at colorectal cancer screening: testing the efficacy of a mailed, psychoeducational intervention in a community sample of older adults. Health Psychology, 22(1), 99-105.
Zapka, J. G., Lemon, S. C., Puleo, E., Estabrook, B., Luckmann, R., & Erban, S.
(2004) Patient education for colon cancer screening: a randomized trial of a video mailed before a physical examination. Annals of Internal Medicine, 141(9), 683-692.
Malcolm Koo received his Ph.D. degree in Public Health Sciences from the University of Toronto, Canada in 2000. From 2000 to 2004, he was an Assistant Professor at the Dalla Lana School of Public Health at the University of Toronto, Canada. Currently, he is an Associate Professor and the director of the Graduate Institute of Natural Healing Sciences at the Nauhua University. He is also an Adjunct Professor in the Dalla Lana School of Public Health at the University of Toronto, Canada.
Alison Tucker received her M.Sc. degree in Epidemiology at the University of Toronto in 2005.
Currently, she is an Epidemiologist and Manager of the Communicable Disease and Epidemiology Division with the St. Charles County Health Department, St. Charles, Missouri, USA.
Michelle Cotterchio holds a Ph.D. in Epidemiology, University of Toronto, a M.S in Biology, and a M.P.H. in Epidemiology, Boston University. Currently, she is a Scientist at the Cancer Care Ontario and an Associate Professor in the Dalla Lana School of Public Health at the University of Toronto, Canada.
Nancy Kreiger received her Ph.D. from Yale University and has been a Fellow of the American College of Epidemiology since 1994. Currently, she is Head of the Division of Epidemiology at the Dalla Lana School of Public Health, University of Toronto and a Senior Scientist for Population Studies and Surveillance at Cancer Care Ontario.
John McLaughlin received his Ph.D. from the University of Toronto. He is the Vice-President of Population Studies and Surveillance at Cancer Care Ontario, and a Professor in the Dalla Lana School of Public Health at the University of Toronto, Canada.
Steve Gallinger is a Senior Investigator in the Program in Molecular Biology and Cancer at the Samuel Lunenfeld Research Institute of Mount Sinai Hospital, and Professor in the Department of Surgery at the University of Toronto, Canada.