Smart Technology for Evaluating Fire Extinguishing Effect of Tert-butyl hydroperoxide
Kuo-Yi Li,
Department of Industrial Engineering and Management, National Chin-Yi University of Technology, 57, Sec. 2, Zhongshan Rd., Taiping Dist., Taichung, Taiwan 41170, ROC
Shu-Yao Tsai, Chun-Ping Lin*,
Department of Health and Nutrition Biotechnology, College of Health Science, Asia University, 500, Lioufeng Rd., Wufeng, Taichung, Taiwan 41354, ROC
Yun-Ting Tsai, Chi-Min Shu
Department of Safety, Health, and Environmental Engineering, National Yunlin University of Science
and Technology, 123,University Rd., Sec. 3, Douliou, Yunlin, Taiwan 64002, ROC
*To whom correspondence should be addressed. Fax: +886-4-2332-1126.
E-mail: cp.lin@asia.edu.tw
Abstract
Tert-butyl hydroperoxide (TBHP) 70 mass%, a solution of liquid peroxide, has been widely
employed in the chemical industry as a polymerization initiator. The smart technology for predicting
the mechanism of thermal decomposition and the inhibitive or hazardous reaction of TBHP by
different calorimetric tests involves using differential scanning calorimetry (DSC) non-isothermal
tests versus DSC isothermal tests and vent sizing package 2 (VSP2) adiabatic tests versus DSC non-
isothermal tests, respectively, for further understanding how to extinguish organic peroxide accidents
under fire scenario or runaway reaction in a chemical plant. Meanwhile, TBHP mixed with inhibitive
and hazardous materials, such as various protic acids to help prevent runaway reactions, was applied
on fires or explosions in the fire system. The results could be available to fire-related agencies as a
reference application. The fire extinguishing system must be well designed for decreasing the degree
of hazard.
1. Introduction
Tert-butyl hydroperoxide (TBHP) is a solution of liquid organic peroxide that has been widely employed in the chemical industry and used to manufacture polymer materials. It is a commercial liquid organic peroxide which needs to be stored under limited temperature. In terms of manufacturing and storage management, many serious explosions and high ambient temperature have occurred from its thermal decomposition and incompatible reaction.
1–4Organic peroxides have caused many serious accidents including fire extinguishing accidents, tank storage, and transportation. One reason for accidents involves the peroxy group (–O–O–) of organic peroxides, due to its thermal instability and high sensitivity to thermal sources or ambient temperature.
5–15Table 1 shows the selected thermal explosion accidents caused by organic peroxides in Asia
10,13, and the thermal runaway reaction and explosion accidents of organic peroxides in chemical plant in Taiwan are displayed in Figure 1.
In particular, organic peroxides cannot be mixed with incompatible materials, such as strong acid strong base and special metal powder, which are used for fire extinguishing the organic peroxides in the chemical plant. Actually, suitable fire-fighting equipment can be applied to reduce losses and protect life and property. The fire extinguishing agent of monoammonium phosphate has been used in the current fire extinguishing system.
16,17This study focused on the organic peroxide mixed with phosphoric acid or the other protic acids, such as sulfuric acid and nitric acid, which can be used for understanding inhibitive and hazardous reactions of organic peroxides under thermal decompositions, and which also could be used to optimize the fire extinguishing system to decrease the degree of hazard of organic peroxide accidents.
TBHP is also very sensitive to heat due to the unstable structure of the peroxy group. If temperature is not well controlled, the system becomes unstable and eventually triggers a runaway reaction in the next stage, potentially leading to various types of accidents.
1,2Organic peroxides have complex decomposition characteristics, and we still lack detailed information about them, especially during the runaway reactions. This aim of this study on TBHP is on inhibitive and hazardous reactions of mixing with various protic acids, and on evaluating the thermal hazard and discussing the phenomenon of runaway reactions.
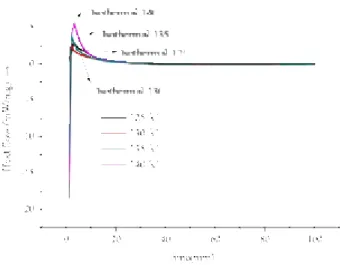
First, we could achieve our aim through a novel and effective thermal analysis technology, a
special method. Via simple differential scanning calorimetry (DSC) tests and kinetics predictions, the
thermal hazard properties of organic peroxides can be evaluated. Comparisons of swift non-
isothermal and isothermal-kinetic model simulations led to a reliable mechanism of thermal
decomposition to predict the parameters of TBHP.
11,12The selected approach was to establish an
effective model for thermal decomposition that included the kinetic parameters and thermal hazard
properties,
8–12such as the activation energy (E
a), frequency factor (lnk
0), heat of decomposition (∆H
d), and reaction order (n), of TBHP.
Secondly, we considered the scenarios when various protic acids are mixed, e.g., monoprotic acid (HNO
3), diprotic acid (H
2SO
4), and triprotic acid (H
3PO
4), respectively, to predict the inhibitive and hazardous materials in TBHP’s thermal stability. The reaction equations of protic acids from Eqs. (1) to (3) are as follows:
18Monoprotic acid
HNO
3+ H
2O ⇌ H
3O
++ NO
3–(1) Diprotic acid
H
2SO
4+ H
2O ⇌ H
3O
++ HSO
4–HSO
4–+ H
2O ⇌ H
3O
++ SO
42–(2) Triprotic acid
H
3PO
4+ H
2O ⇌ H
3O
++ H
2PO
4–H
2PO
4–+ H
2O ⇌ H
3O
++ HPO
42–HPO
42–+ H
2O ⇌ H
3O
++ PO
43–(3)
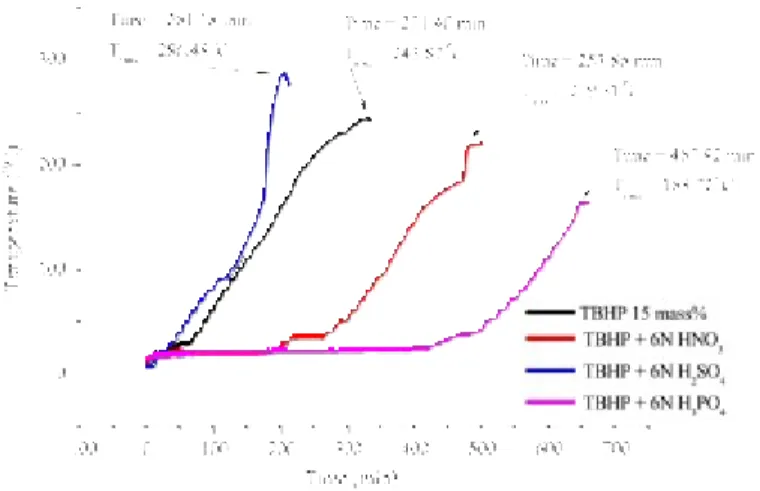
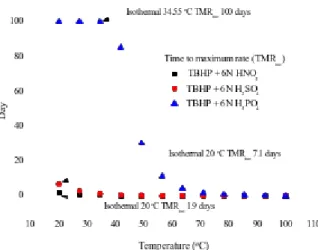
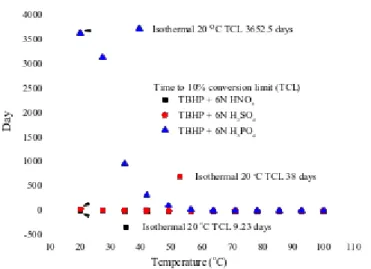
Following the above-mentioned reactions for the prediction results of the original sample of TBHP, we then compared the DSC non-isothermal and the vent sizing package 2 (VSP2) adiabatic- kinetic model simulations to predict the runaway reaction, the inhibitive reaction, and the hazardous reaction of TBHP mixed with various protic acids.
Kinetic model simulation was employed to construct a novel and effective procedure to evaluate the safety parameters for the inhibitive and hazardous reaction of TBHP. The chosen approach could establish a smart technology for thermal decomposition properties that includes the kinetics and hazardous reaction for TBHP, and when mixed with inhibitive or hazardous materials, respectively.
The approach applies the optimal conditions to avoid TBHP’s violent runaway reactions during process manufacturing and storage.
Ultimately, this study’s results could be available to fire-related agencies as a reference application. However, in earlier studies the thermal hazard analysis method was rarely employed to assess and calculate the inhibitive and hazardous reaction for TBHP mixed with various protic acids.
The fire extinguishing system must be well designed for decreasing the degree of hazard. Disaster can be prevented in the first stage, and the results of this study are expected to aid process safety for preventing an accident from occurring.
2. Kinetic simulation
Simulations of kinetic models can be complex multi-stage reactions that may consist of several independent, parallel, and consecutive stages, demonstrated as described by the following equations.
8–12
The initial conditions are as follows:
1 1 1 ( ) 1
d r k T f
dt
(4)
2 1 2 ; 2 2 ( ) 2
d r r r k T f
dt
(5)
3 2 3 ; 3 3 ( ) 3
d r r r k T f
dt
(6)
4 3
d r
dt
(7)
0; i 0; 1, 2, 3, 4
t i
where α
1, α
2, α
3, and α
4are the degrees of conversion of a reaction or stage, r
1, r
2, and r
3are reaction rates of a reaction or stage, k
1, k
2, and k
3are the rate constants of a reaction or stage, and f
1, f
2, and f
3are the kinetic functions of a reaction or stage.
Simple one-step reaction: AB:
0
Ea
d k e
RTf dt
(8)
(1 )
nf n-th order (9)
(1 ) (
n1 n2)
f z autocatalytic (10)
where E
ais the activation energy, k
0is the pre-exponential factor, z is the autocatalytic constant, n
1and n
2are reaction orders of the specific stage.
Reaction including two consecutive stages: ABC:
1 2
1 2
1
(1 ) ;
2( )
E E
n n
RT RT
d d
k e k e
dt dt
(11)
where and are the conversions of the reactant A and product C, respectively, and E
1and E
2are activation energies of stage one and two, respectively.
Two-parallel equations are a useful model of full autocatalysis:
1 2 3
1 1
1 2
2 2
( ) ( )(1 ) ( ) ( );
( ) ( ) (1 )
n n n