Central Diabetes Insipidus in A Patient with Breast Carcinoma Metastatic to the Pituitary Gland
Wei-Yu Chen
1, and Chien-Yu Chen
21Division of Hematology and Oncology, Department of Internal Medicine,
2Department of Radiology, Chi-Mei Medical Center, Yong- Kang Campus, Tainan, Taiwan
Abstract
Pituitary metastatic disease is rare, accounting for 1% of all excised pituitary tumors and generally involving the posterior pituitary. Neoplasms that more often originated pituitary metastases are breast and lung cancer. We describe a case of a 50-year-old woman, diagnosed with breast cancer with liver and bone metastases, who underwent chemotherapy. During hospitalization, she developed polydipsia, polyuria, and nocturia. She had a large volume of 24-hour urine production (8-10L/day) with a very low urine osmolality (109 mosm/kg) compared to that of the plasma (334 mosm/kg). Further biochemical and hormonal workups were normal except for hypernatremia and hyperprolactinemia. Pituitary MRI showed a contrast-enhanced nodular lesion in the pituitary stalk, suggesting a metastatic lesion. The patient was treated with DDAVP and local radiotherapy, which led to the patient’s clinical improvement. Twelve months after her diagnosis of diabetes insipidus, she died of the metastatic disease. (J Intern Med Taiwan 2017; 28: 252-257)
Key Words: Breast cancer, Pituitary metastasis, Diabetes insipidus
Introduction
The pituitary gland and infundibulum can be involved in a variety of medical conditions, including infiltrative diseases, fungal infections, tuberculo- sis, and primary and metastatic tumors. Metasta- ses to the pituitary gland (MP) are absolutely rare, and they are often related to primary breast or lung cancers. Pituitary metastases more commonly affect the posterior lobe and the infundibulum rather than the anterior lobe. The most common sign of this metastatic involvement is diabetes insipidus. A case report of a 50-year-old woman who had metastatic breast cancer, and she presented with polyuria,
polydipsia, and a persistent thirst. She had no elec- trolyte imbalance (such as hyperglycemia, hypoka- lemia, or hypercalcemia) except hypernatremia. But a pituitary stalk metastasis was found on magnetic resonance imaging (MRI) of the brain. A clinical response to nasal vasopressin was achieved and lab- oratory results revealed central diabetes insipidus.
As a result, the urinary output decreased accord- ingly. Supplements of DDAVP combined with local radiotherapy completely resolved the clinical symp- toms of DI of this patient.
Case Report
A 50-year-old premenopausal woman was
Reprint requests and correspondence:Dr. Chien-Yu Chen
Address:Division of Hematology and oncology, Department of Internal medicine, Chi-Mei Medical Center, No.901, Zhonghua Rd, Yongkang Dist, Tainan City 710, Taiwan
diagnosed with carcinoma of the breast (stage IV pT4N3M1b, positive estrogen and progesterone receptors, and negative human epidermal growth factor receptor-2 (HER-2)). She was treated with the first course of chemotherapy with epirubicin and docetaxel on Jun 19 2012. But neutropenic fever developed in Jun 26 2012. She had been alert and well oriented. An empiric antibiotic was given for neutropenic fever. She had a large volume of 24-hour urine production (8-10L/day). Tracing back her his- tory, she had a 2weeks history of polydipsia, poly- uria, and loss of body weight before the admission.
She did not have a headaches or visual complaints.
Physical examination revealed no neurological or visual field deficits. The results of laboratory tests revealed a very low urine-osmolality (109 mOsm/
kg) compared to that of the plasma (334 mOsm/kg), and a plasma sodium of 159 mEq/L, a glucose of 115 mg/dL, and a calcium of 8.0 mg/dL. An Endo- crinological evaluation showed normal cortisol hor- mone, plasma growth hormone, follicle-stimulating hormone, luteinizing hormone, thyroid-stimulating hormone, and adrenocorticotropic hormone, except
mild hyperprolactinemia. Owing to high clinical suspicion and neutropenic fever, water deprivation/
vasopressin tests were not undertaken.
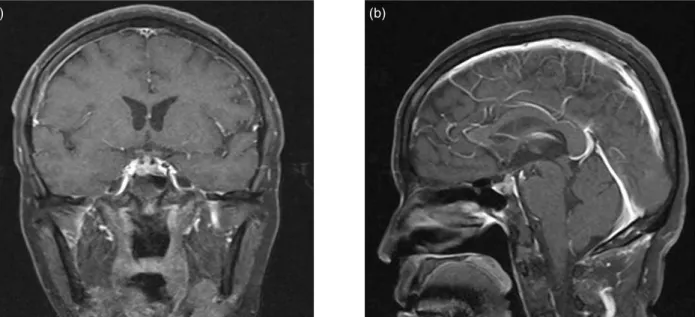
Magnetic resonance imaging (MRI) of the brain revealed poor contrast-enhanced lesion 5mm in the right pituitary gland. This could be of pitu- itary microadenoma or intrasellar metastasis as well as a contrast-enhanced nodular lesion in the pituitary stalk, suggesting metastasis. (Figure. 1a, b). Based upon the clinical symptoms, laboratory results, and radiological findings, the diagnosis of diabetes insipidus without compromising ante- rior pituitary function was caused by a metastasis in the dorsal part of the pituitary gland. She was then treated with nasal spray l-desamino-8-D-argi- nine vasopressin (DDAVP) 20mcg twice daily. The symptoms of polyuria and polydipsia resolved com- pletely under the treatment and local radiotherapy.
Several attempts were made to discontinue DDAVP;
however, the patient experienced a relapse polyuria each time. Twelve months after the diagnosis diabe- tes insipidus, she died of the metastatic disease. Her family declined autopsy.
Figure 1. MRI image of the patient: (a) Coronal image of the Gadolinium-enhanced T1-weighted magnetic reso- nance imaging scan of the brain showed: contrast-enhanced nodular lesion in the pituitary stalk, suggesting metastasis. (b) Sagittal image of the Gadolinium-enhanced T1-weighted magnetic resonance imaging scan of the brain showed: contrast-enhanced nodular lesion in the pituitary stalk, suggesting metastasis.
(a) (b)
Discussion
Diabetes insipidus (DI) is a result of a defi- ciency of vasopressin (ADH) due to a hypotha- lamic-pituitary disorder (central DI [CDI]) or from a resistance of the kidneys to vasopressin (nephro- genic DI [NDI]). Polyuria and polydipsia develop.
CDI must be differentiated from other causes of polyuria, particularly psychogenic polydipsia and NDI. All tests for CDI (and for NDI) are based on the principle that increasing the plasma osmolality in normal people will lead to a decreased excretion of urine with increased urine osmolality. The water deprivation test is the simplest and most reliable method for diagnosing CDI. However it should be done only while the patient is under constant super- vision. A normal response produces maximum urine osmolality after dehydration (often > 1.020 specific gravity or >700 mOsm/L), exceeding the plasma osmolality; osmolality does not increase more than an additional 5% after injections of vasopressin.
Patients with CDI are generally unable to concen- trate urine to greater than the plasma osmolality but are able to increase their urine osmolality by >
50 to >100% after exogenous vasopressin admin- istration. Patients with partial CDI are often able to concentrate urine to above the plasma osmolal- ity but show a rise in urine osmolality of 15 to 50%
after vasopressin administration. Patients with NDI are unable to concentrate urine to greater than the plasma osmolality and show no additional response to vasopressin administration. Owing to high clini- cal suspicion and neutropenic fever, water depriva- tion/vasopressin tests were not undertaken.
Primary diabetes insipidus, due to a defect inherent in the gland itself (no organic lesion), may be familial, occurring as a dominant trait; or, more commonly, sporadic or idiopathic. Secondary dia- betes insipidus is due to destruction of the func- tional unit by trauma, infections (eg, encephalitis, tuberculosis, syphilis, CMV, herpes simplex), breast
cancer or lung cancer (common), vascular accidents (rare), and xanthomatosis (eosinophilic granuloma or Hand-Schuller-Christian disease). Nephrogenic diabetes insipidus is due to a defect in the kidney tubules that interferes with water reabsorption and occurs as an X-linked recessive trait. At times this type is acquired, eg, after pyelonephritis, potassium depletion, or amyloidosis. Certain drugs (eg, dem- eclocycline, lithium) may induce nephrogenic dia- betes insipidus.
The most important differentiation is from the “psychogenic” water-drinking habit. Polydip- sia and polyuria may also be seen in diabetes melli- tus, chronic nephritis, hypokalemia (eg, in primary hyperaldosteronism), and in hypercalcemic states such as hyperparathyroidism.
Pituitary metastases are an uncommon compli- cation of cancer, representing only 1% of the pituitary lesions1,2 and 0.14% to 28% of all brain metastases in reported autopsy series3,4. They are often related to breast or lung cancers, with other sites of primary tumors being reported less often1,2,5,6. According to a recent meta-analysis, it showed breast and lung cancer account for 37.2% and 24.2% of the pituitary metastatic lesions, respectively7. Pituitary metasta- ses are symptomatic in 2.5-18.2% of cases1,2, with the most important signs being diabetes insipi- dus, cranial nerve II deficit, and an anterior pitu- itary insufficiency1. The tumor mass may also cause headaches, and ophthalmoplegia1,2,5,6. Studies, including a recent meta-analysis indicate that DI is the most common clinical manifestation in patients who have pituitary metastasis7. No gender pre- dominance has been reported2. At diagnosis, most patients are elderly in the sixth or seventh decade of life, and have widespread diseases with multiple sites of metastases8,9. Pituitary metastases may also be the first presentation of an occult primary cancer or may be the only site of metastasis4,10-12. They rarely occur in early adulthood13. Metastatic lesion is one of the differential diagnoses. Other causes of
thickened pituitary stalk (TPS) include Langerhans cell histiocytosis, granulomatous disease, germ cell tumor, lymphoma, etc14. Isolated pituitary stalk metastasis accounts for only 2% of pituitary gland metastasis15. An antemortem diagnosis of pituitary stalk metastasis of breast cancer was usually made by MRI without histological confirmation16.
The hematogenous spread (from portal vessels to the pituitary parenchyma or diaphragm sellae) is the most important mechanism for the development of these metastases. Alternative hypotheses for the spread are extension from juxtasellar and skull base metastasis, or a meningeal spread through the supra- sellar cistern1,8,11.
Mc Cormick et al3 reviewed the location of MP in 201 cases and found an involvement of the posterior lobe either alone or in combination with the anterior lobe in 84.6%, whereas the anterior lobe affected only in 15.4%. The predilection for metas- tasis to the posterior lobe is mainly attributed to the lack of direct arterial blood supply of the anterior lobe3,4,9-11,14,15. The posterior lobe is more affected than the anterior lobe, mainly because of the lack of a direct arterial blood supply to the anterior lobe as well as a larger area of contact between the pos- terior lobe and the adjacent dura madre 3,15. For this reason, the most common sign of this metastatic involvement is diabetes insipidus1,2,4,14.
The differential diagnosis of metastases in the hypothalamic pituitary region and pituitary adenoma may be difficult8,14. In contrast to 3.6%
pituitary metastases found in 500 consecutively autopsied patients with cancer, only 1.8% with pituitary adenomas8. However, the general clinical manifestations of metastases in the hypothalamic- pituitary region and differ from pituitary adenoma.
Metastases present with diabetes insipidus or, less commonly, with extra-ocular nerve palsies8,14,17. These symptoms occur in less than 2% of patients with pituitary adenoma18. Thus, diabetes insipidus is the most important criterion for the differentia-
tion of MP from adenomas. Other clinical charac- teristics that raise the suspicion for a pituitary lesion to represent a metastatic lesion rather than a benign adenoma include headaches, visual field deficits due to cranial nerve palsies (especially abducens nerve palsy), presence of skull-bone destruction, demonstration of rapid growth on serial scans, and presence of coexisting focal lesions (metastasis)19. Hormonal assay to be most commonly affected is high prolactin levels, which is however the case in both metastatic lesions and macroadenomas; hence does not help distinguish the two19.
Management of these patients may also be very difficult because the prognosis depends on the stages of the primary neoplasm. Treatments, being basically palliative, depends on the symp- toms and the extent of the systemic disease1,3,11,20,21. Reports of the surgery of MPs indicate that the lesions tend to be firm, diffuse, invasive, vascular, and hemorrhagic. To that, therefore the total resec- tion is unlikely4,9,20,21. In this setting, local radia- tion and/or chemotherapy are recommended for the initial treatment, especially in patients with wide- spread metastases, and in combination with pitu- itary hormone substitution therapy3,4,9,20,21. Surgical exploration and decompression, alone or combined with radiation, is essential if clarification of diag- nosis is likely to affect the therapy or if suprasel- lar extension causes progressive deterioration in vision or pain3,4,9,12,14,20,21. Surgery and radiation are well tolerated in noncompromised patients, being associated with low morbidity and minimal complications11,14. Tumor debulking is beneficial in alleviating the local symptoms, especially head- aches and visual field defect, whereas symptoms such as diplopia, and those related to anterior pitu- itary failure remain unaffected11,14,21-22. The transs- phenoidal approach, completeness of resection, and aggressive treatment (surgery plus local radiation) are associated with better symptom relief but do not affect the survival rates11,22.
In patients reported in the literature, diabe- tes insipidus was usually irreversible after only and anti-tumour therapy only16. The survival of the patients with dural and posterior pituitary metas- tases is better than parenchymal brain metastases or leptomeningeal carcinomatosis14. Prognosis of patients with MPs is still poor, not because of the location per se but because of the aggressiveness of the primary cancers23. Mean survival length in clin- ical series is about 6–7 months5,9,11. Ntyonga-Pono et al12 reviewed 72 patients from the literature, and found that only 10% of patients survived more than 1 year after the diagnosis, with the longest survival being 3 years. Patients with a single MP may have a better outcome5,11. Age over 65 years at presen- tation, metastasis from small-cell lung carcinoma, and short duration (<1 year) between initial diag- nosis of cancer and pituitary invasion have been related to a poorer outcome11. In conclusion, patients with cancers who have symptoms such as polyuria, nocturia and polydipsia should be evaluated for not only metabolic complications like hyperglycemia, hypercalcemia, but also for metastasis of the poste- rior pituitary gland. MRI should be the first choice of imaging for pituitary metastasis. In the context of malignancy, the emergence of CDI should alert the clinician, for the possibility of pituitary’s metastasis.
References
1. Komninos J, Vlassopoulou V, Protopapa D, et al. Tumors met- astatic to the pituitary gland: case report and literature review.
J Clin Endocrinol Metab 2004; 89: 574-80.
2. Fassett DR, Couldwell WT. Metastases to the pituitary gland.
Neurosurg Focus 2004; 16: E8.
3. McCormick PC, Post KD, Kandji AD, et al. Metastatic carci- noma to the pituitary gland. Br J Neurosurg 1989; 3: 71-9.
4. Sioutos P, Yen V, Arbit E. Pituitary gland metastases. Ann Surg Onco 1996; 3: 94-9.
5. Bhatoe HS, Badwal S, Dutta V, et al.Pituitary metastasis from medullary carcinoma of thyroid: case report and review of lit- erature. J Neurooncol 2008; 89: 63-7.
6. Hoellig A, Niehusmann P, Flacke S, et al. Metastasis to pitu- itary adenoma: case report and review of the literature. Cen Eur Neurosurg 2009; 70: 149-53.
7. He W, Chen F, Dalm B, et al. Metastatic involvement of the pituitary gland: A systematic review with pooled individual patient data analysis. Pituitary 2015; 18: 159-68.
8. Max MB, Deck MD, Rottenberg DA. Pituitary metastasis:
incidence in cancer patients and clinical differentiation from pituitary adenoma. Neurology 1981; 31: 998-1002.
9. Houck WA, Olson KB, Horton J. Clinical features of tumor metastasis to the pituitary. Cancer 1970; 26: 656-9.
10. Suganuma H, Yoshimi T, Kita T, et al. Rare case with meta- static involvement of hypothalamo-pituitary and pineal body presenting as hypopituitarism and diabetes insipidus. Intern Med 1994; 33: 795-8.
11. Morita A, Meyer FB, Laws ER. Symptomatic pituitary metas- tases. J Neurosurg 1998; 89: 69-73.
12. Ntyonga-Pono MP, Thomopoulos P, Luton JP. Pituitary metas- tases. Three cases. Presse Med 1999; 28: 1567-71.
13. Ito I, Ishida T, Hashimoto T, et al. Hypopituitarism due to pitu- itary metastasis of lung cancer: case of a 21-year old man.
Intern Med 2001; 40: 414-7.
14. Branch CL, Laws ER. Metastatic tumors of the sella turcica masquerading as primary pituitary tumors. J Clin Endocrinol Metab 1987; 65: 469-74.
15. Teears RJ, Silverman EM. Clinicopathologic review of 88 cases of carcinoma metastatics to the putuitary gland. Cancer 1975; 36: 216-20.
16. Huinink DT, Veltman GA, Huizinga TW, et al. Diabetes insip- idus in metastatic cancer : two case reports with review of the literature. Ann Oncol 2000; 11: 891-5.
17. Roessmann V, Kaufman B, Friede RL. Metastatic lesions in the sella tunica and pituitary gland. Cancer 1970; 25: 478-80.
18. Hollenhorst RW, Younge BR. Ocular manifestations produced by adenomas of the pituitary gland: Analysis of 1000 cases. In Kohler PO, Ross GT (eds): Diagnosis and Treatment of Pitui- taryTumors. Amsterdam: Excerpta Medica 1973; 53-64.
19. Al-Aridi R, El Sibai K, Fu P, et al. Clinical and biochemical characteristic features of metastatic cancer to the sella turcica:
An analytical review. Pituitary 2014; 17: 575-87.
20. Ruelle A, Palladino M, Andrioli GC. Pituitary metastases as presenting lesions of malignancy. J Neurosurg Sci 1992; 36:
51-4.
21. Nelson PB, Robinson AG, Martinez AJ. Metastatic tumor of the pituitary gland. Neurosurgery 1987; 21: 941-4.
22. Pinet C, Raholimina V, Ferri RM, et al. Panhypopituitarism secondary to pituitary metastases. Presse Med 2000; 29: 17-8.
23. Delattre JY, Castelain C, Davila L,et al. Metastasis to the pitu- itary stalk in a case of breast cancer. Rev Neurol (Paris) 1990;
146: 455-6.
乳癌病人因轉移至腦垂腺所導致尿崩症之個案報告
陳威宇1 陳建宇2
奇美醫學中心 血液腫瘤科1 放射診斷科2
摘 要
我們在此報告一個乳癌轉移患者,在接受首次化療後,因發燒而注意到病人有多尿以及 多渴之情形。在經過一系列檢查之後診斷為尿崩症,核磁共振檢查後發現腦垂腺轉移,在經 過藥物治療以及放射線治療之後,病人尿崩症狀完全受到控制。