The ne w engl and
journal of medicine
e s ta b l i s h e d i n 1 8 1 2 j u n e3 0, 2 0 0 5 v o l . 3 5 2 n o . 2 6
Long-Term Therapy with Adefovir Dipivoxil for HBeAg-Negative Chronic Hepatitis B
Stephanos J. Hadziyannis, M.D., Nicolaos C. Tassopoulos, M.D., E. Jenny Heathcote, M.D.,
Ting-Tsung Chang, M.D., George Kitis, M.D., Mario Rizzetto, M.D., Patrick Marcellin, M.D., Seng Gee Lim, M.D., Zachary Goodman, M.D., Jia Ma, M.S., Sarah Arterburn, M.S., Shelly Xiong, Ph.D., Graeme Currie, Ph.D.,
and Carol L. Brosgart, M.D., for the Adefovir Dipivoxil 438 Study Group*
a b s t r a c t
From the Department of Medicine and Hepatology, Henry Dunant Hospital (S.J.H.), and Western Attica General Hos- pital (N.C.T.) — both in Athens; Toronto Western Hospital, University of Toronto, Toronto (E.J.H.); the Department of Inter- nal Medicine, National Chen Kung Uni- versity Hospital, Tainan, Taiwan (T.-T.C.);
Georgios Papanikolaou Hospital, Thessa- loniki, Greece (G.K.); Azienda Osped- aliera San Giovanni Battista, Turin, Italy (M.R.); Service d’Hepatologie, INSERM Unité 481; Centre de Recherche Claude Bernard sur les Hepatites Virales, Hôpital Beaujon, Clichy, France (P.M.); the Division of Gastroenterology, National University Hospital, Singapore (S.G.L.); the Armed Forces Institute of Pathology, Washington, D.C. (Z.G.); and Gilead Sciences, Foster City, Calif. (J.M., S.A., S.X., G.C., C.L.B.).
Address reprint requests to Dr. Hadziyan- nis at the Department of Medicine, Henry Dunant Hospital, 107 Mesogion Ave., Ath- ens 11526, Greece, or at hadziyannis@
ath.forthnet.gr.
*Other members of the Adefovir Dipivox- il 438 Study Group are listed in the Ap- pendix.
N Engl J Med 2005;352:2673-81.
Copyright © 2005 Massachusetts Medical Society.
b a c k g r o u n d
Treatment with adefovir dipivoxil for 48 weeks resulted in histologic, virologic, and bio- chemical improvement in patients with hepatitis B e antigen (HBeAg)–negative chronic hepatitis B. We evaluated the effect of continued therapy as compared with cessation of therapy.
m e t h o d s
One hundred eighty-five HBeAg-negative patients with chronic hepatitis B were assigned to receive 10 mg of adefovir dipivoxil or placebo once daily for 48 weeks (ratio, 2:1). After week 48, patients receiving adefovir dipivoxil were again randomly assigned either to receive an additional 48 weeks of the drug or to switch to placebo. Patients originally assigned to placebo were switched to adefovir dipivoxil. Patients treated with adefovir dipivoxil during weeks 49 through 96 were subsequently offered continued therapy.
The primary end points were changes in hepatitis B virus (HBV) DNA and alanine ami- notransferase levels.
r e s u l t s
Treatment with adefovir dipivoxil resulted in a median decrease in serum HBV DNA of 3.47 log copies per milliliter (on a base-10 scale) at 96 weeks and 3.63 log copies per milliliter at 144 weeks. HBV DNA levels were less than 1000 copies per milliliter in 71 percent of patients at week 96 and 79 percent at week 144. In the majority of patients who were switched from adefovir dipivoxil to placebo, the benefit of treatment was lost (median change in HBV DNA levels from baseline, ¡1.09 log copies per milliliter; only 8 percent of patients had levels below 1000 copies per milliliter at week 96). Side effects during weeks 49 through 144 were similar to those during the initial 48 weeks. Resis- tance mutations rtN236T and rtA181V were identified in 5.9 percent of patients after 144 weeks.
c o n c l u s i o n s
In patients with HBeAg-negative chronic hepatitis B, the benefits achieved from 48 weeks of adefovir dipivoxil were lost when treatment was discontinued. In patients treated for 144 weeks, benefits were maintained, with infrequent emergence of viral resistance.
The n e w e n g l a n d j o u r n a l of m e d i c i n e
n estimated 400 million people worldwide are chronically infected with hepatitis B virus (HBV). One million die each year from complications of infection, includ- ing cirrhosis, hepatocellular carcinoma, or both.1 Hepatitis B e antigen (HBeAg)–negative chronic hepatitis B represents a late phase in the course of HBV infection.2 Mutations in the precore promoter regions, core promoter regions, or both, which pre- vent the formation of HBeAg, are selected during or after HBeAg loss and seroconversion to antibody to HBeAg (anti-HBe). HBeAg-negative chronic hepa- titis B infection is characterized by intermittent pe- riods of exacerbation and quiescence. It frequently follows an aggressive disease course, with low rates of spontaneous recovery.2-4 Epidemiologic data sug- gest that the median prevalence of HBeAg-negative chronic hepatitis B varies considerably, ranging from 14 percent in the United States and Northern Europe to more than 33 percent in the Mediterra- nean area, with an increasing prevalence world- wide.3
Current therapeutic options include treatment with interferon alfa, lamivudine, and adefovir dipiv- oxil. The goal of treatment is HBV DNA suppres- sion, normalization of alanine aminotransferase levels, and reduction in liver necroinflammation.
Longer-term objectives include the prevention of cir- rhosis, end-stage liver disease, hepatocellular car- cinoma, or all of these. It is unknown whether treat- ment can be stopped or whether long-term therapy is needed.5
A one-year regimen of lamivudine has been shown to achieve virologic and biochemical re- sponses.6-8 However, continued therapy results in resistance in approximately 20 percent of patients per year in most studies.9 Interferon alfa and pegy- lated interferon have also shown efficacy; however, the durability of the response after the cessation of treatment is uncertain.8,10-12
In an earlier 48-week, placebo-controlled phase of this study, adefovir dipivoxil, as compared with placebo, resulted in significant histologic improve- ment (in 64 percent of patients vs. 33 percent, re- spectively), biochemical improvement (normaliza- tion of alanine aminotransferase levels, 72 percent vs. 29 percent), and virologic improvement (median reduction in HBV DNA, 3.91 log copies per millili- ter [on a base-10 scale] vs. 1.35 log copies per mil- liliter); no resistance developed in patients treated with adefovir dipivoxil.13,14 Here, we report the out-
comes associated with stopping or continuing treat- ment with adefovir dipivoxil during a second 48- week randomized, controlled period; we also pro- vide long-term data on treatment with this agent over 144 weeks.
Between January 10 and June 7, 2000, 185 patients were enrolled and 184 treated in this internation- al, multicenter, prospective, double-blind, placebo- controlled trial. Patients were randomly assigned to receive 10 mg of adefovir dipivoxil or placebo orally once daily for 48 weeks in a ratio of 2:1. Af- ter week 48, 123 patients who had been assigned to adefovir dipivoxil were randomly assigned ei- ther to continue with adefovir dipivoxil (the con- tinued-adefovir group; 80 patients) or to switch to placebo (the adefovir–placebo group; 40 patients) for an additional 48 weeks. Three patients did not receive treatment during the second 48 weeks. Of the 61 patients who initially received placebo, 60 received adefovir dipivoxil in this second 48- week period (the placebo–adefovir group). Patients who received adefovir dipivoxil in the second 48 weeks were eligible to continue treatment until week 240.
Liver biopsies were required within six months or immediately before treatment and at week 48.
A liver biopsy was optional at weeks 96 and 144.
Serum HBV DNA and alanine aminotransferase were measured and blood chemistry assessments were conducted every 4 weeks until week 96 and then every 12 weeks.
Clinical data were monitored and entered into a database by Quintiles, a contract research organiza- tion. A central reference laboratory (Covance Labo- ratories) assessed all laboratory data. Gilead Sci- ences held all data and conducted the statistical analyses. The academic investigators had full access to the data. Each author contributed to the study de- sign, the interpretation of the results, and the drafts and revisions of the manuscript; all authors had in- put into and approved the final manuscript. Drs.
Hadziyannis, Tassopoulos, Heathcote, Chang, Kitis, Rizzetto, Marcellin, Lim, and Goodman vouch for the veracity and completeness of the data and the data analysis. The study was conducted in compli- ance with the 1975 Declaration of Helsinki and ap- proved by local regulatory bodies. All patients pro- vided written informed consent.
a
m e t h o d s
l o n g - t e r m a d e f o v i r t h e r a p y f o r c h r o n i c h e p a t i t i s b
p a t i e n t s
Full inclusion criteria have been described previous- ly.13 Key criteria included HBeAg-negative, anti- HBeAg–positive status and the presence of compen- sated liver disease, detectable hepatitis B surface antigen (HBsAg) for at least six months, a serum HBV DNA level of at least 100,000 copies per milli- liter on polymerase chain reaction (as measured with the Roche Amplicor Monitor; lower limit of detection, 1000 copies per milliliter, previously 400 copies per milliliter13), and a serum alanine amino- transferase level between 1.5 and 15 times the upper limit of normal.
a s s e s s m e n t o f e f f i c a c y
Primary efficacy end points were the changes from baseline in serum HBV DNA and alanine amino- transferase levels at week 96. Other efficacy end points included the percentage of patients in whom HBV DNA fell below the limit of detection of the as- say, the percentage in whom alanine aminotransfer- ase levels returned to normal, and the percentage in whom there was HBsAg seroconversion (i.e., loss of HBsAg and gain of antibody to HBsAg). End points were evaluated at weeks 96 and 144. In a subgroup of patients, histologic features of liver specimens were evaluated by an independent histopathologist (with the use of both Knodell and Ishak scoring sys- tems, which evaluate necroinflammation and liver fibrosis on scales of 0 to 22 [Knodell] and 0 to 24 [Ishak], with higher scores indicating greater sever- ity) who was blinded to patients’ treatment assign- ments and the date on which the biopsy specimens were obtained.15 Ranked assessment of inflamma- tion and fibrosis was also performed, with severity delineated as improved, no change, or worse as compared with the baseline scores.
a s s e s s m e n t o f s a f e t y
Safety was assessed with the use of laboratory tests and by the reported occurrence of adverse events every 4 weeks for the first 96 weeks and then every 12 weeks. All patients who received at least one dose of adefovir dipivoxil were included in the safety analysis.
r e s i s t a n c e s u r v e i l l a n c e
Genotypic analyses of HBV DNA polymerase mu- tations were performed on serum samples from pa- tients with HBV DNA levels of more than 1000 cop- ies per milliliter in the 123, 134, and 70 patients who received adefovir dipivoxil through weeks 48, 96,
and 144, respectively. The HBV reverse transcriptase (rt) domain (amino acids rt1 to rt344) was se- quenced. Sequences at baseline and after baseline were aligned with the use of the MegAlign program (DNAStar).
s t a t i s t i c a l a n a l y s i s
Statistical analyses included all patients who re- ceived at least one dose of the study drug in the sec- ond 48 weeks. All HBV DNA values less than the lower limit of detection (1000 copies per milliliter) were assigned a value of 999 copies per milliliter.
All tests for significance and resulting P values were two-sided, with a level of significance of 0.05.
c h a r a c t e r i s t i c s o f t h e p a t i e n t s
A total of 180 patients were randomly assigned to re- ceive treatment in the second 48 weeks of the study.
Of these patients, 79 continued to receive adefovir dipivoxil, 40 initially assigned to adefovir dipivoxil received placebo, and 60 were switched from place- bo to adefovir dipivoxil. One patient who had been randomly assigned to the adefovir dipivoxil group withdrew from the study before taking medication in the second 48 weeks. At week 96, 125 patients continued to receive adefovir dipivoxil — 70 in the continued-adefovir group and 55 in the placebo–
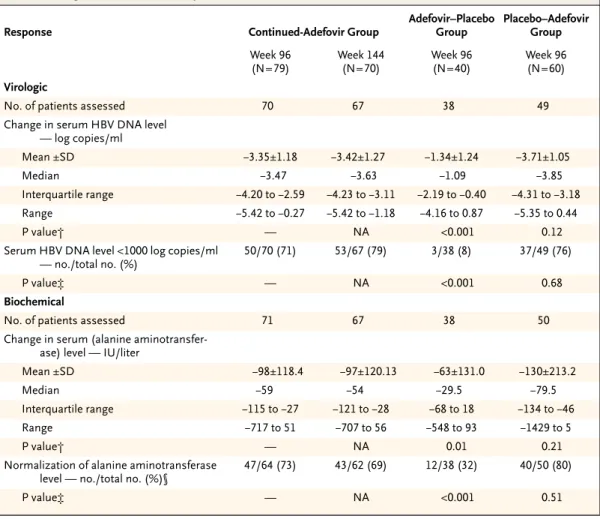
adefovir group. Data are reported up to week 144 for patients who received adefovir dipivoxil from baseline. Baseline demographic characteristics and those related to hepatitis B infection were not statis- tically different among the three groups (Table 1).
v i r o l o g i c r e s p o n s e
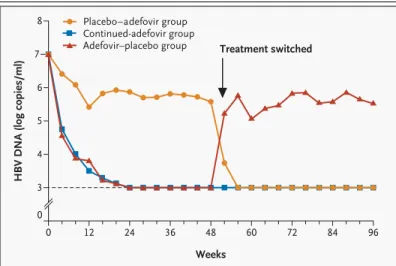
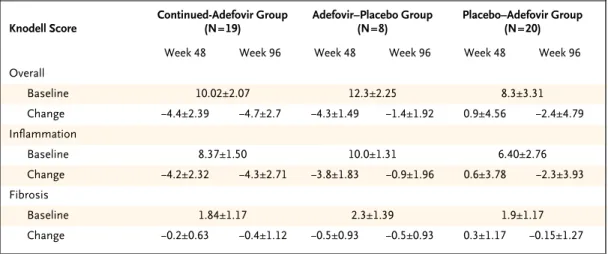
At week 96, serum HBV DNA levels had decreased by a median of 3.47 log copies per milliliter in the continued-adefovir group, as compared with 1.09 log copies per milliliter in the adefovir–placebo group (P<0.001) (Table 2). Undetectable levels of HBV DNA were reported in 71 percent of patients in the continued-adefovir group, as compared with 76 percent and 8 percent, respectively, in the pla- cebo–adefovir and adefovir–placebo groups. There was a rapid reduction in serum HBV DNA levels in patients in the continued-adefovir group, with per- sistent reductions up to week 96. In contrast, the adefovir–placebo group had a rebound in serum HBV DNA levels, with a return to baseline levels within four weeks of the discontinuation of adefo- vir dipivoxil in the majority of patients (Fig. 1).
r e s u l t s
The n e w e n g l a n d j o u r n a l of m e d i c i n e
In the patients who continued adefovir dipivoxil to week 144, HBV DNA levels remained suppressed at week 144 (median reduction in HBV DNA from baseline, 3.63 log copies per milliliter). In 79 percent of these patients, serum HBV DNA levels were less than 1000 copies per milliliter at week 144.
s e r o l o g i c r e s p o n s e
HBsAg seroconversion (i.e., the loss of HBsAg and gain of anti-HBs) occurred in two patients, one in the continued-adefovir group at week 72 and one in the placebo–adefovir group at week 68 (approxi- mately 20 weeks after the start of adefovir dipivoxil).
b i o c h e m i c a l r e s p o n s e
Median reductions in serum alanine aminotransfer- ase levels at week 96 were 59 IU per liter in the con- tinued-adefovir group, as compared with 29.5 IU per liter in the adefovir–placebo group (P=0.01), and 79.5 IU per liter in the placebo–adefovir group (Table 2). At week 96, a return to normal levels of alanine aminotransferase (upper limit of normal, 37 IU per liter for women and 43 IU per liter for men) was achieved in 73 percent of patients in the contin- ued-adefovir group, 80 percent in the placebo–
adefovir group, and 32 percent in the adefovir–
placebo group. Patients in the continued-adefovir group had sustained suppression of alanine amino- transferase throughout the study. In contrast, ala- nine aminotransferase levels returned to pretreat- ment values or higher in the majority of patients in the adefovir–placebo group within eight weeks of stopping therapy. In 32.5 percent of patients, ala- nine aminotransferase levels rose sharply — to more than 10 times the upper limit of normal — before returning to baseline levels. None of these elevations were associated with clinical hepatic de- compensation. In the patients who continued to re- ceive adefovir dipivoxil to week 144, alanine ami- notransferase levels remained suppressed, with normalization in 69 percent of patients.
h i s t o l o g i c r e s p o n s e
A subgroup of 47 patients underwent liver biopsy at week 96. Baseline demographic and disease char- acteristics of these patients were similar to those of patients in the overall study population. Patients in the continued-adefovir group had a mean reduction of 4.7 points from baseline in the overall Knodell score at week 96 (Table 3) (a mean reduction of 4.4 points at week 48). Among patients in the placebo–
adefovir group, there was a mean increase of 0.9 points from baseline at week 48, with a subsequent reduction after the crossover to adefovir dipivoxil of 2.4 points from baseline at week 96, a reversal of the increase observed at week 48. In the adefovir–
placebo group, there was a loss of improvement at week 48, with a median reduction of 1 point from baseline at week 96.
* Race was self-assigned.
† Values were log-transformed with the use of a base-10 scale.
‡ ULN denotes upper limit of the normal range; for men, the level was 43 IU per liter, and for women 34 IU per liter.
§ Some patients had received more than one type of medication.
¶ Lamivudine had been used less than 12 weeks previously in these patients.
Table 1. Baseline Characteristics of the Patients.
Characteristic
Continued- Adefovir Group
(N=79)
Adefovir–
Placebo Group (N=40)
Placebo–
Adefovir Group (N=60) Age — yr
Mean ±SD 46±10 46±9.9 46±10.2
Median 47 47 46
Range 26–65 18–64 22–65
Male sex — no. (%) 65 (82) 33 (82) 50 (83)
Race or ethnic background
— no. (%)*
White 55 (70) 26 (65) 39 (65)
Black 4 (5) 1 (2) 1 (2)
Asian 20 (25) 13 (32) 20 (33)
Weight — kg
Mean ±SD 75±12.2 77±10.0 74±15.2
Median 75 76 74
Range 50–111 60–105 46–135
HBV DNA level — log copies/ml†
Mean ±SD 6.87±0.86 7.03±0.78 6.91±0.94
Median 7.07 7.16 7.05
Range 3.67–8.42 5.28–8.77 4.42–8.45
Alanine aminotransferase level — IU/liter
Mean ±SD 140±120.7 152±140.4 149±196.8
Median 98 86 99
Range 24–742 45–657 29–1459
≤ULN — no. (%)‡ 7 (9) 0 2 (3)
>ULN — no. (%) 72 (91) 40 (100) 58 (97)
Positive for HBsAg
— no. (%)
79 (100) 40 (100) 60 (100)
Prior medications for HBV
— no. (%)§
Interferon alfa 30 (38) 18 (45) 27 (45)
Lamivudine¶ 7 (9) 3 (8) 4 (7)
l o n g - t e r m a d e f o v i r t h e r a p y f o r c h r o n i c h e p a t i t i s b
In the ranked assessment of inflammatory activ- ity, the comparison of scores at baseline and week 96 in the continued-adefovir group showed improve- ment in 17 of 19 patients (89 percent) and no change in 2 of 19 patients (11 percent); in no patients did in- flammation worsen. In the placebo–adefovir group, 14 of 20 patients (70 percent) had improvement, 2 of 20 (10 percent) had no change, and 4 of 20 (20 percent) had a worsening. In the adefovir–placebo group, four of eight patients (50 percent) had im- provement, two of eight (25 percent) had no change, and two of eight (25 percent) had a worsening. Im-
provements were also seen in fibrosis, with patients in the continued-adefovir group having significant reductions from baseline in the Ishak fibrosis score at week 96 (mean [±SD] reduction, 0.63±1.07; me- dian reduction, 1; P=0.031, as compared with the adefovir–placebo group). The improvements in fi- brosis at weeks 48 and 96 were extended in patients who underwent a biopsy at week 144.
r e s i s t a n c e p r o f i l e
A conserved site mutation (rtN236T) was identified in three patients in the continued-adefovir group,
* Negative values indicate a decrease, and positive values an increase. NA denotes not applicable.
† P values were calculated with the use of the Wilcoxon rank-sum test for the comparison between continued treatment with adefovir dipivoxil and the crossover from adefovir dipivoxil to placebo and for the comparison between continued treatment with adefovir dipivoxil and the crossover from placebo to adefovir dipivoxil at week 96. All P values are two-sid- ed, with a level of 0.05 indicating statistical significance; there were no adjustments for multiple comparisons.
‡ Fisher’s exact test was used for the comparison between continued treatment with adefovir dipivoxil and the crossover from adefovir dipivoxil to placebo and for the comparison between continued treatment with adefovir dipivoxil and the crossover from placebo to adefovir dipivoxil at week 96.
§ Patients with baseline alanine aminotransferase levels that exceeded the upper limit of the normal range were included in the analysis.
Table 2. Virologic and Biochemical Responses at Weeks 96 and 144.*
Response Continued-Adefovir Group
Adefovir–Placebo Group
Placebo–Adefovir Group Week 96
(N=79)
Week 144 (N=70)
Week 96 (N=40)
Week 96 (N=60) Virologic
No. of patients assessed 70 67 38 49
Change in serum HBV DNA level
— log copies/ml
Mean ±SD ¡3.35±1.18 ¡3.42±1.27 ¡1.34±1.24 ¡3.71±1.05
Median ¡3.47 ¡3.63 ¡1.09 ¡3.85
Interquartile range ¡4.20 to ¡2.59 ¡4.23 to ¡3.11 ¡2.19 to ¡0.40 ¡4.31 to ¡3.18
Range ¡5.42 to ¡0.27 ¡5.42 to ¡1.18 ¡4.16 to 0.87 ¡5.35 to 0.44
P value† — NA <0.001 0.12
Serum HBV DNA level <1000 log copies/ml
— no./total no. (%)
50/70 (71) 53/67 (79) 3/38 (8) 37/49 (76)
P value‡ — NA <0.001 0.68
Biochemical
No. of patients assessed 71 67 38 50
Change in serum (alanine aminotransfer- ase) level — IU/liter
Mean ±SD ¡98±118.4 ¡97±120.13 ¡63±131.0 ¡130±213.2
Median ¡59 ¡54 ¡29.5 ¡79.5
Interquartile range ¡115 to ¡27 ¡121 to ¡28 ¡68 to 18 ¡134 to ¡46
Range ¡717 to 51 ¡707 to 56 ¡548 to 93 ¡1429 to 5
P value† — NA 0.01 0.21
Normalization of alanine aminotransferase level — no./total no. (%)§
47/64 (73) 43/62 (69) 12/38 (32) 40/50 (80)
P value‡ — NA <0.001 0.51
The n e w e n g l a n d j o u r n a l of m e d i c i n e
two at week 96 and one at week 144. The emergence of rtN236T was associated with a rebound in serum HBV DNA and alanine aminotransferase levels. In vitro susceptibility testing demonstrated a reduction in susceptibility to adefovir that was 3.9 to 13.8 times that of wild-type virus. One patient was switched to lamivudine at week 104; HBV DNA levels, as evalu- ated by the Digene assay (lower limit of detection, 150,000 copies per milliliter), became undetectable, and serum alanine aminotransferase levels were normal after six months.16 Subsequently, resistance to lamivudine developed in this patient; adefovir dipivoxil was restarted, and serum HBV DNA levels again became undetectable.
Another conserved site substitution mutation (rtA181V) in the B domain of HBV polymerase was seen in three additional patients in the continued- adefovir group, two at week 96 and one at week 144.
A rebound in HBV DNA levels occurred in two of the three patients. In vitro susceptibility testing dem- onstrated a reduction in susceptibility that was 2.5 to 3 times that of wild-type virus. For one patient with rtA181V, lamivudine was added to ongoing adefovir therapy; serum HBV DNA levels subse- quently were reduced by more than 2 log copies per milliliter.
Of the six patients in whom resistance developed, four had a reduced response to adefovir dipivoxil (serum HBV DNA reduction from baseline, <2.5 log copies per milliliter). The disease characteristics of these patients at baseline were similar to those of
the overall patient population. The overall cumula- tive rate of resistance to adefovir dipivoxil among all patients at 48, 96, and 144 weeks was 0 percent, 3 percent, and 5.9 percent, respectively.
s a f e t y
Adverse events during weeks 49 to 96 were similar in severity, nature, and frequency to those during the initial 48-week treatment period. At least one ad- verse event was reported in 58 of 79 patients (73 per- cent) in the continued-adefovir group, 41 of 60 pa- tients (68 percent) in the placebo–adefovir group, and 32 of 40 (80 percent) in the adefovir–placebo group. The most common adverse events reported in the continued-adefovir group were headache, ab- dominal pain, and pharyngitis (Table 4).
The study drug was discontinued because of ad- verse events in two patients in the continued-adefo- vir group (a protocol-defined increase in serum cre- atinine levels of ≥0.5 mg per deciliter [44.2 µmol per liter] and hepatocellular carcinoma) and in three pa- tients in the adefovir–placebo group (jaundice, ele- vated alanine aminotransferase levels, and a skin disorder).
No notable differences were seen in laboratory values from week 48, with the exception of increas- es in alanine aminotransferase levels associated with the withdrawal of adefovir dipivoxil therapy. In the adefovir–placebo group, 13 patients (32.5 percent) had alanine aminotransferase levels that were 10 times the upper limit of normal or higher. Elevations of alanine aminotransferase levels were observed in 6 percent of patients who continued to receive adef- ovir dipivoxil over 96 weeks. No patients had clin- ical signs of decompensation or required the inter- vention of an investigator. Of the 13 patients with elevations of alanine aminotransferase levels, 10 had an increase within 12 weeks after the cessation of adefovir dipivoxil therapy.
There were no overall changes in serum creati- nine and phosphorus levels. Two patients in the con- tinued-adefovir group had a confirmed increase in serum creatinine levels of 0.5 mg per deciliter or more from baseline. In one case, the highest value remained within the normal range and resolved with continued treatment. In the other case, the highest value was 2.3 mg per deciliter (203.3 µmol per liter), which returned to normal after discon- tinuation of the study drug. One additional patient in year 3 had a confirmed serum creatinine increase that returned to baseline within eight weeks after the cessation of adefovir dipivoxil. The safety pro-
Figure 1. Median Serum HBV DNA Levels through Week 96.
The dashed line indicates 3 log copies per milliliter, which was the lower limit of detection for the assay.
HBV DNA (log copies/ml)
Weeks
Treatment switched
6
5
4
3
0
0 12 24 36 48 60 72 84 96
Placebo–adefovir group Continued-adefovir group Adefovir–placebo group 8
7
l o n g - t e r m a d e f o v i r t h e r a p y f o r c h r o n i c h e p a t i t i s b
file over 144 weeks remained consistent with that seen earlier in the study.
As shown in other studies, treatment of HBeAg- negative chronic hepatitis B with lamivudine effec- tively suppresses HBV replication and results in bio- chemical remission and histologic improvement in more than two thirds of patients.7,8,13 However, re- lapse has occurred in the majority of HBeAg-nega- tive patients after the cessation of therapy.8,17 Sim- ilarly, in this study, when treatment with adefovir dipivoxil was discontinued, the virologic, biochem- ical, and histologic benefits that had been gained in the first 48 weeks were lost. This finding suggests that because HBsAg seroconversion is rare,2,4,11 long-term therapy will be needed in the majority of patients. Post-treatment flares in serum alanine aminotransferase levels were seen after therapy was stopped. Although these events were self-limiting in this study, it is important to monitor patients care- fully after discontinuation of treatment with adef- ovir dipivoxil.8,18
To ensure a favorable risk–benefit profile, any treatment regimen must provide durable efficacy and limited toxicity, with minimal or no emergence of viral resistance. The development of viral resis- tance over time with the use of lamivudine, which is associated with a loss of clinical response, is com-
mon and may become serious in patients with ad- vanced disease.18 In another study, peginterferon therapy produced a sustained response in terms of normalization of alanine aminotransferase levels for up to 24 weeks after treatment was stopped, and 19 percent of patients had undetectable HBV DNA lev- els at week 24 of follow-up. However, further fol- low-up is required to see if this response will be sus- tained.19
Our study demonstrated that with prolonged therapy, adefovir dipivoxil brought about increasing and persistent virologic, biochemical, and histolog- ic responses, with delayed and infrequent devel- opment of resistance. Among patients who began adefovir dipivoxil in the second 48 weeks, undetect- able HBV DNA levels and normalization of alanine aminotransferase levels were achieved in a signifi- cant proportion of patients. However, comparisons of this subgroup of patients with those treated for 96 weeks should be made cautiously, since differenc- es existed in baseline characteristics at the initiation of treatment with adefovir dipivoxil. Our results also suggest that an additional histologic benefit may occur with extended treatment, whereas cessation of treatment results in a reversal of improvement.
The adverse events associated with extended treatment with adefovir dipivoxil were similar in na- ture, severity, and frequency to those observed over the previous 48 weeks. Although increases in serum creatinine levels have previously been seen with d i s c u s s i o n
* Plus–minus values are means ±SD. Negative values indicate a decrease, and positive values an increase. Patients includ- ed those for whom biopsy specimens could be assessed at baseline, week 48, and week 96. Baseline values were mea- sured before the first 48 weeks of treatment. Knodell scores (ranging from 0 to 22) evaluate necroinflammation and liver fibrosis. A lowering of scores from the initial biopsy indicates histologic improvement, and an increase indicates histo- logic worsening.
Table 3. Changes from Baseline in Knodell Scores at Weeks 48 and 96.*
Knodell Score
Continued-Adefovir Group (N=19)
Adefovir–Placebo Group (N=8)
Placebo–Adefovir Group (N=20)
Week 48 Week 96 Week 48 Week 96 Week 48 Week 96
Overall
Baseline 10.02±2.07 12.3±2.25 8.3±3.31
Change ¡4.4±2.39 ¡4.7±2.7 ¡4.3±1.49 ¡1.4±1.92 0.9±4.56 ¡2.4±4.79
Inflammation
Baseline 8.37±1.50 10.0±1.31 6.40±2.76
Change ¡4.2±2.32 ¡4.3±2.71 ¡3.8±1.83 ¡0.9±1.96 0.6±3.78 ¡2.3±3.93
Fibrosis
Baseline 1.84±1.17 2.3±1.39 1.9±1.17
Change ¡0.2±0.63 ¡0.4±1.12 ¡0.5±0.93 ¡0.5±0.93 0.3±1.17 ¡0.15±1.27
The n e w e n g l a n d j o u r n a l of m e d i c i n e
higher daily doses (>30 mg), the risk is low with a daily dose of 10 mg.
The findings of this study raise two important questions: When should treatment be initiated, and when is it safe to stop? In view of the progressive course of HBeAg-negative chronic hepatitis B1,3 and the progression of liver damage in patients who re- ceived placebo for 48 weeks in this study, it is rea- sonable to suggest that treatment should not be de- layed. However, long-term therapy will be needed for the majority of patients. Therefore, there are sev-
eral important factors to be weighed before treat- ment is begun: the patient’s age, the severity of liver disease, the risk of disease progression, the risk of resistance, the likelihood of compliance, and the costs associated with long-term therapy.
Treatment with adefovir dipivoxil for 144 weeks resulted in continuing benefits in terms of viral sup- pression, normalization of biochemical measures, and histologic improvement. These benefits were associated with a delayed and infrequent emergence of resistance, making adefovir dipivoxil an excellent
* The most common adverse events are those that occurred in 10 percent or more of the patients in any treatment group.
† Fisher’s exact test was used for the comparison with the placebo–adefovir group (P<0.05).
‡ Fisher’s exact test was used for the comparison with the adefovir–placebo group (P<0.05).
Table 4. Proportion of Patients with the Most Common Adverse Events and Renal Events.*
Event Week 49 to Week 96 Continued-Adefovir Group
Continued- Adefovir Group
(N=79)
Adefovir–
Placebo Group (N=40)
Placebo–
Adefovir Group (N=60)
Baseline to Week 96
(N=79)
Baseline to Week 144
(N=70) number of patients (percent)
Any event 58 (73) 32 (80) 41 (68) 67 (85) 60 (86)
General
Headache 12 (15) 4 (10) 5 (8) 23 (29) 19 (27)
Abdominal pain 16 (20) 7 (18) 5 (8) 22 (28) 20 (29)
Asthenia 8 (10) 6 (15) 3 (5) 15 (19) 15 (21)
Flu-like syndrome 6 (8) 4 (10) 5 (8) 14 (18) 14 (20)
Back pain 4 (5) 5 (12) 3 (5) 9 (11) 9 (13)
Pain 4 (5) 2 (5) 4 (7) 11 (14) 12 (17)
Accidental injury 4 (5) 2 (5) 2 (3) 6 (8) 8 (11)
Digestive
Diarrhea 6 (8) 4 (10) 1 (2) 8 (10) 6 (9)
Dyspepsia 4 (5) 5 (12)† 1 (2) 7 (9) 7 (10)
Respiratory
Pharyngitis 14 (18) 8 (20) 8 (13) 23 (29) 25 (36)
Increased cough 3 (4) 4 (10) 2 (3) 6 (8) 7 (10)
Bronchitis 2 (3) 1 (2) 1 (2) 6 (8) 9 (13)
Metabolic and nutritional Increased alanine amino-
transferase levels
2 (3)‡ 6 (15)† 1 (2) 3 (4) 3 (4)
Musculoskeletal
Arthralgia 6 (8) 5 (13)† 1 (2) 7 (9) 6 (9)
Urogenital
Increased creatinine levels 2 (3) 0 0 3 (4) 3 (4)
Hematuria 1 (1) 0 1 (2) 2 (3) 2 (3)
Kidney calculus 0 0 1 (2) 0 1 (1)
Kidney pain 0 0 1 (2) 2 (3) 4 (6)
l o n g - t e r m a d e f o v i r t h e r a p y f o r c h r o n i c h e p a t i t i s b
candidate for the long-term management of HBeAg- negative chronic hepatitis B.
Supported by Gilead Sciences.
Drs. Xiong, Currie, and Brosgart and Ms. Ma and Ms. Arterburn are employees of Gilead Sciences and report having equity owner-
ship in Gilead Sciences. Drs. Hadziyannis, Heathcote, Marcellin, and Goodman report having received consulting fees from Gilead Sciences. Drs. Hadziyannis and Marcellin report having received lecture fees from Gilead Sciences.
We are indebted to John Fry and Michael Wollman for their assis- tance in the preparation of the manuscript.
a p p e n d i x
In addition to the authors, the Adefovir Dipivoxil 438 Study Group includes the following persons: A. Alberti and S. Boccato, Universita di Padova, Padova, Italy; G.D. Anagnostopoulus, Western Attica Hospital, Athens; P. Angus and R. Vaughan, Austin and Repatriation Medical Centre, Melbourne, Australia; K. Barange and J.-M. Conbis, Hôpital Purpan, Toulouse, France; F. Bonino and B. Coco, Azienda Ospedaliera Pisana, Pisa, Italy; C.-H. Wu and K.-C. Tseng, National Cheng Kung University Hospital, Tainan, Taiwan; G. Cooksley and G. Macdonald, Royal Brisbane Hospital, Brisbane, Australia; P. Desmond and S. Brown, St. Vincent’s Hospital, Melbourne, Australia; A. Francavilla, F.
Malcangi, and G. Pastore, Azienda Ospedaliera Consorziale Policlinico, Bari, Italy; G. Papatheodoridis and V. Sevastianos, Henry Dunant Hospital, Athens; K. Kaita and G. Y. Minuk, University of Manitoba Health Sciences Centre, Winnipeg, Man., Canada; Y.-F. Liaw and R.-N.
Chien, Chang Gung Memorial Hospital, Taipei, Taiwan; Y.-Y. Dan, National University Hospital, Singapore; Y. Lurie and R. Pakula, Tel Aviv Sourskey Medical Center, Tel Aviv, Israel; L. Castelnau and N. Boyer, Hôpital Beaujon, Clichy, France; M. Ngu, Concord Repatriation Gen- eral Hospital, Concord, Australia; E. Nussenson and O. Segol, Haemel Hospital, Afula, Israel; M. Lagget, Azienda Ospedaliera Consorziale, San Giovanni Battista, Turin, Italy; J. Farley, Viridae Clinical Sciences, Vancouver, B.C., Canada; D. Samuel and J. Duches-Villie, Hôpital Paul Brousse, Villejuif, France; M. Sherman and A. Bartolucci, Toronto General Hospital, Toronto; W. Sievert, A. Dev, and S. Warner, Monash Medical Center, Clayton, Australia; C. Trepo and M. Maynard, Hotel Dieu, Lyon, France; D. Vetter and S. Metzger, Hôpital Civil de Stras- bourg, Strasbourg, France; J.-P. Villeneuve and B. Willems, Centre Hospitalier Universitaire de Montréal Campus St. Luc, Montreal; M.
Wollman, C. James, and C.G. Chang, Gilead Sciences, Foster City, Calif.; O. Cohen, Quintiles, Rockville, Md.; and D. Hunt, Covance, Lafay- ette, Ind.
r e f e r e n c e s
1. Hepatitis B fact sheet no. 204. Geneva:
World Health Organization, October 2000.
2. Hadziyannis SJ. Hepatitis B e antigen negative chronic hepatitis B: from clinical recognition to pathogenesis and treatment.
Viral Hepat Rev 1995;1:7-36.
3. Funk ML, Rosenberg DM, Lok ASF.
World-wide epidemiology of HBeAg-nega- tive chronic hepatitis B and associated pre- core and core promoter variants. J Viral Hepat 2002;9:52-61.
4. Brunetto MR, Stemler M, Schodel E, et al. Identification of HBV variants which can- not produce precore derived HBeAg and may be responsible for severe hepatitis. Ital J Gastroenterol 1989;21:151-4.
5. Proceedings of the European Associa- tion for the Study of the Liver (EASL) Inter- national Consensus Conference on Hepati- tis B, September 14-16, 2002. J Hepatol 2003;39:Suppl 1:S1-S235.
6. Wai CT, Lok AS. Treatment of hepati- tis B. J Gastroenterol 2002;37:771-8.
7. Hadziyannis SJ, Papatheodoridis GV, Dimou E, Laras A, Papaioannou C. Efficacy of long-term lamivudine monotherapy in patients with hepatitis B e antigen-negative chronic hepatitis B. Hepatology 2000;32:
847-51.
8. Tassopoulos NC, Volpes R, Pastore G, et al. Efficacy of lamivudine in patients with
hepatitis B e antigen-negative/hepatitis B vi- rus DNA-positive (precore mutant) chronic hepatitis B. Hepatology 1999;29:889-96.
9. Richman DD. The impact of drug resis- tance on the effectiveness of chemotherapy for chronic hepatitis B. Hepatology 2000;
32:866-7.
10.Hadziyannis S, Bramou T, Makris A, Moussoulis G, Zignego L, Papaioannou C.
Interferon alfa-2b treatment of HBeAg-neg- ative/serum HBV DNA positive chronic ac- tive hepatitis B. J Hepatol 1990;11:Suppl 1:
S133-S136.
11.Lampertico P, Del Ninno E, Manzin A, et al. A randomized, controlled trial of a 24-month course of interferon alfa 2b in pa- tients with chronic hepatitis B who had hep- atitis B virus DNA without hepatitis B e anti- gen in serum. Hepatology 1997;26:1621-5.
12.Papatheodoridis GV, Manesis E, Had- ziyannis SJ. The long-term outcome of inter- feron-alpha treated and untreated patients with HBeAg-negative chronic hepatitis.
J Hepatol 2001;34:306-13.
13.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen–negative chronic hepatitis B. N Engl J Med 2003;348:
800-7. [Erratum, N Engl J Med 2003;348:
1192.]
14.Westland CE, Yang H, Delaney WE IV, et
al. Week 48 resistance surveillance in two phase 3 clinical studies of adefovir dipivoxil for chronic hepatitis B. Hepatology 2003;
38:96-103.
15.Knodell RG, Ishak KG, Black WC, et al.
Formulation and application of a numerical scoring system for assessing histological ac- tivity in asymptomatic chronic active hepati- tis. Hepatology 1981;1:431-5.
16.Angus P, Vaughan R, Xiong S, et al. Re- sistance to adefovir dipivoxil therapy associ- ated with the selection of a novel mutation in the HBV polymerase. Gastroenterology 2003;125:292-7.
17.Santantonio T, Mazzola M, Iacovazzi T, Miglietta A, Guastadisegni A, Pastore G.
Long-term follow-up of patients with anti- HBe/HBV DNA-positive chronic hepatitis B treated for 12 months with lamivudine.
J Hepatol 2000;32:300-6.
18.Di Marco V, Marzano A., Lampertico P, et al. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology 2004;
40:883-91.
19.Marcellin P, Lau GK, Bonino F, et al.
Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in pa- tients with HBeAg-negative chronic hepati- tis B. N Engl J Med 2004;351:1206-17.
Copyright © 2005 Massachusetts Medical Society.