The role of human papillomavirus infection in carcinogenesis of oral squamous cell carcinoma with evidences of prognostic association
Running title: HPV associated with OSCC
Keywords: oral squamous cell carcinoma; human papillomavirus; p16
INK4A; p53; prognosis
Su-Feng Chen 1
, Fu-Shun Yu 1
, Yaoh-Shiang Lin 2,*
, Yun-Ching Chang 3
, Earl Fu 4 , Shin
Nieh 5,*
1 Department of Dental Hygiene, China Medical University, Taichung, Taiwan; 2
Department of Otolaryngology-Head and Neck Surgery, National Defense Medical Centre & Tri-Service General Hospital, Taipei, Taiwan; 3
Graduate Institute of Life Sciences, National Defense Medical Centre, Taipei, Taiwan; 4
Department of Dentistry, National Defense Medical Centre &
Tri-Service General Hospital, Taipei, Taiwan; 5
Department of Pathology, National Defense Medical Centre & Tri-Service General Hospital, Taipei, Taiwan
*
Correspondence: Professors S. Nieh and Y.-S. Lin, Department of Pathology and Department of Otolaryngology-Head and Neck Surgery, National Defense Medical Centre & Tri-Service General Hospital
Email: niehshin1014@yahoo.com.tw and yaohshiang@ndmctsgh.edu.tw Fax: +886 2 66000309
Su-Feng Chen and Fu-Shun Yu are equal contribution to this paper.
S.N. and Y-S L. contributed equally to this work.
Abstract
BACKGROUND: Betel nut chewing, cigarette smoking, and alcohol drinking are thought to be major environmental risk factors responsible for the development of oral squamous cell carcinomas. Oncogenic human papillomavirus infections have a well-established association with uterine cervical carcinoma. However, little is known about the exact role of human papillomavirus infections in oral squamous cell carcinomas. This study is designed to elucidate the role of human papillomavirus infections in cancer development and prognosis of oral squamous cell carcinomas.
METHODS: Molecular techniques including in situ hybridization and immunohistochemistry of p16
INK4Aand p53 for evidences of human papillomavirus in tissue micro-arrays were investigated.
RESULTS: 24 out of 65 cases of oral squamous cell carcinomas were found positive for in situ hybridization and 14 were found positive for p16
INK4A. The majority of cases without the evidence of human papillomavirus were related to p53 over-expression. There were statistically significant correlations between the results of human papillomavirus test and size or extent of the tumor (p=0.003) or the stage of oral squamous cell carcinomas (p=0.015). Kaplan-Meier plot analysis demonstrated a tendency of longer survival in cases of oral squamous cell carcinomas with the evidence of human papillomavirus or positive p16
INK4A.
CONCLUSION: Human papillomavirus infections may play a unique role in oral carcinogenesis.
Our data strongly suggest that human papillomavirus-positive oral squamous cell carcinomas
comprise a distinct clinical and pathological disease entity that appears related to a better
outcome with longer survival and bears a causally associated relationship different from other
carcinogenic mechanisms
Introduction
Oral squamous cell carcinomas (OSCC) are one of the most common and lethal head and neck malignancies with increasing morbidity and mortality in Taiwan (1). In spite of substantial improvements in both diagnosis and therapy in recent decades, the prognosis of OSCC remains generally poor (2). Oral habits like betel nut chewing, cigarette smoking, and alcohol drinking are thought to be major environmental risk factors responsible for the development of OSCC in Taiwan (3, 4). However, some patients develop OSCC without exposure to these three risk factors, which suggests that additional factors, such as genetic predisposition, diet, or oncogenic viruses, may help cells escape the physiological mechanisms of proliferation control (5, 6).
The relevance of the role of human papillomavirus (HPV) infection in cervical and anal cancer is well established and, by way of analogy, oncogenic HPV viruses might play a role in malignant transformation of squamous epithelia in any body region (7). Although still controversial, such evidence implies that carcinogenesis by environmental chemical carcinogens is not the only cause of oral squamous cell carcinoma. Presence of HPV in variable proportions in the oral or oro-pharyngeal squamous cell carcinoma tissues other than the uterine cervix, especially those genotypes with known high oncogenic potential (such as HPV16 and 18), has been demonstrated by several worldwide studies (8-11) (8-13). Recent studies suggest that HPV, especially type 16, may be responsible for a small subgroup of oral squamous cell carcinoma and up to 50% of oro-pharyngeal squamous cell carcinomas, especially the tonsillar squamous cell carcinoma (11, 14). Some reports emphasize the influences of HPV in predicting a better disease prognosis in oro-pharyngeal and tonsillar cancers (15, 16). Furthermore, a strong association between the presence of HPV and expression of key cell cycle proteins has been recently reported in a series of tonsillar cancer studies (15, 16) (17-20). These data seem to strongly suggest that HPV-positive tonsillar squamous cell carcinoma represents a distinct molecular, clinical and pathologic entity.
However, little is reported about the exact role of HPV in carcinogenesis and the associated
prognosis of OSCC. What is well known is that persistent high-risk HPVs infections may promote malignant transformation of stratified squamous lining mucosal epithelium which is mediated by inactivation of p53 and retinoblastoma (pRb) by the viral gene products E6 and E7 (21). The inactivation of pRb results in an over-expression of p16
INK4A(22). The p16 protein, encoded by the CDKN2A (MTS1, INK4A) tumor suppressor gene located on chromosome 9p21, decelerates the cell cycle by inactivating the function of cdk4 and cdk6-cyclin D complexes.
These complexes regulate the G1 check-point by phosphorylation and subsequent inactivation of
pRb, which in turn releases E2F allowing the cell to enter into the S phase (23, 24).In all
HPV-positive neoplasms, pRb has been shown to be functionally inactivated as a consequence
of HPV E7 protein expression, and as a result, a strong over-expression of p16
INK4Adevelops
(25, 26). p16
INK4Aover-expression is easily detectable with immunohistochemical stains and
has been proposed as a biomarker that would allow unambiguous identification of truly
neoplastic cells and would help in the identification of a subset of tumors related to HPV
infection in a variety of genital and extra-genital areas such as the penis, anus, tonsils, and
rhino-sinusal cavities (26-30). It has even been shown that in particular settings, such as the oral
cavity, immunohistochemical p16
INK4Adetection is a simple, but not frequently used technique
applicable for a histological diagnostic routine that is equivalent to HPV detection (29). The
aim of the current study is to elucidate the role of HPV in carcinogenesis of OSCC through ISH
and IHC of p16
INK4Aand p53 and to further correlate the clinical parameters with each
individual case.
Materials and methods
Patients and tissue specimens
Tissue specimens of 88 patients with OSCC were collected and retrieved from the archives of the Department of Pathology, Tri-Service General Hospital, Taipei, Taiwan from January 2003 to June 2004. Tissue micro-arrays (TMA) with 1.5 mm-sized cores were then constructed and examined from the paraffin blocks. The majority of the cases were selected from the radically surgical specimens of OSCC. 23 cases were lost to follow-up or had insufficient clinico-pathological data for analysis and were therefore excluded. Thus, a total of 65 cases of OSCC were included in the study. All of the collection samples conformed to the ethical approval of Tri-Service General Hospital Institutional Review Board.
Immunohistocemistry
The formalin-fixed paraffin-embedded specimens were made in 4-μm-thick sections. After de-paraffinization and re-hydrated, antigen retrieval was carried out by incubation in a citrate buffer (10mM citrate acid, pH 6.0) at 95℃ for 40 minutes. Endogenous peroxidase and nonspecific antibody reaction were blocked with 3% H
2O
2and 5% normal horse serum in PBS.
For implementation of the labeled streptavidin–biotin method (LASB2 kit/HRP, DakoCytomation), primary mouse anti-Human p16
INK4aantibody (clone E6H4, DakoCytomation) and monoclonal mouse anti-human p53 antibody (clone Dc-7, DakoCytomation) were used at dilution of 1:100 and incubated overnight at 4℃. Subsequently, all sections were counterstained with Mayer’s hematoxylin, dehydrated, and mounted.
In situ hydridization
In situ hybridization was performed using labeled probes (for types 6, 11, 16, 18, 31, 33, 35, 39,
45, 51 and 52) with wide spectrum HPV biotinylated DNA (DakoCytomation) and tyramide
signal amplification system (DakoCytomation GenPoint
TM). Tissue sections 4μm thick were cut
onto coating slides and dried overnight at 65° C. Tissues were dewaxed in two 10-minute
changes of xylene and then washes by degraded (100–70%) ethanol. After rinsing in water, tissues were immersed in 10 mM sodium citrate, pH 6.0, at 95.0° C for 40 minutes. The slides were rinsed with water and air dried. Tissues were then digested at 25° C with 400μg pepsin (Sigma P-7012) in 0.2 N HCl for 10 minutes. Slides were rinsed three times for 5 minutes each in TBST and air dried. Endogenous peroxidase was blocked with 3% H
2O
2at room temperature for 10 minutes. Pre-hybridization mixture was applied directly to the tissue section and comprised 50% formamide; 10% dextran sulfate; 10% 20x SSC, pH 7.0 ; 1000μ g/L salmon sperm signa-strade DNA (Sigma). Cover- slipped slides were placed into Terasaki plates (Nunc) containing 5x SSC at 95° C for 10 minutes to enable simultaneous denaturation of probe and target sequences. Tissues were hybridized overnight at 37° C. Samples were washed stringently three times (2x SSC at 55° C, twice for 5 min; 1x SSC at 37° C, twice for 5 min; 0.1x SSC at 37° C, twice for 5 min). Biotinyl-tyramide and secondary streptavidin– horseradish peroxidase conjugate were used as supplied in the GenPoint kit. Aminoethylcarbazole was (DakoCytomation) applied to sections for 10 minutes. Sections were counterstained with hematoxylin (DakoCytomation) and mounted in a glycerol gelatin (Sigma).
Histopathological evaluation
All of the histopathological slides on TMAs were concurrently reviewed and evaluated
independently by two qualified pathologists using the same type of microscope without any
prior knowledge of each patient’s clinical details. When the opinions of the two evaluators
differed, consensus was reached by discussion. Subsequent interpretations of cases of both ISH
and IHC findings also followed the aforementioned procedure. In each TMA, a positive control
with a case of cervical squamous carcinoma for p16
INK4Awas included. Cases with more than
50% of tumor cells showing strong or moderate nuclear staining along with cytoplasmic staining
were considered positive for p16
INK4Aexpression. In the meantime, a positive control with a case
of poorly differentiated gastric adenocarcinoma for p53 was also prepared. Cases with more than
50% of tumor cells showing strong or moderate nuclear staining were considered positive for p53 expression. ISH was performed using individual plasmid probes including types 6, 11, 16, 18, 31, 33, 35, 39, 45, 51 and 52. Cases with a more than 10% integrated form (nuclear dots or punctuate staining) or episomal (diffuse staining) form throughout the nucleus were considered positive. A discrete, punctate signal pattern within a cell nucleus correlated with integration, whereas a diffuse signal throughout the nucleus indicated episomal HPV (20, 31). The results were measured according to the previous study. Cases with more than 10% of tumor cells displaying punctate or diffuse signal throughout the nucleus were considered positive (11).
Statistical analysis
All statistical analyses were done using SPSS software v.13.0 (SPSS UK Ltd, Woking, UK) for
windows. Chi-square (χ
2) tests (with adequate Yates’ correction for 2x2 tables and d.f. =1, or
with Fisher’s exact test when expected value < 5 in > 20% table cells) were used to measure the
significance between clinical parameters and ISH of HPV. Chi-square (χ
2) tests were also used
to measure the significance between ISH of HPV and p16
INK4Aor p53. The log rank test was
used to compare differences in survival between two groups and survival curves were obtained
by the Kaplan-Meier method. P < 0.05 was considered to be significant.
Results
24 out of 65 cases of OSCC were positive for ISH showing evidence of HPV on TMA. Most of the ISH-positive cases demonstrated the integrated viral DNA by a dot-like or punctate merging signal within the nucleus. However, there were only four cases showing episomal viral DNA by a diffuse signal throughout the nucleus. Among these 24 positive cases, 14 were found positive for p16
INK4Aby IHC. There was a close association between positive p16
INK4Aimmunostaining and positive ISH for evidence of HPV infection (14/24). However, there were 10 cases out of 24 that were positive for ISH but negative for p16
INK4Aimmunostaining. In addition, among these 24 positive cases, p53 immunostaining was variably expressed. There were 11 cases over-expressed for p53 including 3 positive p16
INK4Aimmunostaining and 8 positive ISH. There were 41 cases negative for ISH without evidence of HPV. The majority of ISH-negative cases were related to p53 over-expression (37/41). However, there were 4 cases negative both for p53 and for ISH (Fig. 1 and 2).
The presentation and correlation of the clinico-pathological data and the results of HPV tests of 65 patients with OSCC is summarized in Table 1. When comparing the results of the HPV test with the clinico-pathological parameters, there was no significant correlation between the results of the HPV test and gender, age, location, status of lymph node metastasis and histological grading. However, there were statistically significant correlations between the results of the HPV test and size or extent of the tumor (p=0.003) or the stage of OSCC (p=0.015).
Together with three main test parameters, there were statistically significant correlations between the results of the HPV test with p16
INK4Aand/or p53 (Table 2).
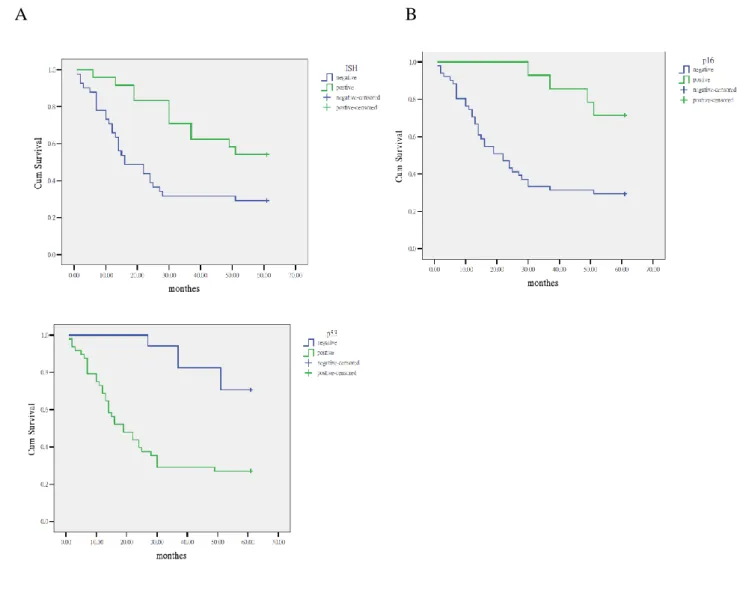
Follow-up of 65 cases with OSCC from initial diagnosis to 60 months was performed and
the overall survival rate estimated at 38.5%. There was a statistical significance between the
patient’s survival and results of ISH of HPV and IHC of p16
INK4Aby the log rank test and
Kaplan-Meier plots (Fig. 3). Cases of OSCC with positive HPV and/or positive p16
INK4Aresults
were associated with better prognoses; however, cases of OSCC with over-expressed p53 were
associated with worse prognoses.
Discussion
Oncogenic HPV infections have a well-established association with uterine cervical and ano-genital carcinomas. Their relationship to OSCC, however, is less clear. Based on our data, there were more than 1/3 (24/65) cases of OSCC showing evidence of HPV via ISH. Each positive ISH showing punctate or diffuse signals within the nucleus was variably expressed.
Among these 24 positive cases, 14 were found positive for p16
INK4A. ISH for evidence of HPV
infection in OSCC appeared more sensitive than immunohistochemical detection of p16
INK4A.
The use of molecular assays between ISH by HPV probes and IHC for p16
INK4Acomprised
different sensitivities and specificities for viral or its oncoprotein detection. Nevertheless IHC
for p16
INK4Adetection is a simple technique applicable for histologically diagnostic tool only
and exclusively indicative of E7 oncoprotein, this useful method thus results in less sensitive
detection for HPV (28, 32). The majority (37/41) of HPV-negative cases of OSCC were related
to over-expression of p53. In addition to 11 cases co-expressed with ISH for HPV and p53, there
were a total of 48 cases of OSCC showing over-expression of p53 (48/65). It was generally
agreed that the role of p53 mutations in the pathogenesis of certain head and neck cancers may
be substituted by HPV infection because viral E6 protein can inactivate p53 by targeting the
protein for ubiquitination and degradation (30, 32, 33). Mutant p53 proteins tended to
accumulate and thus become over-expressed until carcinogenesis occurred in a certain
percentage of all tumors. There was a marked difference in p53 mutation frequency between
HPV-positive and HPV-negative tumors in OSCC. The inverse relationship between p53
mutation with over-expressions and HPV strengthens the etiologic role of HPV in OSCC. In
addition, this inverse relationship further suggests two parallel or overlapping pathways of oral
carcinogenesis: one was thought to be driven by environmental toxins (e.g., betel nut, tobacco
and alcohol) and another driven by viral oncogens (e.g., high-risk HPVs). In addition, cases of
HPV-positive OSCC were more likely to occur in patients without environmental risk factors
than those of HPV-negative OSCC (data not shown). As seen in other reports, the lifestyles of patients with HPV-related OSCC appeared relatively different and ‘healthier’ than those of patients with HPV-negative OSCC (6, 9, 11, 16). Nevertheless, viral infections may also act synergistically with environmental toxins effects. The coexistence of positive ISH and p53 over-expression seen in 11 cases, along with 4 cases negative for ISH and less expressed p53 immunostaining, may be explained by the variable sensitivity of certain p53 mutants to E6 degradation and/or by tumor promotion via p53-independent viral mechanisms such as disruption of pRb function by HPV E7 (33-36).In conjunction with recent studies, our data provide strong evidences that HPV are etiologically linked to a defined subset of OSCC.
Compared to other previous reports where different methods conclude that the HPV-positive rate of OSCC ranges from 43 to 75%, our data with nearly 37% HPV-positive rate seems obviously lower than those in oro-phargnx and pharynx, as reported by others (11, 15-18).The difference strongly suggests anatomical site specificity. It also implies that the evidences of HPV in mucosal tissues of head and neck other than oral cavity are far more easily detected. The motile nature of oral cavity along with saliva secretion and cleaning perhaps, at least in part, is responsible for the lower detection rate of HPV.
As shown in Figure 3, like previous reports, significant differences were found between
the presence or absence of HPV and survival in OSCC (15, 16, 37, 38). The reason for the
favorable prognoses with longer survival in patients with HPV-positive OSCC is still unclear. A
recent in vitro study disclosed that HPV oncoproteins E6 and E7 mediate suppression of NF-kB
transcriptional activity and may further contribute to HPV escaping from the immune system in
cases of HPV-related carcinogenesis (39). Evidences also revealed that HPV-positive cancers
may have an intact apoptotic response to radiation and chemotherapy (40). Aforementioned
theoretical statements may also be referred to the statistical significance between the results of
HPV test and tumor size as well as the clinical stage as seen in our study. The reason why other
clinico-pathological parameters are not involved still needs further investigation and additional
cases.
Along with other recent reports, our study suggests that HPV-positive OSCC should be regarded as a unique subset of all squamous cell carcinomas that arise in the head and neck (23, 37). As seen in recent reports for prevention of persistent high risk HPV-induced cervical dysplasia and cancer an alternative strategy using prophylactic vaccine for disease prevention of HPV-related OSCC, although still in animal model experiments, may be served the similar protective effect in the near future (41).
In our study we investigated and analyzed two molecular techniques including ISH and IHC via TMAs which provide powerful tools for high-throughput in situ analysis allowing the simultaneous evaluation of tumor by histology. All slides on TMAs are prepared and stained under the same conditions and analyzed accordingly. In addition, ISH is an emerging technology that allows sensitive direct visualization of HPV at the light microscopic level. Comparatively, ISH test for HPV appears qualitatively better than an assay of PCR as seen in our study. ISH is
an emerging technology that allows direct visualization of the viral particles of HPV in the nucleus with satisfactory sensitivity and specificity at the light microscopic level. Many studies for detection of HPV are measured by PCR and the test result of PCR is based on repeated amplification of fragments of viral DNA resulting in the highest sensitivity and HPV detection rate. Nevertheless this technique may also inevitably cause unreliable data following repeated amplification. Comparatively, ISH test for HPV appears qualitatively better than an assay of PCR as shown by other reports. (11, 31)
In conclusion, HPV associated tests including ISH and IHC comprise distinct molecular
techniques which may serve as clinically accessible markers. HPV infections may play a unique
role in oral carcinogenesis distinct from other carcinogenic mechanisms. HPV-positive OSCC
appears related to a better outcome with longer survival.
References
1. Chen YJ, Chang JT, Liao CT, et al. Head and neck cancer in the betel quid chewing area:
recent advances in molecular carcinogenesis. Cancer Sci 2008; 99: 1507-14.
2. Lippman SM, Sudbo J, Hong WK. Oral cancer prevention and the evolution of molecular-targeted drug development. J Clin Oncol 2005; 23: 346–56.
3. Dobrossy L. Epidemiology of head and neck cancer: Magnitude of the problem. Cancer Metastasis Rev 2005; 24: 9-17.
4. Sudbø J. Novel management of oral cancer: a paradigm of predictive oncology. Clin Med Res 2004; 2: 233-42.
5. Bagan JV, Scully C. Recent advances in Oral Oncology 2007: epidemiology, aetiopathogenesis, diagnosis and prognostication. Oral Oncol 2008; 44: 103-8.
6. Sugerman PB, Shillitoe EJ. The high risk human papillomaviruses and oral cancer:
evidence for and against a causal relationship. Oral Dis 1997; 3: 130-47.
7. zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst 2000; 92: 690-8.
8. Angiero F, Gatta LB, Seramondi R, et al. Frequency and role of HPV in the progression of epithelial dysplasia to oral cancer. Anticancer Res 2010; 30: 3435-40.
9. Syrjänen S. The role of human papillomavirus infection in head and neck cancers. Ann Oncol 2010;21 Suppl 7: vii243-5.
10. Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus related and unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 2008; 26: 612–9.
11. Kuo KT, Hsiao CH, Lin CH, Kuo LT, Huang SH, Lin MC. The biomarkers of human
papillomavirus infection in tonsillar squamous cell carcinoma-molecular basis and
predicting favorable outcome. Mod Pathol 2008; 21: 376-86.
12. Lajer CB, von Buchwald C. The role of human papillomavirus in head and neck cancer.
APMIS 2010;118:510–9
13. D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007;356:1944-56.
14. Li W, Thompson CH, Xin D, et al. Absence of human papillomavirus in tonsillar squamous cell carcinomas from Chinese patients. Am J Pathol 2003; 163: 2185–9.
15. Li W, Thompson CH, O’Brien CJ, et al. Human papillomavirus positivity predicts favorable outcome for squamous carcinoma of the tonsil. Int J Cancer 2003; 106: 553–8.
16. Ringström E, Peters E, Hasegawa M, Posner M, Liu M, Kelsey KT. Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin Cancer Res 2002; 8:
3187–92.
17. Li W, Thompson CH, Cossart YE, et al. The expression of key cell cycle markers and presence of human papillomavirus in squamous cell carcinoma of the tonsil. Head Neck 2004; 26: 1–9.
18. Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol
Biomarkers Prev 2005; 14: 467–75.
19. Mannarini L, Kratochvil V, Calabres L, et al. Human Papilloma Virus (HPV) in head and neck region: review of literature. Acta Otorhinolaryngol Ital 2009 ;29:119-26.
20. Laco J, Vosmikova H, Novakova V, et al. The role of high-risk human papillomavirus infection in oral and oropharyngeal squamous cell carcinoma in non-smoking and non-drinking patients: a clinicopathological and molecular study of 46 cases. Virchows Arch 2011;458:179-87.
21. Santos M, Montagut C, Mellado B, et al. Immunohistochemical staining for p16 and p53 in premalignant and malignant epithelial lesions of the vulva. Int J Gynecol Patho. 2004; 23:
206–14.
22. Riethdorf S, Neffen EF, Cviko A, Löning T, Crum CP, Riethdorf L. p16
INK4Aexpression as biomarker for HPV 16-related vulvar neoplasias. Hum Pathol 2004; 35: 1477–83.
23. Lerma E, Matias-Guiu X, Lee SJ, Prat J. Squamous cell carcinoma of the vulva: study of ploidy, HPV, p53, and pRb. Int J Gynecol Pathol 1999; 18: 191–7.
24. Mo′nica Santos, Stefania Landolfi, Anna Olivella, et al. p16 Overexpression Identifies HPV-positive Vulvar Squamous Cell Carcinomas. Am J Surg Pathol 2006; 30: 1347–56.
25. Agoff SN, Lin P, Morihara J, Mao C, Kiviat NB, Koutsky LA. p16
INK4Aexpression correlates with degree of cervical neoplasia: a comparison with Ki-67 expression and detection of high-risk HPV types. Mod Pathol 2003; 16: 665–73.
26. Klaes R, Benner A, Friedich T, et al. p16INK4A Immunohistochemistry improves interobserver agreement in the diagnosis of cervical intraepithelial neoplasia. Am J Surg Pathol 2002; 26: 1389–99.
27. Cubilla AL, Lloveras B, Alejo M, et al. The basaloid cell is the best tissue marker for human papillomavirus in invasive penile squamous cell carcinoma: a study of 202 cases from Paraguay. Am J Surg Pathol 2010; 34: 104-14.
28. Lu DW, El-Mofty S, Wang HL. Expression of p16, Rb, and p53 proteins in squamous cell carcinomas of the anorectal region harboring human papillomavirus DNA. Mod Pathol 2003; 16: 692–9.
29. Klussmann JP, Gultekin E, Weissenborn SJ, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol 2003; 162: 747–53.
30. El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus DNA in non-keratinizing (cylindrical cell) carcinoma of the sinonasal tract. A distinct
clinicopathologic and molecular disease entity. Am J Surg Pathol 2005; 29: 1367–72.
31. Evans MF, Mount SL, Beatty BG, Cooper K. Biotinyl-tyramide-based in situ hybridization
signal patterns distinguish human papillomavirus type and grade of cervical intraepithelial
neoplasia. Mod Pathol 2002;15:1339-47.
32. Rapp L, Chen JJ. The papillomavirus E6 proteins. Biochem Biophys Acta 1998;1 378:
F1–19.
33. Maura L. Gillison, Wayne M. Koch, Randolph B. Capone, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000; 92: 709-20.
34. Gardiol D, Banks L. Comparison of human papillomavirus type 18 (HPV-18) E6-mediated degradation of p53 in vitro and in vivo reveals significant differences based on p53 structure and cell type but little difference with respect to mutants of HPV-18 E6. J Gen Virol 1998; 79: 1963–70.
35. Magal S, Jackman A, Pei XF, Schlegel R, Sherman L. Induction of apoptosis in human keratinocytes containing mutated p53 alleles and its inhibition by both the E6 and E7 oncoproteins. Int J Cancer 1998; 75: 96–104.
36. Chen X, Sturgis EM, Lei D, Dahlstrom K, Wei Q, Li G. Human papillomavirus seropositivity synergizes with MDM2 variants to increase the risk of oral squamous cell carcinoma. Cancer Res 2010; 70: 7199-208.
37. Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000; 92:
709–20.
38. Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus— associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 2006; 24: 736–47.
39. Spitkovsky D, Hehner SP, Hofmann TG, Möller A, Schmitz ML. The human papillomavirus oncoprotein E7 attenuates NF-kappa B activation by targeting the Ikappa B kinase complex. J Biol Chem 2002; 277: 25576–82.
40. Ferris RL, Martinez I, Sirianni N, et al. Human papillomavirus-16 associated squamous cell
carcinoma of the head and neck (squamous cell carcinoma HN): a natural disease model provides insights into viral carcinogenesis. Eur J Cancer 2005; 41: 807–15.
41. Maeda H, Kubo K, Sugita Y, et al. DNA vaccine against hamster oral
papillomavirus-associated oral cancer. J Int Med Res 2005; 33: 647–53.
Acknowledgements
This study was partly supported by the Tri-Service General Hospital, Grant
No.TSGH-C99-009-10-S02 and Department of Dental Hygiene, China Medical University,
Grant No. CMU99-N1-04-1 and by the National Science Council, Republic of China, and Grant
No. NSC 99-2320-B-039-028-MY3.
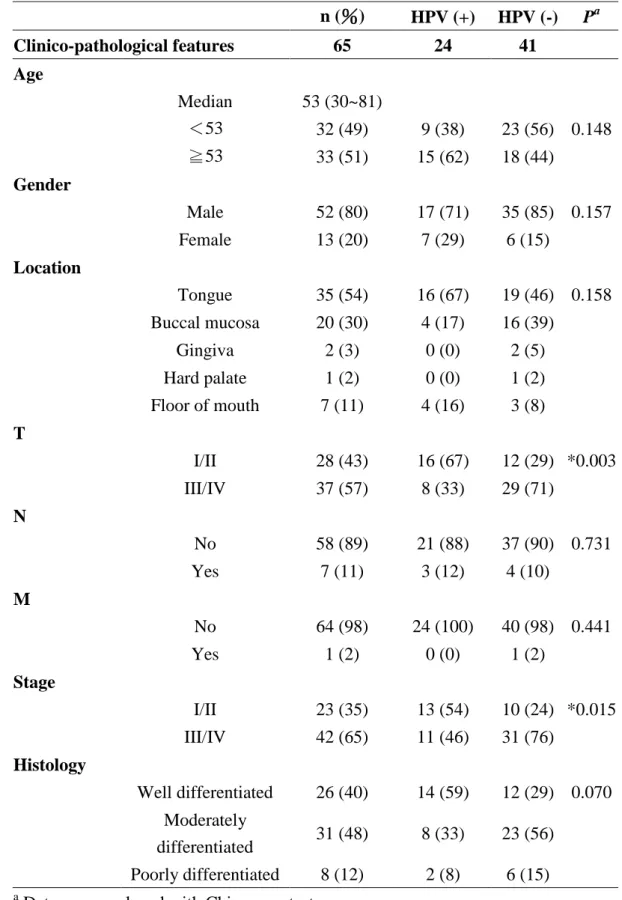
Table 1. Correlations and analysis of clinico-pathological parameters with the test results of HPV in 65 patients with OSCC.
Table 2. Correlations between the results for HPV by ISH and IHC for p16
INK4Aand p53 in 65 n (%) HPV (+) HPV (-) P
aClinico-pathological features 65 24 41
Age
Median 53 (30~81)
<53 32 (49) 9 (38) 23 (56) 0.148
≧53 33 (51) 15 (62) 18 (44)
Gender
Male 52 (80) 17 (71) 35 (85) 0.157
Female 13 (20) 7 (29) 6 (15)
Location
Tongue 35 (54) 16 (67) 19 (46) 0.158 Buccal mucosa 20 (30) 4 (17) 16 (39)
Gingiva 2 (3) 0 (0) 2 (5)
Hard palate 1 (2) 0 (0) 1 (2)
Floor of mouth 7 (11) 4 (16) 3 (8) T
I/II 28 (43) 16 (67) 12 (29) *0.003
III/IV 37 (57) 8 (33) 29 (71)
N
No 58 (89) 21 (88) 37 (90) 0.731
Yes 7 (11) 3 (12) 4 (10)
M
No 64 (98) 24 (100) 40 (98) 0.441
Yes 1 (2) 0 (0) 1 (2)
Stage
I/II 23 (35) 13 (54) 10 (24) *0.015 III/IV 42 (65) 11 (46) 31 (76)
Histology
Well differentiated 26 (40) 14 (59) 12 (29) 0.070 Moderately
differentiated 31 (48) 8 (33) 23 (56) Poorly differentiated 8 (12) 2 (8) 6 (15)
a
Data were analyzed with Chi-square test.
patients with OSCC.
FIGURE 1. Distributions of the test results of HPV by ISH and IHC of p16
INK4Aand p53 in 65
patients with OSCC.
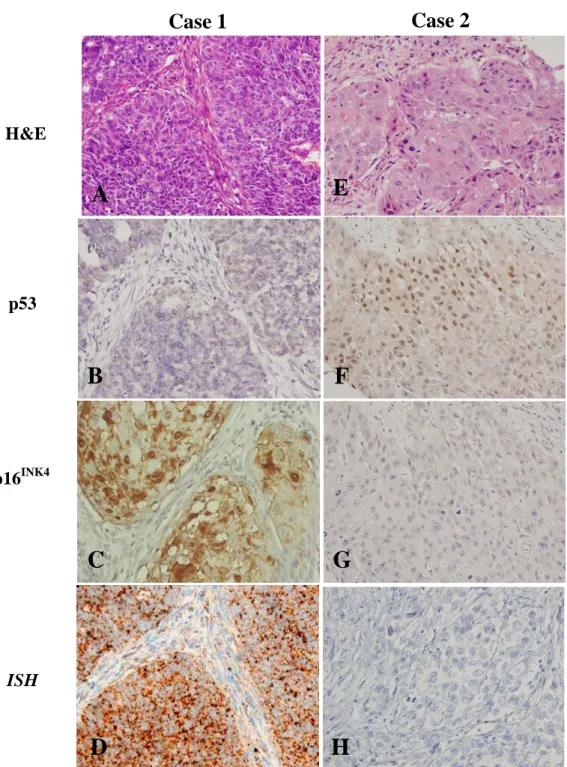
FIGURE 2. The microscopic illustrations with comparisons of two representative cases of OSCC were shown in left panel from A-D (case 1) and right panel from E-H (case 2). The histological findings of case 1 elicited a poorly differentiated squamous cell carcinoma in A, compared with the case 2 presenting a moderately differentiated squamous cell carcinoma in E (X200);
The corresponding immunohistochemical stains for p53 showed a negative result (B) in case 1, compared with over-expressed p53 (F) in case 2 (X200); while p16
INK4Astains demonstrated a positive result in case 1 (C) (X400) and a negative result in case 2 (G) (X200); In situ hybridization studies for evidence of HPV showed a positive result with nuclear punctate pattern in case 1 (D) (X400) and a negative result in case 2 (H) (X200).
A
B
C
D
E
F
G
H C
H&E
ISH p16
INK4A