Original article Version 2 January, 2012
Telbivudine plus pegylated interferon alfa-2a in HBeAg-positive chronic hepatitis B:
potent antiviral efficacy but risk of peripheral neuropathy
Patrick Marcellin
1, Karsten Wursthorn
2, Heiner Wedemeyer
2, Wan-Long Chuang
3, George Lau
4, Claudio Avila
5, Cheng-Yuan Peng
6, Edward Gane
7, Seng Gee Lim
8, Hugo Fainboim
9, Graham Foster
10, Rifaat Safadi
11, Mario Rizzetto
12, Michael Manns
2, Weibin Bao
13, Nikolai Naoumov
131
Service d’Hépatologie, INSERM-CRB3, Hôpital Beaujon, Clichy, France
2
Clinic for Gastroenterology, Hepatology, and Endocrinology, Hannover Medical School, Hannover, Germany
3
Hepatobiliary Division, Department of Internal Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
4
Humanity and Health GI and Liver Clinic, Hong Kong, SAR
5
Novartis Pharma AG, Basel, Switzerland
6
School of Medicine, China Medical University, and Division of Hepatogastroenterology, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan Liver Research Unit, Chang Gung Memorial Hospital, Chang Gung University, Tao Yuan, Taiwan
7
New Zealand Liver Unit, Auckland City Hospital, Auckland, New Zealand
8
Department of Gastroenterology and Hepatology, National University Health System, Yong Yoo Lin School of Medicine, National University of Singapore, Kent Ridge, Singapore
9
Unidad 4, Hepatopatias Infecciosas, Hospital Francisco Muñiz, AAEEH, Ciudad Autónoma de Buenos Aires, Buenos Aires, Argentina
10
Queen Mary University of London, the Liver Unit, Blizard Institute of Cellular and
Molecular Science, Barts, and the London School of Medicine, The Royal London Hospital, London, UK
11
Liver Unit, Department of Medicine, Hadassah-Hebrew University Medical Center, Jerusalem, Israel
12
Department of Gastroenterology, University of Turin, Turin, Italy
13
Novartis Pharmaceuticals, East Hanover, New Jersey, USA
Correspondence:
Patrick Marcellin, MD, PhD Service d’Hépatologie INSERM-CRB3 Hôpital Beaujon
100, Boulevard du Général Leclerc Clichy, France
Tel: +33 1 40 87 53 38 Fax: +33 1 47 30 94 40 patrick.marcellin@bjn.aphp.fr
Word Counts: 261 (abstract)
3561 (text)
ABSTRACT
Aim and Background. We investigated the antiviral efficacy and safety of telbivudine in combination with pegylated interferon (PegIFN) alpha-2a in chronic hepatitis B (CHB).
Patients and Methods. This was a randomized, open-label, multicenter study comparing, in treatment-naïve patients with HBeAg-positive CHB, the efficacy and safety of 1) telbivudine 600 mg in combination with pegylated interferon (PegIFN) alpha-2a 180 μg, 2) PegIFN alpha-2a monotherapy and 3) telbivudine monotherapy. The study was terminated early due to increased rates of peripheral neuropathy in the combination therapy group.
Results. Of the 159 patients randomized from 300 planned (50 to combination therapy, 55 to telbivudine, 54 to PegIFN), 110 (18, 49 and 43, respectively) reached week 24. At week 24, undetectable HBV DNA (< 300 copies/mL) was achieved by 71%, 35% and 7% of patients receiving combination therapy, telbivudine monotherapy and PegIFN monotherapy, respectively (p = 0.022 for combination therapy vs. telbivudine; p <0.0001 for combination therapy vs.
PegIFN). The rate of patients with HBsAg decline ≥ 0.5 log
10IU/mL at week 24 (a predictor of subsequent HBsAg clearance) was significantly higher in combination-therapy group (61%) than in telbivudine (40%) and PegIFN (28%) groups (p = 0.03). Both monotherapies were well tolerated; peripheral neuropathy occurred in 7/50, 1/54 and 0/54 patients in the three groups, respectively. No drug-drug interactions or correlation between peripheral neuropathy and serum alanine aminotransferase or creatine kinase levels were observed.
Conclusion. Combination therapy provided rapid and profound reductions in HBV DNA levels
and faster declines in HBsAg. However, combination therapy carried an increased risk of
peripheral neuropathy and should not be used.
Key Words: Hepatitis B virus, nucleoside analog, pegylated interferon, telbivudine, hepatitis
surface antigen
INTRODUCTION
The ultimate goal of chronic hepatitis B (CHB) therapy is to prevent the progression of the disease to cirrhosis and hepatocellular carcinoma. Effective and sustained suppression of hepatitis B virus (HBV) replication and hepatitis B e antigen (HBeAg) seroconversion are correlated with clinical remission of liver disease and have been established as milestones of CHB therapy in the clinical practice (1-5). Although higher levels of HBV DNA suppression and HBeAg seroconversion can be attained with newer anti-CHB agents, the achievement of hepatitis B surface antigen (HBsAg) seroconversion, considered the “cure” for CHB, remains an elusive goal (6).
Monotherapy for a finite period with pegylated interferon (PegIFN) yields to HBeAg seroconversion in 33% of patients and HBsAg seroconversion in 4%, but response rates are influenced by a number of factors, including baseline viral load, alanine aminotransferase (ALT) levels, HBV genotype, patient age and duration of treatment (7-11).
The main attribute of immunomodulators is in seroconversion and the strength of nucleoside or
nucleotide analogs is in the potent reduction of HBV DNA. The fact that no currently available
single medication can induce both potent HBV DNA suppression and high rates of HBeAg and
HBsAg clearance has prompted interest in combination therapy with agents having additive or
synergistic effects. Initial trials evaluating PegIFN and lamivudine demonstrated slightly
enhanced viral suppression and lower resistance rates with the combination compared with either
drug alone (8, 10-11). However, a clear advantage of combination therapy over single-agent
treatment for long-term outcomes such as HBeAg or HBsAg kinetics or seroconversion has not
been demonstrated in studies conducted to date. This may be due to limitations of study design
these studies were performed, new sensitive markers of virologic response, such as HBeAg and HBsAg quantitation, were not available.
Telbivudine (LdT), a highly specific and potent inhibitor of HBV replication, has demonstrated superior virologic and biochemical responses with a similar safety profile to lamivudine in patients with CHB (12-13). LdT induces high rates of HBeAg seroconversion after 2 years of continuous treatment in HBeAg-positive patients, particularly those with undetectable HBV DNA at week 24. This seroconversion is durable off treatment in most patients (12-13).
The aim of the present study was to determine whether combination therapy with LdT and PegIFN had superior antiviral therapy efficacy and provided higher HBeAg and HBsAg seroconversion rates than either agent alone.
METHODS
Study Design and Patients
This randomized, open-label, international, multicenter, 3-arm study compared combination of
LdT plus PegIFN versus each treatment alone in adults with HBeAg-positive CHB. Eligible
patients were randomized (1:1:1) to receive one of the following treatments: LdT 600 mg once
daily for 104 weeks plus PegIFN 180 µg subcutaneously once per week for the first 52 weeks,
LdT 600 mg once daily for 104 weeks or PegIFN 180 µg subcutaneously once per week for 52
weeks with off-treatment follow-up to week 104. For patients who were unable to tolerate the
protocol-specified dosing scheme for PegIFN, protocol-specified dose adjustments and
interruptions were permitted in order to keep the patient on study drug.
Written informed consent was obtained from each patient. The protocol was conducted in accordance with the Declaration of Helsinki and approved by each local independent Ethics Committee. This trial is registered with ClinicalTrials.gov, number NCT00412750.
The study enrolled patients ≥ 18 years of age with documented CHB defined by all of the following: a clinical history compatible with CHB, detectable serum HBsAg at screening and ≥ 6 months prior, HBeAg positivity and HBeAb negativity, a history of chronic liver inflammation documented by at least two elevated serum ALT levels measured ≥ 6 months apart, serum ALT levels 1.3 to 10.0 times the upper limit of normal (ULN), serum HBV DNA levels
≥ 6 log
10copies/mL as determined by the COBAS
®Amplicor HBV polymerase chain reaction (PCR) assay (Roche Diagnostics, Branchburg, NJ), chronic liver inflammation on liver biopsy.
Key exclusion criteria were coinfection with HIV-1 or hepatitis C or D virus, previous nucleos(t)ide therapy or any immunomodulatory treatment in the 12 months before screening, evidence of hepatocellular carcinoma, a history of or clinical signs or symptoms of hepatic decompensation, myopathy, myositis, persistent muscle weakness or peripheral neuropathy, a creatinine clearance < 50 mL/min, a hemoglobin level < 11 g/dL in men and < 10 g/dL in women, a neutrophil count < 1,500 mm
3and liver decompensation or failure. Women who were pregnant, breastfeeding or of child-bearing potential who were not using two methods of birth control were also ineligible.
Assessments
E
FFICACYThe original primary efficacy endpoint was the proportion of patients who achieved HBV DNA
non-detectability by PCR at week 52. Secondary efficacy evaluations included measurement of
serum HBV DNA levels, ALT levels, HBeAg loss, HBeAg seroconversion, HBsAg loss and HBsAg seroconversion.
HBsAg levels were quantified retrospectively from frozen serum samples in a specialized laboratory from all patients who reached week 24 of treatment with serum samples available.
HBsAg quantitation was performed with Abbott Architect
®HBsAg assay (Abbott Diagnostics, Abbott Park, IL) at baseline and treatment weeks 12 and 24. The lower limit of detection of this assay is 0.05 IU/mL.
HBeAg levels were quantified retrospectively in frozen serum samples at baseline and treatment weeks 4, 8, 12, 18 and 24 using the Abbott Architect
®HBeAg assay modified for quantitative use (14). The lower limit of detection of this assay is 0.17 PE IU/mL.
P
HARMACOKINETICSPK analysis was performed on plasma samples from 82 patients (50 receiving LdT and 32 receiving combination therapy) to characterize the possible PK interaction between LdT and PegIFN at the steady state. PK parameters included plasma minimum concentration, maximum concentration and average concentration (area under the concentration-time curve from time of administration to 24 hours at steady state divided by 24). A population PK model was used to simulate prediction bands for the true plasma concentration-time profiles at steady state.
LdT in plasma was analyzed by a validated high-performance liquid chromatography/tandem
mass spectrometry (HPLC/MS/MS) methodology by Quest Pharmaceutical Services (Newark,
DE). The lower limit of quantification was 10 ng/mL. PegIFN alfa-2a levels were analyzed by a
validated HPLC/MS/MS methodology by DMPK Bioanalytics Novartis (East Hanover, NJ). The
lower limit of quantification was 7.2 ng/mL.
S
AFETYThe safety analyses included all patients who received at least one dose of a study drug. Adverse events and laboratory abnormalities were monitored throughout the treatment phase and graded for severity according to criteria adapted from the Division of Acquired Immunodeficiency Syndrome, National Institute of Allergy and Infectious Disease (15). ALT flares were defined as ALT elevations > 2 × baseline and > 10 × ULN, as per the American Association for the Study of Liver Diseases (AASLD) practice guidelines (16). Grade 3-4 creatine kinase (CK) elevations were defined as CK levels > 7 × ULN.
Statistical Analysis
The primary objective and the key secondary objective of this study was to show superior antiviral efficacy (proportion of patients with HBV DNA non-detectability at week 52) of combination therapy over PegIFN monotherapy and LdT monotherapy over PegIFN monotherapy, respectively. Due to the reduced number of patients reaching week 52, the main analysis focused on week 24.
Data for sample size calculation were obtained from LdT and PegIFN alpha-2a pivotal studies (10, 13). A sample size of 100 randomized patients per group was estimated to provide sufficient power (≥ 80%) to detect a 20% treatment difference (HBV DNA non-detectability at Week 52) between combination therapy and telbivudine monotherapy.
The analyzed populations included the population of randomized patients, the intent-to-treat
(ITT) population, which consisted of all patients who received at least one dose of study drug
and underwent at least one post-baseline assessment and the safety population, which consisted
of all patients who received at least one dose of study drug and underwent at least one post- baseline safety assessment.
Pre-specified efficacy analysis (HBV DNA undetectability, ALT normalization, HBeAg loss and HBsAg seroconversion) were compared between treatment groups of the ITT population using the Fisher’s exact test. Changes from baseline of HBV DNA at different times were assessed on all available patients and comparisons between groups were performed using analysis of covariance (ANCOVA). HBeAg and HBsAg quantitative evaluations were performed in all randomized patients who reached week 24.
All statistical tests were performed at a 2-sided 0.05 level.
RESULTS
Disposition of patients and characteristics at baseline
Due to increased rates of serious adverse events (specifically peripheral neuropathies) reported for patients receiving combination therapy, the Data Safety Monitoring Board (DSMB) suspended the combination group in order to review the data. All patients in this group were stopped at the same date, but at varying study visits due to different enrollment times.
Subsequently, the DSMB, together with the sponsor, decided to terminate the study prematurely.
All patients in the monotherapy groups were stopped at the same date, but also at varying study visits due to different enrollment times.
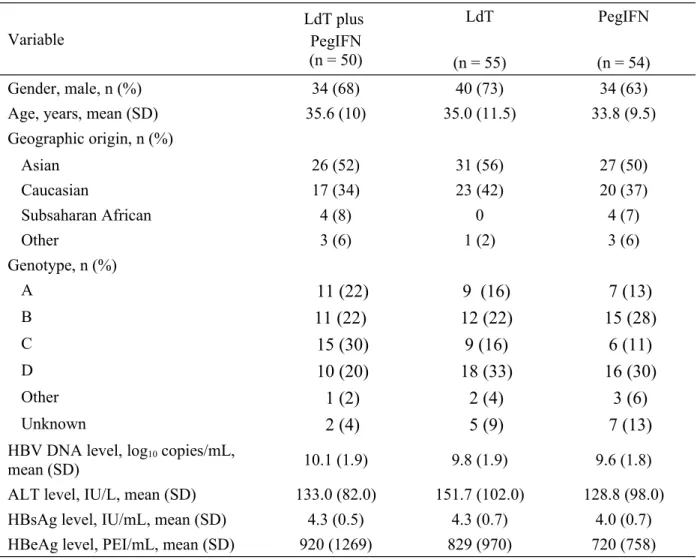
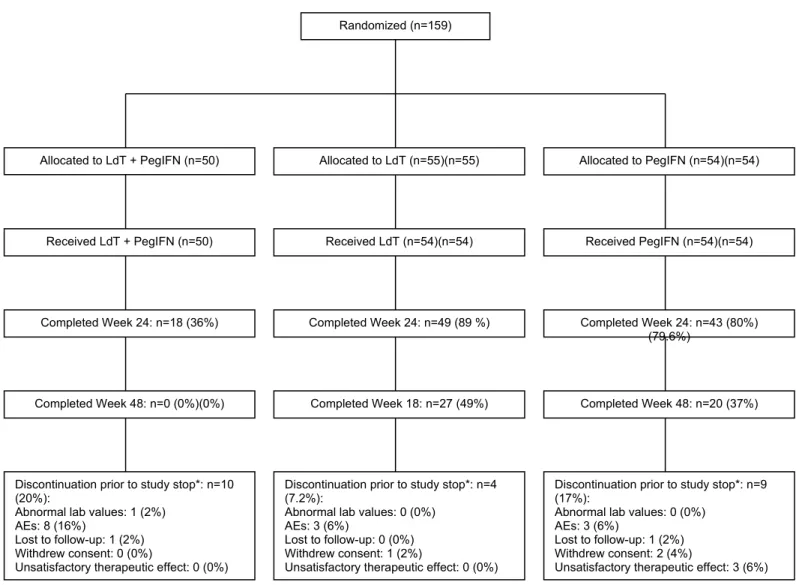
Overall, 159 patients were randomized (50 to combination therapy, 55 to LdT monotherapy, and
54 to PegIFN monotherapy) (Figure 1). One patient in the LdT monotherapy group
did not receive study medication and was excluded from the safety
population. The ITT population included 49 patients to combination therapy, 53 patients in LdT monotherapy group and 54 patients in PegIFN monotherapy group.
A total of 18 (36%) patients in the combination-therapy group, 49 (89%) patients in the LdT monotherapy group and 43 (80%) patients in the PegIFN monotherapy group completed 24 weeks of study. Forty-eight weeks of study were completed by 27 (49%) patients in the LdT monotherapy group and 20 (37%) patients in the PegIFN monotherapy group, while no patients in the combination group reached 48 weeks
Twenty-three patients (15%) discontinued the study before study discontinuation by the sponsor, 10 patients (20%) in the combination-therapy group, 4 (7%) in the LdT monotherapy group and 9 (17%) in the PegIFN monotherapy group; the most frequent reason of early discontinuation was adverse events (14/23 [61%]). The treatment groups were well balanced with respect to baseline demographic and clinical characteristics (Table 1). Mean baseline HBV DNA level was
~10 log
10copies/mL.
Efficacy
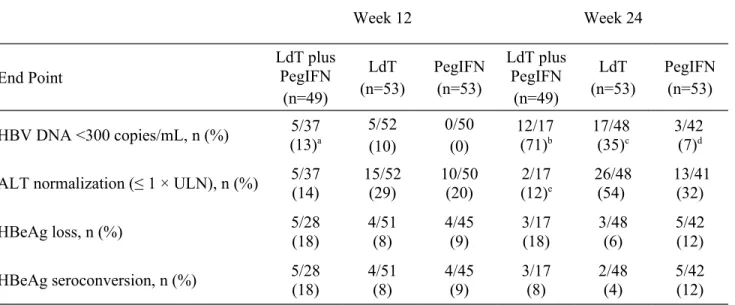
HBV DNA R
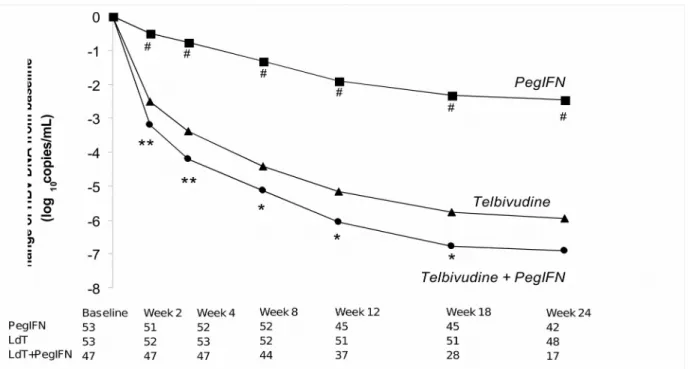
ESPONSECombination therapy was associated with a faster suppression of viral load and higher rate of HBV undetectability (HBV DNA < 300 copies/mL) compared with either monotherapy (Figure 2 and Table 2). From treatment week 2, the reduction from baseline was significantly greater with combination treatment compared to LdT or PegIFN (Figure 2). At week 24, a significantly higher proportion of patients in the combination therapy group (71%) had undetectable HBV DNA compared with either LdT monotherapy (35%; p = 0.022) or PegIFN monotherapy (7%;
p < 0.0001) (Table 2). At week 48, out of the patients still available for analysis, higher rates of
viral suppression were observed among patients in the LdT monotherapy compared with the PegIFN monotherapy group (58% [11/19] vs. 17% [2/12]).
Up to week 52, virologic breakthrough was reported in 8% of patients in the LdT monotherapy group and 9% of patients in the PegIFN monotherapy group but in no patient in the combination therapy group.
ALT N
ORMALIZATIONA significantly greater proportion of patients in the LdT monotherapy group than in the combination therapy group normalized ALT levels at week 24 (54 vs. 12%; p = 0.0036) (Table 2); the rates of ALT normalization were not significantly different between combination therapy and PegIFN monotherapy (12 vs 32%, respectively; p = 0.1878) and between LdT monotherapy and PegIFN monotherapy (54 vs 32%, respectively; p = 0.0531).
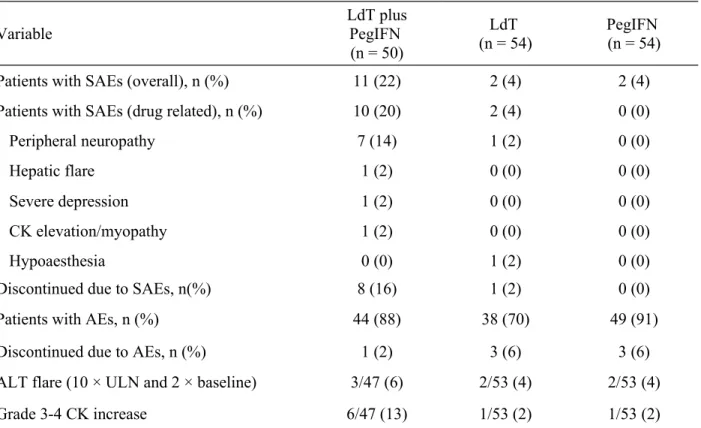
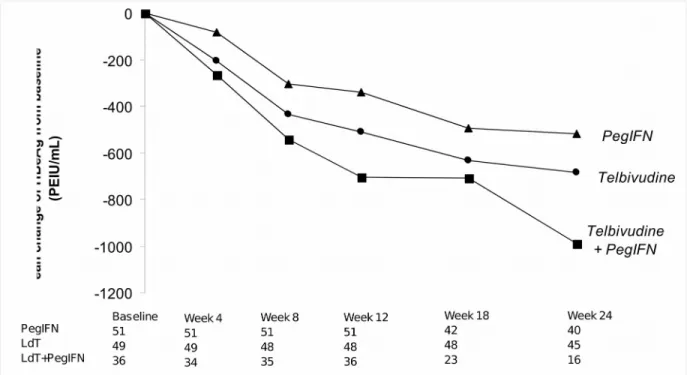
HB
EA
GANDHB
SA
GR
ESPONSESThe combination of LdT and PegIFN appeared to result in a more rapid decline in HBeAg than did either drug as monotherapy, but this difference did not achieve statistical significance (Figure 3). At week 24, rates of HBeAg loss and HBeAg seroconversion were not statistically different between treatment groups (Table 2); at week 48, in the limited number of evaluable patients receiving LdT or PegIFN monotherapy, rates of HBeAg loss were 37% (7/19) for LdT and 25% (3/12) for PegIFN and rates of HBeAg seroconversion were 37% (7/19) for LdT and 25% (3/12) for PegIFN.
The rate of patients with rapid HBsAg decline (≥ 0.5 log
10IU/mL) at week 24 was significantly
higher in combination therapy group (61%) compared to either monotherapy groups: 40% in the
LdT monotherapy group and 28% in the PegIFN monotherapy group (p = 0.03 for all
comparisons) (Table 3). No patient in any treatment group achieved HBsAg loss or seroconversion before the study was terminated.
Pharmacokinetic analysis
Pharmacokinetic analysis indicated no significant interaction between LdT and PegIFN. In addition, there were no specific PK or pharmacodynamic findings in patients who developed peripheral neuropathy. The clearance of LdT was estimated to be 9% higher (90% CI, +1% to +17%) when given in combination with PegIFN alfa-2a compared with LdT monotherapy. Mean trough levels of PegIFN alfa-2a did not differ significantly when it was combined with LdT and when used alone. Thus, there was no evidence that the addition of LdT had an impact on PegIFN alfa-2a pharmacokinetics. In the subgroup reporting clinical symptoms commonly associated with peripheral neuropathy, mean LdT PK parameters (n = 10) and mean PegIFN alfa-2a trough levels (n = 9) were comparable to those in the overall analysis populations.
Safety
Safety results are summarized in Table 4. Due to the earlier discontinuation of the LdT+PegIFN combination group, exposure to study drug was shorter for the combination therapy group (18.2 weeks) compared to the LdT (46.1 weeks) and PegIFN (36.6 weeks) monotherapy groups. The percentage of patients who experienced adverse events was higher in the PegIFN monotherapy group (90.7%) and the combination-therapy group (88.0%) than in the LdT monotherapy group (70.4%) despite the different drug exposure times.
No patient died during the study and the rates of serious adverse events in the combination-
therapy, LdT monotherapy and PegIFN monotherapy groups were 22%, 4% and 4%,
respectively (Table 4).
Peripheral neuropathy was the most often reported serious adverse event (Table 4). Eight cases of peripheral neuropathies were recorded as serious adverse events and were judged as being drug related; 7 occurred in patients receiving combination therapy and, at last follow up, 3 patients had improved and 4 had completely recovered (Table 5). One case of peripheral neuropathy occurred in LdT monotherapy group and at last follow-up the patient had improved.
No relationship between the occurrence of peripheral neuropathy and other variables (e.g., PK data of LdT and PegIFN, treatment efficacy, ALT levels, CK elevations) were observed.
Comparing peripheral neuropathy events in the combination group with previously reported peripheral neuropathy cases in LdT monotherapy in an analysis across the full Novartis clinical study database confirmed, that peripheral neuropathy is an unusual finding with telbivudine monotherapy with 0.45% (10/2200) of cases of serious adverse events (17), while it is a more frequent serious adverse event with combination of PegIFN and telbivudine (7/50; 14%). Time of onset to peripheral neuropathy was an average of 3.8 months in combination (this study) while it was 14 months treatment for LdT monotherapy event (Novartis database) (17).
On-treatment CK elevations of grade 3-4 were reported in 6/47 patients (13%) of the
combination group and 1 patient (2%) in each monotherapy group (Table 4). Grade 3-4 CK
elevations were not found to predict peripheral neuropathy events in combination or
monotherapy groups. On-treatment muscle and connective tissue adverse events were assessed in
patients who had a new onset Grade 3-4 CK elevation and in patients who had no Grade 3-4
elevation. This analysis revealed that there was no relationship between the timing of the clinical
adverse events and the occurrence of the Grade 3-4 CK elevation.
Paraesthesia and myalgia were more frequently reported in the LdT+Peg-INF combination group (12% and 28%) compared to LdT (2% and 9%) and PEG-INF (4% and 17%, respectively) monotherapy groups.
The most frequent adverse events in the PegIFN monotherapy groups were headache (17 [32%]), pyrexia (14 [26%]) and alopecia (15 [28%]), while discontinuations due to adverse event occurred in 3 patients (feeling abnormal, neutropenia and impotence). In the LdT monotherapy group, the most frequent adverse events were upper respiratory tract infections (n=11, 20%) and headache (n=9, 17%).
On-treatment ALT flares were 6% (3/47) in the LdT+PegIFN combination group and 4% (2/53) in each monotherapy group.
No cases of rhabdomyolysis or lactic acidosis were reported.
DISCUSSION
Conclusions regarding the efficacy of combination therapy with LdT plus PegIFN compared with either agent alone in patients with CHB in this trial must be certainly tempered by the early termination and small sample size. Nevertheless, the combination therapy demonstrated additive efficacy over LdT monotherapy as reflected not only by significantly higher and faster HBV DNA decline but also serological responses. Moreover, treatment with LdT monotherapy resulted in faster HBV DNA reduction and similar HBeAg decline, HBeAg seroconversion and HBsAg decline as PegIFN monotherapy.
The present results suggest that the combination of LdT plus PegIFN may be more effective than
the combination of lamivudine plus PegIFN, which has demonstrated only limited additional
benefit over PegIFN monotherapy in previous clinical trials (8, 10-11, 18-19).
Thus, the study of Janssen et al in HBeAg-positive CHB patients concluded that PegIFN alfa-2b (100 µg/week) in combination with lamivudine for 52 weeks was not superior to PegIFN alfa-2b alone due to the high relapse of patients off treatment (8). In the study of Marcellin et al in HBeAg-negative CHB patients and in the study of Lu Lau et al in HBeAg-negative positive CHB patients, addition of lamivudine to PegIFN alfa-2a (180 µg/week) did not improve post- therapy response rates compared to PegIFN alfa-2a alone (10-11).
The difference between efficacy reported in the literature for lamivudine plus PegIFN and efficacy observed in our study for LdT plus PegIFN may be related to the greater antiviral efficacy of LdT as demonstrated in prior clinical trials (12-13).
An unexpected result from our study was the similar HBeAg and HBsAg response to LdT monotherapy and PegIFN monotherapy. ALT and HBV DNA levels at baseline suggest that the severity of the disease was comparable in both monotherapy groups. Despite the unavailability of long-term results of HBeAg or HBsAg clearance due to premature termination of the study, kinetics of decline for both of these parameters might suggest similar response for both agents.
HBsAg decline to undetectable levels with subsequent HBsAg clearance and seroconversion is
the major long-term goal of anti-CHB therapy. Kinetics, predictors and possible mechanisms of
HBsAg decline with various anti-HBV agents are accumulating (14, 20). However, direct head-
to-head comparisons of HBsAg kinetics between active agents are limited. Results from previous
studies showed that nucleos(t)ide analogs such as lamivudine, entecavir and tenofovir are
associated with HBsAg decline of around 0.3 log IU/mL at 6 months (21-22) and are associated
generally with lower HBsAg long-term seroconversion than with PegIFN (10-11). Rapid HBsAg
decline has been shown to be a predictor of subsequent HBsAg clearance (14, 20). Although the
treatment duration in the present study was too short to evaluate HBsAg clearance, the high
percentage of patients with rapid HBsAg decline (≥ 0.5 log
10IU/mL) at week 24 suggests that the combination of LdT plus PegIFN has the potential to achieve higher rates of HBsAg clearance than any other agent. A comparable synergistic effect between LdT and PegIFN was not observed in trials using the combination of lamivudine and PegIFN (8, 10-11).
The safety profile of this combination prevents however further evaluations in this direction.
Indeed, the significant improvement of efficacy results using LdT plus PegIFN combination was eclipsed by the high occurrence of peripheral neuropathies. Serious peripheral neuropathy cases were found mostly in the combination group (only one in the LdT monotherapy group).
Peripheral neuropathy has been rarely reported in untreated CHB and it is more common in end- stage liver disease (23). However, in our trial, patients were treated and another mechanism for peripheral neuropathy could be mitochondrial toxicity. All nucleoside analogs used for the treatment of patients with CHB have the potential for inhibiting the human DNA polymerase-γ involved in mitochondrial DNA replication, which may lead to clinical manifestations of mitochondrial toxicity, including neuropathy, myopathy and lactic acidosis (24). However, studies carried out to date have indicated that LdT monotherapy has rarely been associated with peripheral neuropathy (< 1% of patients) (17, 25). The mechanisms of LdT-associated peripheral neuropathy when coadministrated with PegIFN are unknown. In vitro studies have shown that LdT concentrations up to 10-fold higher than the maximal clinical exposure have no effect on neuronal cells and do not result in mitochondrial toxicity (25). The present results underline that combination of two well-tolerated therapies may produce unexpected adverse events.
Even though the mechanism responsible for peripheral neuropathy remains unclear, further
research into this area is limited by this safety concern. Dosing modification or appropriate
timing of PegIFN and LdT administration might overcome the risk of peripheral neuropathy
while preserving high antiviral efficacy. Nevertheless such trials would have to be closely monitored. At the moment, the concomitant use of PegIFN and LdT is not recommended and a warning was added to the LdT product label to ensure prescribing physicians are aware of these risks.
Despite the appropriate decision of the DSMB to terminate the study prematurely, efficacy
results suggest that the combination of LdT and PegIFN enhances HBV DNA, HBeAg and
HBsAg decline and therefore is scientifically important. However, the optimal combination
regimen of a nucleoside analog and PegIFN remains to be determined. Results from this
shortened trial also suggest that LdT monotherapy has serologic effects comparable to PegIFN,
which has not been observed with any other nucleos(t)ide analog. Preclinical animal studies and
in vitro experiments may help explain the mechanisms of the remarkable efficacy of combination
of LdT and PegIFN and the increased risk of peripheral neuropathy.
ACKNOWLEDGMENTS
The authors thank Kathleen Covino, PhD (Bluesparks), Mechthild Jung, Ph.D. (Novartis Pharma
AG) and Charles Koehne, MSc (Novartis Pharma Corporation) for editorial assistance in the
development of this manuscript, which has been supported by Novartis Pharma AG.
REFERENCES
1. Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med. 2004; 116: 829-34.
2. Chu CM, Liaw YF. Chronic hepatitis B virus infection acquired in childhood: special emphasis on prognostic and therapeutic implication of delayed HBeAg seroconversion. J Viral Hepat. 2007; 14: 147-52.
3. Chu CM, Liaw YF. Predictive factors for reactivation of hepatitis B following hepatitis B e antigen seroconversion in chronic hepatitis B. Gastroenterology. 2007; 133: 1458-65.
4. Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B.
Hepatology. 2002; 35: 1522-7.
5. Krastev ZA. The "return" of hepatitis B. World J Gastroenterol. 2006; 12: 7081-6.
6. Fung J, Lai CL, Yuen MF. To stop or not to stop: the quest for long-term viral suppression. J Gastroenterol Hepatol. 2011; 26: 420-2.
7. Brunetto MR, Moriconi F, Bonino F, Lau GK, Farci P, Yurdaydin C, et al. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology. 2009; 49: 1141-50.
8. Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, et al.
Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005; 365: 123-9.
9. Kau A, Vermehren J, Sarrazin C. Treatment predictors of a sustained virologic response
in hepatitis B and C. J Hepatol. 2008; 49: 634-51.
10. Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, et al.
Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005; 352: 2682-95.
11. Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, et al. Peginterferon alfa- 2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004; 351: 1206-17.
12. Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007; 357: 2576-88.
13. Liaw YF, Gane E, Leung N, Zeuzem S, Wang Y, Lai CL, et al. 2-Year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B.
Gastroenterology. 2009; 136: 486-95.
14. Wursthorn K, Jung M, Riva A, Goodman ZD, Lopez P, Bao W, et al. Kinetics of hepatitis B surface antigen decline during 3 years of telbivudine treatment in hepatitis B e antigen-positive patients. Hepatology. 2010; 52: 1611-20.
15. Division of AIDS. Table for grading severity of adult adverse experiences. Bethesda, MD: National Institute of Allergy and Infectious Diseases, 1992.
16. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009; 50: 661-2.
17. Goncalves J, Laeufle R, Avila C. Increased risk with combination of telbivudine and pegylated-interferon Alfa-2A in study CLDT600A2406, compared to uncommon rate with telbivudine monotherapy from the Novartis global database. J Hepatol. 2009; 50:
S329, Abstract #907.
18. Kaymakoglu S, Oguz D, Gur G, Gurel S, Tankurt E, Ersoz G, et al. Pegylated interferon
Alfa-2b monotherapy and pegylated interferon Alfa-2b plus lamivudine combination
therapy for patients with hepatitis B virus E antigen-negative chronic hepatitis B.
Antimicrob Agents Chemother. 2007; 51: 3020-2.
19. Papadopoulos VP, Chrysagis DN, Protopapas AN, Goulis IG, Dimitriadis GT, Mimidis KP. Peginterferon alfa-2b as monotherapy or in combination with lamivudine in patients with HBeAg-negative chronic hepatitis B: a randomised study. Med Sci Monit. 2009; 15:
CR56-61.
20. Moucari R, Korevaar A, Lada O, Martinot-Peignoux M, Boyer N, Mackiewicz V, et al.
High rates of HBsAg seroconversion in HBeAg-positive chronic hepatitis B patients responding to interferon: a long-term follow-up study. J Hepatol. 2009; 50: 1084-92.
21. Gane EJ, Marcellin P, Jacobson I, Heathcoate EJ, Dusheiko G, de Man R. HBsAg kinetics of decay and baseline characteristics of HBeAg-positive patients with chronic hepatitis B following 3 years of tenofovir disoproxil fumarate (TDF) treatment [Abstract]. J Hepatol. 2010; 52: S388.
22. Jung YK, Kim JH, Lee YS, Lee HJ, Yoon E, Jung ES, et al. Change in serum hepatitis B surface antigen level and its clinical significance in treatment-naive, hepatitis B e antigen-positive patients receiving entecavir. J Clin Gastroenterol. 2010; 44: 653-7.
23. Inoue A, Tsukada N, Koh CS, Yanagisawa N. Chronic relapsing demyelinating polyneuropathy associated with hepatitis B infection. Neurology. 1987; 37: 1663-6.
24. Fontana RJ. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology.
2009; 49: S185-95.
25. Fleischer RD, Lok AS. Myopathy and neuropathy associated with nucleos(t)ide analog
therapy for hepatitis B. J Hepatol. 2009; 51: 787-91.
Table 1. Patient demographics and baseline characteristics (randomized population).
Variable
LdT plus PegIFN (n = 50)
LdT (n = 55)
PegIFN (n = 54)
Gender, male, n (%) 34 (68) 40 (73) 34 (63)
Age, years, mean (SD) 35.6 (10) 35.0 (11.5) 33.8 (9.5)
Geographic origin, n (%)
Asian 26 (52) 31 (56) 27 (50)
Caucasian 17 (34) 23 (42) 20 (37)
Subsaharan African 4 (8) 0 4 (7)
Other 3 (6) 1 (2) 3 (6)
Genotype, n (%)
A
11 (22) 9 (16) 7 (13)
B
11 (22) 12 (22) 15 (28)
C
15 (30) 9 (16) 6 (11)
D
10 (20) 18 (33) 16 (30)
Other
1 (2) 2 (4) 3 (6)
Unknown
2 (4) 5 (9) 7 (13)
HBV DNA level, log10 copies/mL,
mean (SD) 10.1 (1.9) 9.8 (1.9) 9.6 (1.8)
ALT level, IU/L, mean (SD) 133.0 (82.0) 151.7 (102.0) 128.8 (98.0)
HBsAg level, IU/mL, mean (SD) 4.3 (0.5) 4.3 (0.7) 4.0 (0.7)
HBeAg level, PEI/mL, mean (SD) 920 (1269) 829 (970) 720 (758) ALT, alanine aminotransferase; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B s antigen; HBV, hepatitis B virus; LdT, telbivudine; PegIFN, pegylated interferon.
Table 2. Virologic and biochemical end points at weeks 12 and 24 (ITT population).
Week 12 Week 24
End Point
LdT plus PegIFN
(n=49)
LdT (n=53)
PegIFN (n=53)
LdT plus PegIFN
(n=49)
LdT (n=53)
PegIFN (n=53) HBV DNA <300 copies/mL, n (%) 5/37
(13)a
5/52 (10)
0/50 (0)
12/17 (71)b
17/48 (35)c
3/42 (7)d ALT normalization (≤ 1 × ULN), n (%) 5/37
(14)
15/52 (29)
10/50 (20)
2/17 (12)e
26/48 (54)
13/41 (32)
HBeAg loss, n (%) 5/28
(18)
4/51 (8)
4/45 (9)
3/17 (18)
3/48 (6)
5/42 (12) HBeAg seroconversion, n (%) 5/28
(18)
4/51 (8)
4/45 (9)
3/17 (8)
2/48 (4)
5/42 (12) LdT, telbivudine; PegIFN, pegylated interferon
a p = 0.012 for PegIFN+LdT vs. PegIFN
b p = 0.022 for PegIFN+LdT vs. LdT
c p = 0.002 for LdT vs. PegIFN
d p < 0.0001 for PegIFN vs. PegIFN+LdT
e p = 0.0036 for PegIFN+LdT vs. LdT.
Table 3. HBsAg decline during 24 weeks (in patients who reached week 24).
HBsAg decline from baseline at week 24 (log10 IU/mL)
LdT plus PegIFN N=18
LdT n=47
PegIFN n=43
≥ 0.5, n (%) 11 (61)* 19 (40) 12 (28)
]0–0.5[, n (%) 5 (28) 16 (34) 17 (40)
≤ 0, n (%) 2 (11) 12 (26) 14 (33)
LdT, telbivudine; PegIFN, pegylated interferon
* p=0.03 (chi-square non zero correlation between all three treatment groups).
Table 4. Safety and selected laboratory abnormalities (randomized population)
Variable
LdT plus PegIFN (n = 50)
LdT (n = 54)
PegIFN (n = 54)
Patients with SAEs (overall), n (%) 11 (22) 2 (4) 2 (4)
Patients with SAEs (drug related), n (%) 10 (20) 2 (4) 0 (0)
Peripheral neuropathy 7 (14) 1 (2) 0 (0)
Hepatic flare 1 (2) 0 (0) 0 (0)
Severe depression 1 (2) 0 (0) 0 (0)
CK elevation/myopathy 1 (2) 0 (0) 0 (0)
Hypoaesthesia 0 (0) 1 (2) 0 (0)
Discontinued due to SAEs, n(%) 8 (16) 1 (2) 0 (0)
Patients with AEs, n (%) 44 (88) 38 (70) 49 (91)
Discontinued due to AEs, n (%) 1 (2) 3 (6) 3 (6)
ALT flare (10 × ULN and 2 × baseline) 3/47 (6) 2/53 (4) 2/53 (4)
Grade 3-4 CK increase 6/47 (13) 1/53 (2) 1/53 (2)
AE, adverse event; ALT, alanine aminotransferase; CK, creatine kinase; LdT, telbivudine; PegIFN, pegylated interferon; SAE, serious adverse event; ULN, upper limit of normal.
Patients could have multiple events and are counted once for each event, but only listed once for the overall number of patients with SAEs.
Table 5. Details for serious adverse events involving peripheral neuropathy.
Age/gender Preferred term Time to onset Outcome at last
follow-up LdT plus PegIFN combination
24/F Neuropathy peripheral 77 days Complete recovery
47/M Peripheral neuropathy paresthesia 99 days Improved
21/M Polyneuropathy 67 days Improved
24/F Peripheral sensory neuropathy 128 days Improved
45/M Peripheral sensory neuropathy 89 days Complete recovery
55/M Demyelinating polyneuropathy, PN 168 days Complete recovery
32/M Peripheral sensory neuropathy 186 days Complete recovery
LdT monotherapy
23/F Peripheral sensory neuropathy 115 days Improved
F, female; LdT, telbivudine; M, male; PegIFN, pegylated interferon.
Figure 1. Patient disposition.
* All remaining patients discontinued from study when the sponsor decided to discontinue the study
Randomized (n=159)
Discontinuation prior to study stop*: n=10 (20%):
Abnormal lab values: 1 (2%) AEs: 8 (16%)
Lost to follow-up: 1 (2%) Withdrew consent: 0 (0%)
Unsatisfactory therapeutic effect: 0 (0%)
Discontinuation prior to study stop*: n=4 (7.2%):
Abnormal lab values: 0 (0%) AEs: 3 (6%)
Lost to follow-up: 0 (0%) Withdrew consent: 1 (2%)
Unsatisfactory therapeutic effect: 0 (0%)
Discontinuation prior to study stop*: n=9 (17%):
Abnormal lab values: 0 (0%) AEs: 3 (6%)
Lost to follow-up: 1 (2%) Withdrew consent: 2 (4%)
Unsatisfactory therapeutic effect: 3 (6%) Allocated to LdT (n=55)(n=55) Allocated to PegIFN (n=54)(n=54)
Received LdT + PegIFN (n=50) Received LdT (n=54)(n=54) Received PegIFN (n=54)(n=54)
Completed Week 24: n=18 (36%) Completed Week 24: n=49 (89 %) Completed Week 24: n=43 (80%) (79.6%)
Completed Week 48: n=0 (0%)(0%) Completed Week 18: n=27 (49%) Completed Week 48: n=20 (37%) Allocated to LdT + PegIFN (n=50)
Figure 2. Changes from baseline in HBV DNA by PCR assay through treatment week 24 (all available patients).
* p < 0.05 for telbivudine (LdT) vs. telbivudine + pegylated interferon (PegIFN)
** p < 0.01 for telbivudine vs. telbivudine + pegylated interferon
# p < 0.0001 for pegylated interferon vs telbivudine
Figure 3. Decline in on-treatment patients of HBeAg levels (all available patients)
LdT, telbivudine; PegIFN, pegylated interferon.