慢性運動對於肥胖鼠血管功能之影響(1/2)
計畫類別: 個別型計畫
計畫編號: NSC94-2320-B-006-062-
執行期間: 94 年 08 月 01 日至 95 年 07 月 31 日 執行單位: 國立成功大學物理治療學系
計畫主持人: 楊艾倫 計畫參與人員: 賴詠淳
報告類型: 精簡報告
處理方式: 本計畫可公開查詢
中 華 民 國 95 年 5 月 25 日
ABSTRACT
OBJECTIVE: Insulin and insulin-like growth factor-1 (IGF-1) have vasorelaxant effects in
vivo, which is dependent on nitric oxide (NO) production. The aim of this study was to investigate the vasorelaxant responses mediated by insulin and/or IGF-1 in aortas of obese Zucker rats. METHODS: The thoracic aortas of 8 lean and 8 obese Zucker rats (6 months old) were isolated for vasorelaxation analysis. Insulin-induced and IGF-1-induced vasorelaxant responses were evaluated by the isometric tension of aortic rings in the organ bathes. The roles of phosphatidylinositol 3-kinase (PI3K) and nitric oxide synthase (NOS) in vasorelaxant responses were examined by treating selective inhibitors, such as wortmannin (an inhibitor of PI3K) and Nω-nitro-L-arginine methyl ester (L-NAME, a NOS inhibitor). In addition, the vascular responses to sodium nitroprusside (SNP), a direct vasodilator of vascular smooth muscle, were examined. RESULTS: The insulin-induced vasorelaxation in aortas of obese rats was significantly decreased, whereas the IGF-1-induced vasorelaxation was significantly increased, compared with that in lean rats. After the pre-administration of wortmannin or L-NAME, the altered insulin-induced or IGF-1-induced vasorelaxation was abolished. There was no significant difference in the SNP-induced vasorelaxation between lean and obese rats.
CONCLUSION: Our findings suggested that the decreased insulin-mediated vasorelaxation
in obese rats appeared to be counteracted by the increased IGF-1-mediated vasorelaxation.
Furthermore, the NO-dependent pathway was involved in the altered vasorelaxant responses.
However, the SNP-induced vasorelaxation was not changed in obese rats.
Keywords: insulin, IGF-1, phosphatidylinositol 3-kinase, nitric oxide, vasodilation, obesity
INTRODUCTION
The obese Zucker rat, a genetic model of morbid obesity, presents many of the same metabolic and cardiopulmonary deficits as noted in obese humans, including increased risks of type II diabetes mellitus 1, hypertension 2, respiratory dysfunction 3-5, upper airway narrowing 6 and poor exercise capacity 7, 8. Various factors, such as insulin resistance, nocturnal hypoxia, hypertension and poor exercise capacity, often observed in the obesity, may directly or indirectly contribute to the excessive rates of cardiovascular diseases and vascular dysfunctions 9-12. Abnormalities in vascular functions have been described in obese animals and obese humans such as increased risks of atherosclerosis, endothelial abnormalities, altered vascular inflammatory markers, altered vascular contractile properties and the imbalance of vasodilators and vasoconstrictors 13-15. However, the precise mechanisms of vasodilatory functions in the obesity were not totally understood.
Insulin and IGF-1 have specific and physiological functions in regulating vascular contractilities in experimental animals and humans as well as they have been shown to cause decreases in vascular resistances 16. In experimental animals, insulin has differential effects in different vascular beds, exerting vasorelaxant effects on the femoral, aortic, coronary and tail vasculature 16, 17. IGF-1 has effects on the regulation of vascular tone that are similar to those of insulin, with regional differences in vasorelaxant responses 18. The endothelium-derived relaxing factor, nitric oxide (NO), appears to be an important mediator of insulin-induced and IGF-1-induced vascular relaxations 17, 19, 20. Both insulin and IGF-1 stimulate NO production and diminish in vivo and in vitro vascular contractilities in intact vessels 17, 19, 20. Using a specific inhibitor of NOS, N-monomethyl-L-arginine, the investigators have demonstrated that insulin-mediated and IGF-1-mediated vasorelaxations are highly dependent on vascular NO production 20, 21. The signaling pathways of NO formation involve tyrosine kinase and
phosphatidylinositol 3-kinase (PI3K) activities 21. Both insulin and IGF-1 mediate vascular relaxations mainly through activating PI3K and NOS to further modulate vascular tones 16, 22. In previous study, a diminished ability of insulin to attenuate vasocontractile responses was found in obese rats 23. Disturbances of NO have been reported in both obese animals and humans 1, 24. Nevertheless, the roles of the PI3K and NOS on insulin-mediated and IGF-1-mediated vascular relaxations in the obesity are still unexplored.
Sodium nitroprusside (SNP), a direct vasodilator of vascular smooth muscle, induces endothelium-independent vasodilatory responses. The arteriolar dilation of skeletal muscle in response to SNP was reduced in hypertensive diabetic obese Zucker rats, compared with lean rats 25. However, SNP-mediated vasorelaxation of the descending thoracic aorta was not changed in insulin-resistant obese rats 26. Until now, the vascular responses to SNP are still controversial in the obese animal models.
Therefore, the purpose of this study was to investigate whether the altered insulin-mediated or IGF-1-mediated vasorelaxation occurs in the isolated thoracic aortas of obese Zucker rats. Also, the roles of PI3K and NOS in the NO-dependent vasorelaxant pathway were examined. Furthermore, the SNP-induced vasorelaxation in aortic rings of obese rats was observed. We hypothesized that altered insulin-mediated and IGF-1-mediated vasorelaxations occurred in morbidly obese Zucker rats, compared with lean rats. A parallel study design was used, with lean, age-matched Zucker rats serving as controls.
METHODS Animals
The studies were performed on 8 lean (Fa/Fa or Fa/fa) and 8 obese (fa/fa) age matched 6 months old male Zucker rats. Animals were born by female (Fa/fa) and male (Fa/fa) breeders purchased from Charles River Lab in France. One lean and one obese rat were obtained from the same breeder and were housed per cage. Ambient temperature was maintained at 25 °C and the animals were kept on an artificial 12-h light-dark cycle. The light period began at 7:00 A.M. Rats were provided with standard laboratory chow (Lab Diet 5001; PMI Nutrition International Inc., Brentwood, MO, USA) and water ad libitum. All protocols were approved by the Institutional Animal Care and Use Committee of National Cheng Kung University, Tainan, Taiwan and the principles of laboratory animal care (NIH publication) were followed.
Evaluation of Vasorelaxant Responses
All rats were weighed and sacrificed under general anesthesia with ether inhalation. The thoracic aortas were immediately isolated for various experiments described below. The vasorelaxant responses were recorded using the isometric tension of aortic rings, of which complete details had previously been provided 11, 12. The isolated vessel rings of thoracic aortas (3 mm long) were mounted on force-displacement transducers (Grass Instrument, Rhode Island, USA) and submerged in organ chambers containing Krebs-Ringer solution (compositions in mmol/L: 118 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 24 NaHCO3, 0.03 Na2-EDTA, and 11 glucose) bubbling with 95% O2-5% CO2 at 37oC. They were stretched to the optimal passive tension (i.e. 2 g) at which the contraction evoked by phenylephrine was maximal. The vessel rings were equilibrated for at least 90 min, precontracted with phenylephrine (10-7 mol/L, Sigma Chemical, St. Louis, MO, USA), and exposed to various concentrations of insulin (3x10-7-10-5 mol/L, Sigma Chemical) or IGF-1 (3x10-9-10-7 mol/L, CytoLab, Rehovot, Israel) to evoke vasorelaxant responses. The
vasorelaxant responses (i.e. vasorelaxation), which is defined as reduction in tension of the walls of the blood vessels, were expressed as percentages of the precontractile force.
In some phenylephrine-precontracted vessels, the vasorelaxant responses to 10-9 mol/L sodium nitroprusside (SNP, a NO donor) (Merck, Darmstadt, Germany), a direct vasodilator of vascular smooth muscle, were also examined to observe whether the vascular responses were affected in obese rats.
Examination of PI3K and NOS in Vasorelaxant Responses
The possible roles of PI3K and NOS in the insulin-induced or IGF-1-induced vasorelaxant responses were examined by no inhibitor or pre-administration of either wortmannin (3x10-7 mol/L; an inhibitor of PI3K) (Sigma Chemical), or Nω-nitro-L-arginine methyl ester (L-NAME; 3x10-7 mol/L; a NOS inhibitor) (Sigma Chemical) into the oxygenated organ chambers 15 min before the administration of phenylephrine.
Statistical Analysis.
All data presented in the figures and texts were means ± SEM. Sample sizes were indicated by “n”. Body weights of lean and obese rats were tested by unpaired Student’s t test.
The responses of vasorelaxation were analyzed by the analysis of variance using the general linear model (GLM) in a one between (lean and obese) and one within (different drugs) design. Differences in the responses of vasorelaxation among different drugs were subsequently tested as single group repeated measures with contrast transformation.
Differences of SNP-induced vasorelaxations between lean and obese rats were tested by unpaired Student’s t test. In all cases, a difference at P < 0.05 was considered statistically significant.
RESULTS
Obese rats weighed about 29% more than age-matched lean animals (380.4±11.4 g vs.
498.9±14.0 g for lean vs. obese groups, respectively; n = 8, P < 0.01). Obese rats had a higher level of blood glucose than lean rats (118.5±5.8 mg/dL vs. 188.3±27.1 mg/dL for lean vs.
obese groups, respectively; n=8, P < 0.05).
Insulin-induced Vasorelaxation. When the phenylephrine (10-7 mol/L)-induced contraction had reached a plateau level, insulin (3x10-7-10-5 mol/L) was added cumulatively.
In aortic rings from lean and obese Zucker rats, insulin (3x10-7-10-5 mol/L) caused a concentration-dependent vasorelaxation (Fig. 1). Downward shift of the concentration-response curve for insulin was found in obese Zucker rats (Fig. 1). The insulin-induced vasorelaxation was significantly (P < 0.05) reduced in thoracic aortas of obese rats compared with that in lean rats.
IGF-1-induced Vasorelaxation. Similarly, IGF-1 (3x10-9-10-7 mol/L) was added cumulatively when the aortic ring was pre-contracted with phenylephrine (10-7 mol/L). The administration of IGF-1 (3x10-9-10-7 mol/L) induced a concentration-dependent vasorelaxation in lean and obese Zucker rats (Fig. 2). Upward shift of the concentration-response curve for IGF-1 was found in obese Zucker rats (Fig. 2) In contrast with the insulin-induced vasorelaxation, the IGF-1-induced vasorelaxation was significantly (P < 0.05) elevated in thoracic aortas of obese Zucker rats compared with that in lean rats.
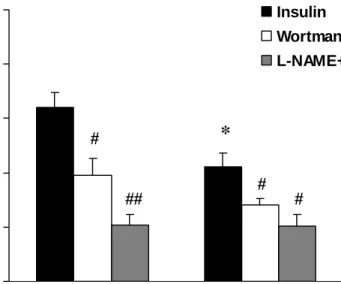
Roles of PI3K and NOS in Insulin-induced Vasorelaxation. The insulin-induced
vasorelaxation in lean and obese Zucker rats was greatly diminished by the pre-incubation with wortmannin (3x10-7 mol/L; an inhibitor of PI3K) or L-NAME (3x10-7 mol/L; a NOS inhibitor). Prior to the administration of wortmannin or L-NAME, the vascular response to 3x10-6 mol/L of insulin in the obese rats was significantly lower than that in the lean rats (P <
0.05; Fig. 3). When wortmannin was added, insulin-evoked vasorelaxation was significantly
reduced in either lean or obese groups, and the group difference between lean and obese rats disappeared. Similar results were observed when the vessels were pretreated with L-NAME (Fig. 3). The pretreatment of L-NAME also inhibited insulin-evoked vasorelaxation in either lean or obese groups and attenuated the group difference between lean and obese rats.
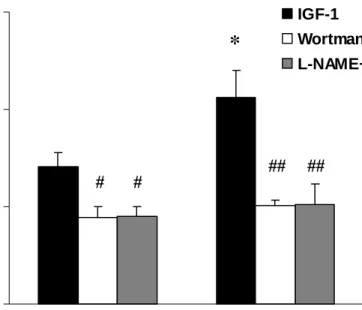
Roles of PI3K and NOS in IGF-1-induced Vasorelaxation. The IGF-1-induced
vasorelaxation in lean and obese Zucker rats was also greatly diminished by the pre-incubation with wortmannin (3x10-7 mol/L) or L-NAME (3x10-7 mol/L). In contrast with insulin-induced vasorelaxation, the vascular response to 10-8 mol/L of IGF-1 in the obese rats was significantly higher than that in the lean rats before the administration of wortmannin or L-NAME (P< 0.05; Fig. 4). Moreover, the pretreatment of wortmannin or L-NAME significantly inhibited IGF-1-evoked vasorelaxation and attenuated the group difference between lean and obese rats (Fig. 4).
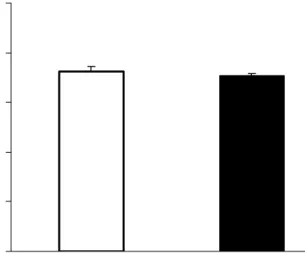
Relaxation Responses to SNP. The relaxation responses to SNP (10-9 mol/L), a direct vasodilator of vascular smooth muscle, was the same between lean and obese Zucker rats (Fig.
5). It indicated that obesity did not affect the downstream signaling pathways of NO in rat smooth muscle.
DISSCUSSION
Our results clearly demonstrated that 1) in comparison with age-matched lean rats, the insulin-induced vasorelaxation was significantly decreased in thoracic aortas of obese Zucker rats whereas the IGF-1-induced vasorelaxation was significantly increased; 2) after the administration of wortmannin or L-NAME, the altered insulin-induced or IGF-1-induced vasorelaxation was abolished; 3) the SNP-induced vasorelaxation was not affected in obese Zucker rats compared with lean rats.
The isolated thoracic aortas measured by the isometric tension of aortic rings precontracted with phenylephrine in the organ bathes were used in the present study. All vasorelaxant responses were presented in % of the precontractile force of phenylephrine.
Phenylephrine, a synthetic sympathomimetic agent, is an alpha-adrenergic agonist vasoconstrictor and is a well developed agonist for pre-contracting vascular rings. The insulin and IGF-1 concentrations used in this study were modified according to the previous study 27. Consistently, lower sensitivity of insulin-induced vasorelaxation and higher sensitivity of IGF-1-induced vasorelaxation were found in both lean and obese rats. Many reports have indicated that the fasting level of insulin was significantly increased in obese Zucker rats, compared with lean rats. Otherwise, the circulating IGF-1 level was similar between lean and obese rats 28, 29. However, whether the vascular sensitivity to insulin or IGF-1 is associated with the circulating level of insulin or IGF-1 remain unknown. Both insulin and IGF-1 have been found to stimulate NO production from endothelial and smooth muscle cells 16. Since the vessel ring contains both endothelium and smooth muscle, any effect of the insulin-mediated or IGF-1-mediated vasorelaxation in the current study can not be clarified whether this response exists in endothelial, smooth muscle cells or both. Further studies are required to look into specific mechanisms of vasorelaxant responses mediated by insulin and IGF in endothelium or smooth muscle of obese rats. Besides, our data obtained from obese rats in six
months of age may only represent the altered vasorelaxant mechanism in mature obesity, which may not be suitable for younger obesity or aging obesity. The vascular function may be potentially interacted with various factors, often seen in the obesity, such as hypertension, volume overload, hypoxia, other hormonal disorders, oxidative stress and physical inactivity 9-15. Therefore, we have to add a note of caution that any effect on vascular changes in the obesity noted in the present investigation cannot be isolated to any specific interacting factors, such as hypertension, volume overload, nocturnal hypoxemia, oxidative stress, or other unclear factors.
The present study indicated that, compared with lean rats, the insulin-induced vasorelaxation in thoracic aortas of obese Zucker rats was significantly decreased whereas the IGF-1-induced vasorelaxation was significantly increased. In consistent with previous study, a diminished ability of insulin to attenuate vasocontractile responses was reported in obese rats
23. Besides, IGF-1 has been demonstrated to function as a vasodilator that are similar to those of insulin 18. Therefore, the increased IGF-1-induced aortic vasorelaxation in obese Zucker rats might imply that the impaired insulin-mediated vasorelaxation appear to be counteracted with the up-regulated IGF-I-mediated vasorelaxation in obese rats. We speculate that our findings in obese Zucker rats could be useful models for investigating similar vascular pathophysiologic conditions occurred in obese animals or obese human.
Insulin and IGF-1 mediate vascular relaxation mainly through the NO-dependent pathway involving the PI3K and NOS activities 16, 22. In our study, after the administration of wortmannin (an inhibitor of PI3K) or L-NAME (a NOS inhibitor), no significant difference of the insulin-induced or IGF-1-induced vasorelaxation was found between lean and obese groups. These findings suggested that the decreased insulin-mediated vasorelaxation in obese Zucker rats was mainly due to the reduced release of PI3K and NOS in the NO-dependent vasorelaxant pathway. On the contrary, the increased IGF-1-mediated vasorelaxation in the
obesity was due to the elevated level of PI3K and NOS. Similar results in human leg vessels were found in the previous study. In 20 lean and 20 obese human subjects, intra-arterial infusions of N-monomethyl-L-arginine (L-NMMA; an inhibitor of NOS) alone produced an approximately 40% increase in the leg vascular resistance, which was not statistically different between two groups 14. In another study, it has been reported that the IGF-1-induced vasorelaxation was greater, the IGF-1 receptor was elevated, and the endothelial NOS expression was also increased in thoracic aortas of streptozotocin (STZ)-induced diabetic rats
27. It indicated that this change of vasorelaxant response was associated with the alteration of IGF-1 receptor level in diabetic rats. In addition, insulin treatment could further increase the aortic IGF-1 receptors in STZ-induced diabetic rats 27. However, whether hyperinsulinemia or other biochemical factors contribute to the up-regulation of vascular IGF receptors in diabetic rats or diabetic obese rats remain unknown. Further studies should be conducted to explore, in the obesity, whether the levels of insulin or IGF-1 receptors and their underlying mechanisms may contribute to the change of the insulin-induced or IGF-1-induced vasorelaxation.
The SNP-induced relaxation in the thoracic aortas of obese Zucker rats was not altered in our study. It indicated that the endothelium-independent NO pathway in vascular smooth muscle was not affected in the obesity. Vasorelaxation in response to SNP in different obese animal models appeared to be still controversial. In insulin-resistant obese rats, the SNP-induced vasorelaxation of the descending thoracic aorta was not changed 26. In addition, no significant difference was observed in vascular response to SNP in adiponectin-knockout obese mice compared with wild type mice 30. However, the arteriolar dilation of skeletal muscle in response to SNP was reduced in hypertensive diabetic obese Zucker rats compared with lean rats 25. We speculated that different findings of the vascular responses to SNP may be due to different pathophysiologic conditions (ex. hypertensive or diabetic), different age, or different vascular beds.
Obesity has recently been recognized as a major modifiable risk factor for cardiovascular disease, second only to cigarette smoking 31. Excess weight and obesity markedly increase the risk for hypertension, diabetes, coronary artery disease, congestive heart failure and abnormalities in the vascular function in both men and women 13-15, 31. Our current findings indicating “the decreased insulin-mediated vasorelaxation in obese rats appear to be counteracted with the increased IGF-1-mediated vasorelaxation” might provide one of possible mechanisms to explain the development and compensation of vascular dysfunction in the obesity. Also, we found that the effects of obesity on vasorelaxant responses mediated by insulin or IGF-1 were mainly due to the altered release of PI3K and NOS. Clinically, when considering possible therapeutic agents to control or prevent the development of vascular dysfunction in the obesity, it should be paid attention on actions of insulin and IGF-1 on the vasorelaxant responses.
ACKNOWLEDGEMENTS
This study was supported by the grant NSC94-2320-B-006-062 and was partially supported by NSC94-2314-B-040-007 from the National Science Council, Taiwan.
REFERENCES
1 Bohlen HG, Nase GP. Obesity lowers hyperglycemic threshold for impaired in vivo endothelial nitric oxide function. Am J Physiol Heart Circ Physiol 2002; 283: H391-7.
2 Alonso-Galicia M, Brands MW, Zappe DH, Hall JE. Hypertension in obese Zucker rats.
Role of angiotensin II and adrenergic activity. Hypertension 1996; 28: 1047-54.
3 Lee SD, Magalang UJ, Krasney JA, Farkas GA. Opioidergic modulation of ventilatory response to sustained hypoxia in obese Zucker rats. Obes Res 2001; 9: 407-13.
4 Lee SD, Nakano H, Farkas GA. NMDA receptor-mediated modulation of ventilation in obese Zucker rats. Int J Obes Relat Metab Disord 2001; 25: 997-1004.
5 Lee SD, Nakano H, Farkas GA. Adenosinergic modulation of ventilation in obese zucker rats. Obes Res 2005; 13: 545-55.
6 Nakano H, Magalang UJ, Lee SD, Krasney JA, Farkas GA. Serotonergic modulation of ventilation and upper airway stability in obese Zucker rats. Am J Respir Crit Care Med 2001;
163: 1191-7.
7 Lee SD, Nakano H, Farkas GA. GABAergic modulation of ventilation and peak oxygen consumption in obese Zucker rats. J Appl Physiol 2001; 90: 1707-13.
8 Lee SD, Nakano H, Gosselin LE, Krasney JA, Schlenker EH, Farkas GA. Endogenous opioids modulate ventilation and peak oxygen consumption in obese Zucker rats. Am J Respir Crit Care Med 2000; 162: 1009-15.
9 Steinberg HO, Baron AD. Vascular function, insulin resistance and fatty acids.
Diabetologia 2002; 45: 623-34.
10 Zhang L. Adaptation of pharmacomechanical coupling of vascular smooth muscle to chronic hypoxia. Comp Biochem Physiol A Mol Integr Physiol 1998; 119: 661-7.
11 Yang AL, Chen HI. Chronic exercise reduces adhesion molecules/iNOS expression and partially reverses vascular responsiveness in hypercholesterolemic rabbit aortae.
Atherosclerosis 2003; 169: 11-7.
12 Yang AL, Jen CJ, Chen HI. Effects of high-cholesterol diet and parallel exercise training on the vascular function of rabbit aortas: a time course study. J Appl Physiol 2003; 95:
1194-200.
13 Wright GL, Morrison R, Fultz ME, Wright G, McCumbee W, Wehner P, et al. Effect of fasting on vascular contractility in lean and obese Zucker rats. Clin Nutr 2003; 22: 359-63.
14 Mather KJ, Lteif A, Steinberg HO, Baron AD. Interactions between endothelin and nitric oxide in the regulation of vascular tone in obesity and diabetes. Diabetes 2004; 53: 2060-6.
15 Vigili de Kreutzenberg S, Kiwanuka E, Tiengo A, Avogaro A. Visceral obesity is characterized by impaired nitric oxide-independent vasodilation. Eur Heart J 2003; 24:
1210-5.
16 Sowers JR. Insulin and insulin-like growth factor in normal and pathological cardiovascular physiology. Hypertension 1997; 29: 691-9.
17 Zeng G, Quon MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest 1996; 98: 894-8.
18 Wu HY, Jeng YY, Yue CJ, Chyu KY, Hsueh WA, Chan TM. Endothelial-dependent vascular effects of insulin and insulin-like growth factor I in the perfused rat mesenteric artery and aortic ring. Diabetes 1994; 43: 1027-32.
19 Walsh MF, Barazi M, Pete G, Muniyappa R, Dunbar JC, Sowers JR. Insulin-like growth factor I diminishes in vivo and in vitro vascular contractility: role of vascular nitric oxide.
Endocrinology 1996; 137: 1798-803.
20 Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 1994; 94: 1172-9.
21 Schini-Kerth VB. Dual effects of insulin-like growth factor-I on the constitutive and inducible nitric oxide (NO) synthase-dependent formation of NO in vascular cells. J Endocrinol Invest 1999; 22: 82-8.
22 Isenovic E, Walsh MF, Muniyappa R, Bard M, Diglio CA, Sowers JR.
Phosphatidylinositol 3-kinase may mediate isoproterenol-induced vascular relaxation in part through nitric oxide production. Metabolism 2002; 51: 380-6.
23 Zemel MB, Reddy S, Sowers JR. Insulin attenuation of vasoconstrictor responses to phenylephrine in Zucker lean and obese rats. Am J Hypertens 1991; 4: 537-9.
24 Nakano H, Lee SD, Ray AD, Krasney JA, Farkas GA. Role of nitric oxide in thermoregulation and hypoxic ventilatory response in obese Zucker rats. Am J Respir Crit Care Med 2001; 164: 437-42.
25 Frisbee JC, Stepp DW. Impaired NO-dependent dilation of skeletal muscle arterioles in hypertensive diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol 2001; 281:
H1304-11.
26 Brooks-Asplund EM, Shoukas AA, Kim SY, Burke SA, Berkowitz DE. Estrogen has opposing effects on vascular reactivity in obese, insulin-resistant male Zucker rats. J Appl Physiol 2002; 92: 2035-44.
27 Kobayashi T, Kamata K. Short-term insulin treatment and aortic expressions of IGF-1 receptor and VEGF mRNA in diabetic rats. Am J Physiol Heart Circ Physiol 2002; 283:
H1761-8.
28 Chang SP, Chen YH, Chang WC, Liu IM, Cheng JT. Merit of physical exercise to reverse the higher gene expression of hepatic phosphoenolpyruvate carboxykinase in obese Zucker rats. Life Sci 2006.
29 Melian E, Gonzalez B, Ajo R, Gonzalez N, Sanchez Franco F. Tissue-specific response of IGF-I mRNA expression to obesity-associated GH decline in the male Zucker fatty rat. J Endocrinol 1999; 160: 49-56.
30 Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension 2003; 42: 231-4.
31 Sharma AM. Obesity and cardiovascular risk. Growth Horm IGF Res 2003; 13 Suppl A:
S10-7.
FIGURE LEGENDS
Figure 1. Concentration-response curves for insulin (3x10-7-10-5 mol/L)-induced vasorelaxation in thoracic aortas of lean and obese Zucker rats. The relaxation response to insulin was significantly decreased in obese rats (*P < 0.05). Numbers in parentheses indicate the numbers of animals used in each group.
Figure 2. Concentration-response curves for IGF-1 (3x10-9-10-7 mol/L)-induced vasorelaxation in thoracic aortas of lean and obese Zucker rats. The relaxation response to IGF-1 was significantly increased in obese rats (*P < 0.05). Numbers in parentheses indicate the numbers of animals used in each group.
Figure 3. Vascular responses to insulin (3x10-6 mol/L) without or with the pretreatment of wortmannin (3x10-7 mol/L) or L-NAME (3x10-7 mol/L). *P < 0.05, obese vs. lean; #P < 0.05 and ##P < 0.01, with the inhibitor (wortmannin or L-NAME) vs. without the inhibitor. The group difference between lean and obese rats was disappeared after the pretreatment of wortmannin or L-NAME (n = 6).
Figure 4. Vascular responses to IGF-1 (10-8 mol/L) without or with the pretreatment of wortmannin (3x10-7 mol/L) or L-NAME (3x10-7 mol/L). *P < 0.05, obese vs. lean; #P < 0.05 and ##P < 0.01, with the inhibitor (wortmannin or L-NAME) vs. without the inhibitor. The group difference between lean and obese rats was disappeared after the pretreatment of wortmannin or L-NAME (n = 6).
Figure 5. Vascular responses to SNP (10-9 mol/L) in thoracic aortas of lean and obese Zucker rats. No significant difference was found between these two groups (n = 6).
Fig 1.
0 20 40 60 80
-6.5 -6 -5.5 -5
[ Insulin ] (LogM)
Vasorelaxation (% precontraction)
Lean (8) Obese (8)
*
Fig 2.
0 20 40 60 80
-8.5 -8 -7.5 -7
[ IGF-1 ] (LogM)
Vasorelaxation (% precontraction)
Lean (8) Obese (8)
*
Fig 3.
0 10 20 30 40 50
Lean Obese
Vasorelaxation (% precontraction)
Insulin
Wortmannin+insulin L-NAME+insulin
# *
#
## #
Fig 4.
0 20 40 60
Lean Obese
Vasorelaxation (% precontraction)
IGF-1
Wortmannin+IGF-1 L-NAME+IGF-1
*
#
##
##
#
Fig 5.
0 20 40 60 80 100
Lean Obese
SNP-induced Vasorelaxation (% precontaction)
Self-Evaluation
This research is consistent with my previously proposed first-year project for NSC. Our results indicating “the decreased insulin-mediated vasorelaxation in obese rats appear to be counteracted with the increased IGF-1-mediated vasorelaxation” might provide one of possible mechanisms to explain the development and compensation of vascular dysfunction in the obesity. Also, we found that the effects of obesity on vasorelaxant responses mediated by insulin or IGF-1 were mainly due to the altered release of PI3K and NOS. Clinically, when considering possible therapeutic agents to control or prevent the development of vascular dysfunction in the obesity, it should be paid attention on actions of insulin and IGF-1 on the vasorelaxant responses.
The plan in my NSC proposal was executed, and the findings support the assumptions in my proposal.
This paper had been accepted for publication by the International Journal of Obesity on 15th Mar 2006.