行政院國家科學委員會專題研究計畫 成果報告
半導體廢水以雙重膠凝處理之研究(第 2 年) 研究成果報告(完整版)
計 畫 類 別 : 個別型
計 畫 編 號 : NSC 95-2221-E-011-051-MY2
執 行 期 間 : 96 年 08 月 01 日至 97 年 07 月 31 日 執 行 單 位 : 國立臺灣科技大學化學工程系
計 畫 主 持 人 : 劉志成
計畫參與人員: 此計畫無其他參與人員:
此計畫無其他參與人員:
報 告 附 件 : 出席國際會議研究心得報告及發表論文
處 理 方 式 : 本計畫可公開查詢
中 華 民 國 97 年 10 月 28 日
行政院國家科學委員會補助專題研究計畫期末報告
※※※※※※※※※※※※※※※※※※※※※※※※
※ ※
※
半導體廢水以雙重膠凝處理之研究(2/2)※
※
※
※ ※
※※※※※※※※※※※※※※※※※※※※※※※※
計畫類別:;個別型計畫 □整合型計畫 計畫編號:NSC 95-2211-E-011-051-MY2
執行期間: 96 年 8 月 1 日至 97 年 7 月 31 日
計畫主持人:劉志成 共同主持人:
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
執行單位:國立台灣科技大學化學工程技術系
中 華 民 國 96 年 07 月 31 日
2
行政院國家科學委員會專題研究計畫期末報告
半導體廢水以雙重膠凝處理之研究(2/2)
計畫編號:NSC 95-2211-E-011-051-MY2 執行期限:96 年 8 月 1 日至 97 年 7 月 31 日 主持人:劉志成 國立台灣科技大學化學工程技術系
摘要
化學機械研磨是目前能夠將 250 奈米以下製程的 8 吋晶圓完全平坦化的技 術。然而,此程序消耗大量的超純水,即產生大量難以處理之廢水。本研究利用 PDA2000 來觀察不同高分子對於膠羽在雙重混凝程序中的影響。此外,經由導 入高剪力可以獲得膠羽重新絮凝的能力,實驗結果發現,當雙重調理使用陽電性 高分子絮凝劑 PDADMAC 搭配陰電性聚電解質(PAA, AP410, MagnaFloc156) 時,絮凝效果會變好,同時亦提供了更廣的加藥範圍。但是,PDADMAC 與非 離子性聚電解質(PAM)組合,卻無法有效的幫助絮凝。另一方面,結合 PDADMAC 與兩性聚電解質(T204)可以比任一聚電解質單獨使用下,使用更低的劑量來達到 相同的絮凝效果,並且也增加了加藥劑量範圍。從 FI 値與數據分析得知,濁度、
總懸浮固體、界達電位與溶解矽酸與膠羽大小無關。膠羽破碎與再生成的實驗中 發現,使佣 PDADMAC 搭配高劑量的 AP410 或是 MagnaFloc156,膠羽破碎之後 會再重新生成,然而,如果使用 PDADMAC 搭配 T204 或是低劑量的 AP410 或 MagnaFloc156,則膠羽經破碎之後就不會重新生成新膠羽。。
關鍵字:研磨廢水、半導體、絮凝、雙重絮凝、聚電解質
。
Abstract
The current study was conducted to study the effect of dual flocculation using different kind of polymers in treating CMP wastewater of semiconductor manufacturer with Photometric Dispersion Analyser (PDA2000) to continuously monitor the flocculation. In addition, the reformation of flocs after introducing high shear rate was investigated.
The experimental results showed that dual flocculation using combination of cationic polyelectrolyte (PDADMAC) and anionic polyelectrolyte (PAA, AP410, MagnaFloc156) enhanced the flocculation and gave broader range of optimum dosage at the expense of increasing the dosage of chemicals. Combination of PDADMAC and non-ionic polyelectrolyte (PAM) hardly enhanced the flocculation. On the other hand, combination of PDADMAC and polyampholyte (T204) also can enhance the flocculation with less dosage than the optimum dosage in single flocculation and gave broader optimum dosage. Turbidity, TSS, zeta potential, and dissolved silica were not related to floc size based on Flocculation Index (FI) and data analysis. Breakage and reformation results indicated that when using combination of PDADMAC and high dosage of AP410 or MagnaFloc156, reformation of flocs occurred. However, in dual flocculation using combination of PDADMAC with T204, low-dosage AP410, and low-dosage MagnaFloc156 did not show any reformation of flocs.
Keywords:
Chemical mechanical polishing CMP), dual flocculation, flocs, photometric dispersion analyzer PDA), semiconductor, wastewater.
4
1. Introduction
The semiconductor manufacturing processes include photolithography, oxidation, etching, doping, planarization, washing, and cleaning [Chuang et al., 2002; Lin and Yang, 2004; Lien and Liu, 2006b]. As IC size smaller than 250 nm and wafer size changes to 8 in. and beyond, chemical mechanical polishing (CMP) is the only polishing technique that provides global planarization [Yang et al., 2003; Lien and Liu, 2006b]. However, the CMP has a major drawback in that it consumes a large amount of ultra pure water and produces approximately an equal amount of wastewater that is difficult to treat [Lin and Yang, 2004; Yang and Yang, 2004; Hu et al., 2005].
More than 90% of CMP wastewater generated in Taiwan is oxide-CMP type [Yang et al., 2003]. Normally, oxide-CMP wastewater is high in alkalinity, total solids content, and turbidity [Yang et al., 2003; Lin and Yang, 2004]. When all effluent from the CMP tools are mixed, then a high volume of wastewater with a high turbidity and total solids content has to be managed before discharge [Yang, 2002;
Lien and Liu, 2006b]. The quality of CMP wastewater does not meet the current effluent standards if it is not treated. For this kind of wastewater, the potential treatment methods are coagulation / flocculation, flotation, microfiltration /
ultrafiltration, and etc [Yang, 2002; Yang et al., 2003; Yang and Yang, 2004].
Coagulation and flocculation are effective for destabilization of some soluble and suspended particles, so that the particles can be filtered or separated by other means [Lin and Yang, 2004]. Most semiconductor manufacturers in Taiwan use coagulation flocculation in treating the CMP wastewater. However, that process faces some problems. Since the range of optimum coagulant dosage is narrow, controlling of coagulant dosage becomes difficult [Lo and Lo, 2004; Lien and Liu, 2006b]. Yang et al. [2003] investigated the electro-microfiltration process which can effectively
treat polishing wastewater and generate electrolyzed water. Electrocoagulation has been shown to be effective in removing total solids, Cu, and COD simultaneously [Lai and Lin, 2003; Lin and Yang, 2004]. In addition, dispersed and dissolved air flotation also showed effective removal of particles [Lien and Liu, 2006a; 2006b].
Currently, semiconductor manufacturers in Taiwan in the Science Parks are required to recycle 80-85% of process water and 65-75% of total water used [Lien and Liu, 2006a]. It is estimated that 30-40% of ultrapure water is used in CMP operation unit in semiconductor fabricator [Yang, 2002; Lien and Liu, 2006b]. Research and development on water conservation and wastewater treatment always have a high priority [Lien and Liu, 2006a].
The objectives of the current work were to study the effect of dual flocculation using different kind of polymers in treating CMP wastewater of semiconductor manufacturer with Photometric Dispersion Analyser (PDA2000) to continuously monitor the flocculation. In addition, the reformation of flocs generated from different combination of polymers after subjecting to high shear rate during slow mixing of flocculation was investigated.
6
The wastewater was taken from a semiconductor company in Hsinchu Science Park of Taiwan. The CMP wastewater was combination of oxide CMP wastewater and metal CMP wastewater. Polyelectrolyte used in this experiment were poly(diallyldimethylammonium) chloride, KP201C, poly(acryl acid), poly(acryl amide), MagnaFloc156 and polyampholyte T204.
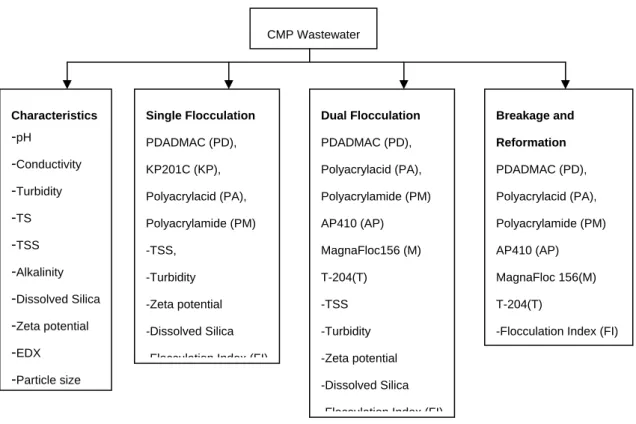
Experiment was divided into 4 parts: analyzing the characteristics of CMP wastewater, single flocculation, dual flocculation, and breakage and reformation as shown in Figure 1.
Figure 1 Block Diagram of Experiments
The test suspension was contained in 1 liter rectangular beaker with stirrer unit from jar test device [Phipps & Bird PB-700]. For dynamic monitoring, sample from one beaker was circulated through transparent plastic tubing (3mm ID) by means of a peristaltic pump. The pump was located after the PDA instrument to prevent floc
CMP Wastewater
Characteristics
-pH
-Conductivity
-Turbidity
-TS
-TSS
-Alkalinity
-Dissolved Silica
-Zeta potential
-EDX
-Particle size
Dual Flocculation PDADMAC (PD),
Polyacrylacid (PA),
Polyacrylamide (PM)
AP410 (AP)
MagnaFloc156 (M)
T-204(T)
-TSS
-Turbidity
-Zeta potential
-Dissolved Silica
Flocculation Index (FI) Single Flocculation
PDADMAC (PD),
KP201C (KP),
Polyacrylacid (PA),
Polyacrylamide (PM)
-TSS,
-Turbidity
-Zeta potential
-Dissolved Silica
Flocculation Index (FI)
Breakage and Reformation PDADMAC (PD),
Polyacrylacid (PA),
Polyacrylamide (PM)
AP410 (AP)
MagnaFloc 156(M)
T-204(T)
-Flocculation Index (FI)
breakage in the pinch portion of pump. The tubing was clamped in the PDA2000 instrument so that the flowing sample illuminated by a narrow light beam (850 nm wavelength). Figure 2 illustrates the experimental equipment arrangement. Before conducting any experiment, PDA2000 need to be warm up for at least 10 minutes [Yukselen and Gregory, 2002a].
Sample was pumped from a stirrer beaker at about 100 ml/min through the tubing and the average (dc) and fluctuating (rms) components of the transmitted light intensity were monitored by the PDA2000 instrument. Readings were taken every 2 seconds and the results were stored in a computer for subsequent spreadsheet analysis.
After allowing 1 minute for steady-state readings to be established, the flocculant was dosed and the suspension was stirred at 100 rpm for 1 minute. Then, stirring speed was reduced to 40 rpm for 10 minutes. Let the flocs to sediment for 30 minutes before collecting sample for other analysis. For dual flocculation, after first polymer was dosed and mixed for 30 seconds, second polymer was dosed. In order to investigate the floc breakage and reformation, after the suspension was slowly stirred for 5 minutes, the stirring speed was suddenly increased to 300 rpm for 10 seconds then reduced back to 40 rpm. Samples for other analysis were taken after allowing flocs to sediment for 30 minutes.
P
Stirrer
PDA2000
Pump Beaker
Flow Cell
8
Figure 2 Schematic diagram of PDA2000 experiment
3. Methods and Materials
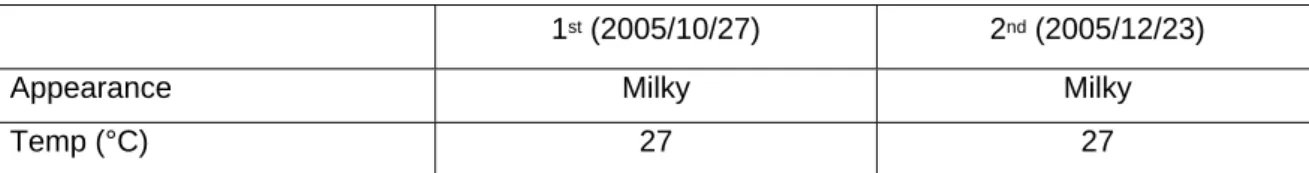
The CMP wastewater was characterized and shown in Table 1. Both CMP wastewater had pH value higher than 9.5. It might be due to the KOH or NH4OH content in CMP wastewater [Yang and Yang, 2004]. Zeta potential result showed that the CMP wastewater contains high silica content. This analysis showed that first and second CMP wastewater mostly contains 40-48% silica and 52-60% oxygen with traces of other elements. The composition from EDS results indicated that the CMP wastewater contain mostly oxide CMP slurry rather than metal CMP slurry. This result agrees with some study [Hu et al., 2005] but disagrees with others [Yang et al., 2003]. Total solids in CMP wastewater were 2100-2230 mg/L. This value agrees with previous works [Yang et al., 2003; Hu et al., 2005] that find total solids higher than 1500 mg/L. TSS in CMP wastewater was 1200-1437 mg/L. The TSS values were much higher than previous works [Lai and Lin, 2003; Yang et al., 2003; Hu et al., 2005]. Dissolved silica in this CMP wastewater (147.5-187.5 mg/L) was lower than previous study [Hu et al., 2005]. The phenomenon may occur because of the different process used by different semiconductor manufacturers and larger particles were resulted from the removed materials from the wafer surfaces during the CMP process [Yang and Yang, 2004].
Table 1 Characteristics of CMP wastewater
1st (2005/10/27) 2nd (2005/12/23)
Appearance Milky Milky
Temp (°C) 27 27
pH 9.78 9.92
Alkalinity (mg/L) 354.92±1.42 177.4±5
Conductivity (mS/cm) 287 222
Turbidity (NTU) 175 165
Zeta potential (mV) -42.7±3.7 -43.9±0.9
TS (mg/L) 2230±9 2130±17
TSS (mg/L) 1437±124 1200±36
Dissolved Si (mg/L) 187.5 147.5
3.1 Single Flocculation
The flocculation experiments are divided into 3 parts: single flocculation to obtain the optimum operating condition, dual flocculation to investigate the enhanced flocculation using two polymers, and breakage and reformation experiment to check if the flocculation is reversible.
The main mechanisms of flocculation are charge neutralization and bridging [Gregory, 1988; Palmer et al., 1994; Yu and Somasundaran, 1996]. If the polymer acts by a bridging mechanism, then the number of chains adsorbed on the particles must provide enough bridges to form flocs of adequate strength. Polyelectrolytes which act by charge neutralization need to adsorb sufficiently to overcome electrical repulsion between particles [Gregory, 1988].
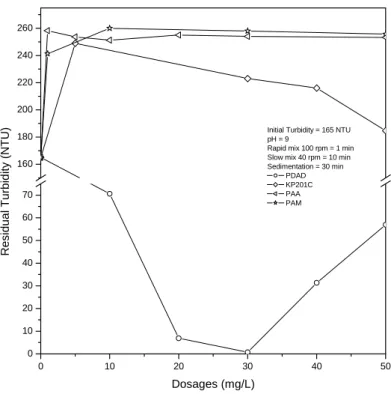
The residual turbidity results can be seen in Figure 3. When PDADMAC dosages increased, the turbidity reached the lowest value at dosages around 30-40 mg/L, and then it slowly increased again. It was very effective in removing fine particles so that turbidity decreased from 165 NTU to 0.7 NTU. It might be due to high positive charge density of PDADMAC that reduced electric repulsion force between particles. At still higher concentrations, the suspension becomes stable again because of charge reversal of surfaces [Runkana et al., 2006]. In contrast, KP201C, PAA, and PAM were not effective. At dosages of 1-5 mg/L, KP201C, PAA and PAM
10
made turbidity higher to 240-260 NTU. The increase in turbidity is a result of limited aggregation of submicron particles. Residual turbidity gradually decreased while KP201C dosages kept increasing until 50 mg/L. However, it still had higher residual turbidity than initial turbidity. It seemed KP201C can achieve good removal efficiency only if the dosage was increased 2-3 times than PDADMAC [Huang, 2001].
It might be due to charge density of KP201C is lower than PDADMAC. PAA and PAM did not show any decrease in turbidity even though the polymer dosages were increased.
Figure 3 Residual turbidity of single flocculation as a function of polymer dosages.
Flocculation Index (FI) from PDA 2000 as shown in Figure 4, revealed the change of floc size from steady state reading to the end of slow mixing. Steadier floc size was found for PDADMAC than for KP201C. The floc size increased as the dosages of PDADMAC increased until it reached 30 mg/L, then it decreased. FI when using
0 10 20 30 40 50
0 10 20 30 40 50 60 70 160 180 200 220 240 260
Residual Turbidity (NTU)
Dosages (mg/L)
Initial Turbidity = 165 NTU pH = 9
Rapid mix 100 rpm = 1 min Slow mix 40 rpm = 10 min Sedimentation = 30 min
PDAD KP201C PAA PAM
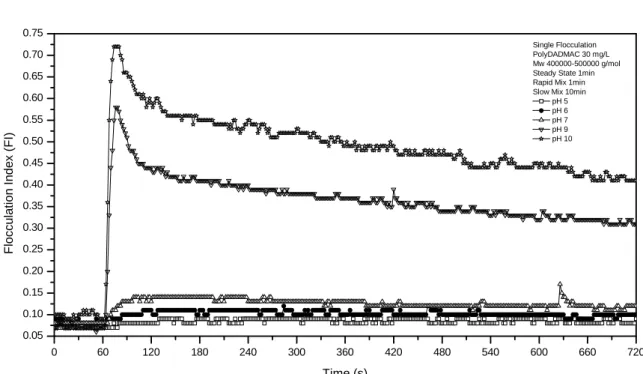
KP201C was totally different from that of PDADMAC, larger floc size (more than two times) was found. It can be understood because KP201C has higher molecular weight (10 times than PDADMAC). Although KP201C had larger floc size, it did not guarantee good result in term of turbidity and TSS removal. The main mechanism of KP201C may be polymer bridging, which makes the floc size larger, yet with inferior efficiency in fine particle capture. Increase in polymer molecular weight gradually changes the mechanism of floc formation from that of charge neutralization to polymer bridging, and shifts the floc size distribution toward bigger size [Chen and Berg, 1993]. Addition of PAA and PAM did not lead to FI because PAA and PAM did not form any floc at all. Flocculation Indexes also show the kinetic of floc formation. Thirty mg/L of KP201C needed 60 seconds to form flocs. It was 2 times than 40 mg/L of KP201C which only needed 30 seconds. The largest floc size occurred when the pH value was at 10 (Figure 5). The floc size decreased as the pH decreased to acidic range. The optimum condition of single flocculation, 30 mg/L of PDADMAC at pH 9, was chosen for dual flocculation.
0 60 120 180 240 300 360 420 480 540 600 660 720
0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 1.1 1.2 1.3 1.4 1.5
Flocculation Index (FI)
Time (s)
Single Flocculation pH = 9 Steady State 1min Rapid Mix 1min Slow Mix 10min PD 10mg/L PD 20mg/L PD 30mg/L PD 40mg/L PD 50mg/L KP 30mg/L KP 40mg/L
12
Fig. 4 Flocculation Index of single flocculation using PDADMAC and KP201C with different dosages.
Fig. 5. Flocculation Index of single flocculation using PDADMAC and KP201C at
different pH.
0 10 20 30 40 50
0 1 2 3 4 5 6 7 30 45 60 75 90
-40 -35 -30 -25 -20 -15 0 5 10 15 20
Residual Turbidity (NTU)
PAA Dosages (mg/L)
Dual Flocculation pH = 9 Residual Turbidity
PD 10 mg/L PD 30 mg/L PD 40 mg/L PD 50 mg/L Zeta Potential
PD 10 mg/L PD 30 mg/L PD 40 mg/L PD 50 mg/L
Zeta Potential (mV)
0 60 120 180 240 300 360 420 480 540 600 660 720
0.05 0.10 0.15 0.20 0.25 0.30 0.35 0.40 0.45 0.50 0.55 0.60 0.65 0.70 0.75
Flocculation Index (FI)
Time (s)
Single Flocculation PolyDADMAC 30 mg/L Mw 400000-500000 g/mol Steady State 1min Rapid Mix 1min Slow Mix 10min
pH 5 pH 6 pH 7 pH 9 pH 10
Figure 6. Residual turbidity and zeta potential of dual flocculation using combination of PDADMAC and PAA.
3.2 Dual Flocculation
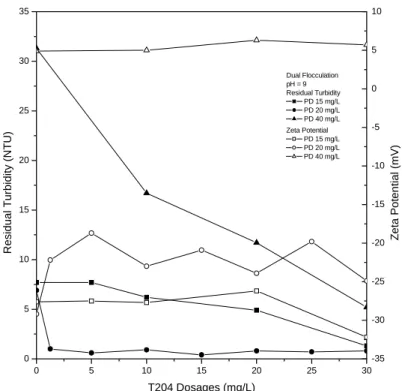
Combination of PDADMAC and PAA can enhance flocculation with broader range of dosages (Fig. 6). However, this combination had a drawback of chemical dosage and cost, and probably increased conductivity in treated effluent. It has found out the negative charges of particles has to be reversed when pre-conditioned with the cationic polymer, so that the subsequent adsorption of anionic polymer is facilitated [Lee and Liu, 2000; 2001; Wang et al., 2005]. This combination had better enhancement than combination of PDADMAC and PAA because it used less dosage of polymer to get the same result. Higher molecular weight produces better flocculation whose mechanism is polymer bridging [Fan et al., 2000]. But it still had the same drawback with combination of PDADMAC and PAA. Last combination was PDADMAC and T204 as given in Figure 7. Interestingly, this combination had different phenomenon than the others. It gave good enhancement in low residual turbidity at PDADMAC dosage of 20 mg/L with very low T204 dosages (1.25 mg/L).
The residual turbidity remained below 1 NTU even when T204 dosage increased. In contrast, 40 mg/L of PDADMAC gave little enhancements than the identical dosage in other combinations. The zeta potential did not give any significant change
14
0 5 10 15 20 25 30
0 5 10 15 20 25 30 35
-35 -30 -25 -20 -15 -10 -5 0 5 10
Residual Turbidity (NTU)
T204 Dosages (mg/L)
Dual Flocculation pH = 9 Residual Turbidity
PD 15 mg/L PD 20 mg/L PD 40 mg/L
Zeta Potential (mV)
Zeta Potential PD 15 mg/L PD 20 mg/L PD 40 mg/L
because T204 is amphoteric polyelectrolyte. When wastewater was pre-conditioned with the cationic polyelectrolyte, it became adsorbed on the particles and formed more compact primary flocs. The amphoteric polyelectrolyte then became adsorbed on the loops and tails of the cationic polyelectrolyte by hydrogen bonding and van der Waals force. It provides bridging of the primary flocs to form aggregates [Lee and Liu, 2000; 2001]. That was the reason the zeta potential did not change much. Hydrogen bonding and van der Waals force were the probable driving force for the non-ionic polyelectrolyte adsorption on particles [Somasundaran and Yu, 1994; Somasundaran and Krishnakumar, 1997; Lee and Liu, 2000] and the slipping plane moved outwards from the surface of the particles, resulting in a decrease in the absolute value of the zeta potential [Shimabayashi et al., 1992; Csempesz et al., 1998; Lee and Liu, 2000].
Therefore, the zeta potential was more positive than samples pre-coagulated with PDADMAC.
Figure 7. Residual turbidity and zeta potential of dual flocculation using combination
of PDADMAC and T204.
3.3 Breakage and reformation
In order to compare floc formation, breakage and reformation under different combination of polymers and dosages, it is convenient to define certain parameters, derived from measured values of flocculation index at different stages. For CMP wastewater, the initial value is quite low but not negligible. The FI values shown are [Fitzpatrick et al., 2004; Yukselen and Gregory, 2004]:
FI1 – This is the value corresponding to the plateau floc size, before breakage.
FI2 – This represents the minimum floc size reached on increasing the shear rate.
FI3 – The plateau value after re-formation at low shear Use these parameters to define the following factors:
(FI1-FI2) Breakage Factor (%) = x100
FI1 (FI3-FI2) Recovery Factor (%) = x100
(FI1-FI2)
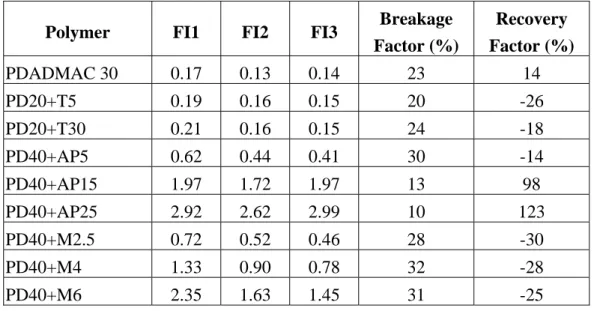
These factors are defined so that 100% represents complete breakage and complete recovery. These factors of all combination of various combinations of polymers are given in Table 2.
Table 2 Breakage and recovery factors for single and dual flocculation system.
Polymer FI1 FI2 FI3 Breakage
Factor (%)
Recovery Factor (%)
PDADMAC 30 0.17 0.13 0.14 23 14
PD20+T5 0.19 0.16 0.15 20 -26
PD20+T30 0.21 0.16 0.15 24 -18
PD40+AP5 0.62 0.44 0.41 30 -14
PD40+AP15 1.97 1.72 1.97 13 98
PD40+AP25 2.92 2.62 2.99 10 123
PD40+M2.5 0.72 0.52 0.46 28 -30
PD40+M4 1.33 0.90 0.78 32 -28
PD40+M6 2.35 1.63 1.45 31 -25
16
PD40+M8 2.16 1.53 3.32 29 283
PD40+M10 1.56 1.12 2.92 28 411
The results showed that single flocculation of PDADMAC 30 mg/L and dual flocculation with 20 mg/L of PDADMAC and T204 had breakage factor of around 20-24%. Thirty mg/L of PDADMAC had low recovery factor than the combination of PDADMAC and T204 which had a negative value. Higher dosage of T204 seemed to induce slightly higher recovery factor even though still in negative value.
Breakage factor for PDADMAC and AP410 had higher value at lower dosage (5 mg/L), and decreased with increase in AP410 dosage. It showed that higher dosage can produce larger and stronger floc. The recovery factor had opposite trend with breakage factor. Its value increased when using higher dosage of AP410, it even reached higher than 100% (123%). Cationic – anionic dual polymer system generated flocs with lower breakage factors and higher recovery factors (flocs with higher strength) than single polymers [Yukselen et al., 2005]. In this case, it is valid only when using higher dosage.
The combination of PDADMAC and MagnaFloc156 gave constant value of breakage factor of around 28-32%. A change in second polymer dosage did not affect the breakage factors. However, it had some effects on recovery factors. When using 2.5-6 mg/L of MagnaFloc156, the effect on recovery factors was insignificant.
Addition of higher dosage of MagnaFloc156 to 8-10 mg/L increased the recovery factor higher than 100%.
This phenomenon occurred because MagnaFloc156 at higher dosage did not disperse well and form non uniform flocs. Some large flocs were too large to go through the 3mm ID tube of PDA2000, which made the FI before breakage steeply decreased after MagnaFloc156 was added. The breakage actually made the flocs to
have more uniform and smaller so that they still could go trough the tube of PDA2000.
The combination of PDADMAC and MagnaFloc156 did not have the same result with previous study [Yukselen et al., 2005]. Identical polymers of low molecular weight (Mw 50000 g/mol) PDADMAC and MagnaFloc156 were used in dual flocculation as applied to synthetic kaolin clay suspension. Their mixing regime was rapid mixing at 400 rpm for 5 seconds for each polymer, slow mixing at 50 rpm for up to 60 min, and breakage at 400 rpm for 10 seconds. In this research, CMP wastewater may contain many chemicals such as surfactants, corrosion inhibitor, and etc.
Different mixing regime of rapid mixing at 100 rpm for 30 seconds for each polymer, slow mixing at 40 rpm for 10 min, and breakage at 300 rpm for 10 seconds was utilized. These differences may result in different results.
4. Conclusions
Based on results from the single flocculation, dual flocculation, and breakage experiments, there are some conclusions in the current study:
1. Single flocculation of CMP wastewater by using PDADMAC induced good removal efficiency of fine particles. However, PDADMAC had low removal efficiency for dissolved silica and the range of optimum dosage was very narrow.
Meanwhile, single flocculation using other polymers such as KP201C, PAA, and PAM was not effective.
2. Dual flocculation using combination of PDADMAC with PAA, AP410, and MagnaFloc156, respectively, enhanced the flocculation at the expense of increasing the dosage of chemicals. But it gave broader range of optimum dosage.
The combination of PDADMAC and PAM hardly enhanced the flocculation.
18
3. On the other hand, dual flocculation using combination PDADMAC and T204 also can enhance the flocculation with less dosage than the optimum dosage in single flocculation and gave broader optimum dose.
4. Increasing shear rate could cause breakage of flocs to smaller size. After breakage, reformation of flocs may occur on restoring the previous low shear rate. When using PDADMAC and high dosage of AP410 or MagnaFloc156, reformation of flocs occurred. In dual flocculation using PDADMAC with T204, low dosage of AP410, and low dosage MagnaFloc156 did not show any reformation of flocs.
References
Chen, W. and Berg, J. C., "The effect of polyelectrolyte dosage on floc formation in protein precipitation by polyelectrolytes", Chemical Engineering Science, vol. 48, No.
10, pp. 1775-1784 (1993).
Chuang, T. C., Huang, C. J., and Liu, J. C., "Treatment of semiconductor wastewater by dissolved air flotation", Journal of Environmental Engineering, vol. 128, No. 10, pp. 974-980 (2002).
Csempesz, F., Nagy, M., and Rohrsetser, S., "Characterization and features of competitive polymer adsorption on colloidal dispersions", Colloids and Surfaces A:
Physicochemical and Engineering Aspects, vol. 141, No. 3, pp. 419-424 (1998).
Fan, A., Turro, N. J., and Somasundaran, P., "A study of dual polymer flocculation", Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 162, No.
1-3, pp. 141-148 (2000).
Fitzpatrick, C. S. B., Fradin, E., and Gregory, J., "Temperature effects on flocculation, using different coagulants", Water Science and Technology, vol. 50, No. 12, pp.
171-175 (2004).
Gregory, J., "Polymer adsorption and flocculation in sheared suspensions", Colloids and Surfaces, vol. 31, No. 1, pp. 231-253 (1988).
Hu, C. Y., Lo, S. L., Li, C. M., and Kuan, W. H., "Treating chemical mechanical
polishing (CMP) wastewater by electro-coagulation-flotation process with surfactant", Journal of Hazardous Materials, vol. 120, No. 1-3, pp. 15-20 (2005).
Huang, X. R., "Treatment of chemical mechanical polishing wastewater of semiconductor manufacturer", Department of Chemical Engineering, National Taiwan University of Science and Technology, Taipei, Taiwan (2001).
Lai, C. L. and Lin, S. H., "Treatment of chemical mechanical polishing wastewater by electrocoagulation: system performances and sludge settling characteristics", Chemosphere, vol. 54, No. 3, pp. 235-242 (2004).
Lee, C. H. and Liu, J. C., "Enhanced sludge dewatering by dual polyelectrolytes conditioning", Water Research, vol. 34, No. 18, pp. 4430-4436 (2000).
Lee, C. H. and Liu, J. C., "Sludge dewaterability and floc structure in dual polymer conditioning", Advances in Environmental Research, vol. 5, No. 2, pp. 129-136 (2001).
Lien, C. Y. and Liu, J. C., "Dissolved air flotation of polishing wastewater from semiconductor manufacturer", Water Science and Technology, vol. 53, No. 7, pp.
133-140 (2006a).
Lien, C. Y. and Liu, J. C., "Treatment of polishing wastewater from semiconductor manufacturer by dispersed air flotation", Journal of Environmental Engineering, vol.
132, No. 1, pp. 51-56 (2006b).
Lin, S. H. and Yang, C. R., "Chemical and physical treatments of chemical mechanical polishing wastewater from semiconductor fabrication", Journal of Hazardous Materials, vol. 108, No. 1, pp. 103-109 (2004).
Lo, R. and Lo, S. L., "A pilot plant study using ceramic membrane microfiltration, carbon adsorption and reverse osmosis to treat CMP wastewater", Water Science and Technology: Water Supply, vol. 4, No. 1, pp. 111-118 (2004).
Palmer, T. S., Campbell, N., Bowman, J. L., and Dewar, P., "Flocculation behavior of some cationic polyelectrolytes", Journal of Applied Polymer Science, vol. 52, No. 9, pp. 1317-1325 (1994).
20
Runkana, V., Somasundaran, P., and Kapur, P. C., "A population balance model for flocculation of colloidal suspensions by polymer bridging", Chemical Engineering Science, vol. 61, No. 1, pp. 182-191 (2006).
Shimabayashi, S., Nishino, K., and Nakagaki, M., "Aggregation/dispersion of amorphous silica particles by simulaneous adsorption of two polymers in an aqueous phase", Colloids and Surfaces, vol. 63, No. 1-2, pp. 121-129 (1992).
Somasundaran, P. and Yu, X., "Flocculation/dispersion of suspensions by controlling adsorption and conformation of polymers and surfactants", Advances in Colloid and Interface Science, vol. 53, No.1, pp. 33-49 (1994).
Somasundaran, P. and Krishnakumar, S., "Adsorption of surfactants and polymers at the solid-liquid interface", Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 123-124, No. 1, pp. 491-513 (1997).
Wang, J. S., Liu, J. C., and Lee, D. J., "Dual conditioning of sludge utilizing polyampholyte", Journal of Environmental Engineering, vol. 131, No. 12, pp.
1659-1666 (2005).
Yang, G. C. C., "CMP wastewater management using the concepts of design for environment", Environmental Progress, vol. 21, No. 1, pp. 57-62 (2002).
Yang, G. C. C., Yang, T. Y., and Tsai, S. H., "Crossflow electro-microfiltration of oxide-CMP wastewater", Water Research, vol. 37, No. 4, pp. 785-792 (2003).
Yang, G. C. C. and Yang, T. Y., "Reclamation of high quality water from treating CMP wastewater by a novel crossflow electrofiltration/electrodialysis process", Journal of Membrane Science, vol. 233, No. 1-2, pp. 151-159 (2004).
Yu, X. and Somasundaran, P., "Role of polymer conformation in interparticle-bridging dominated flocculation", Journal of Colloid and Interface Science, vol. 177, No. 2, pp. 283-287 (1996).
Yukselen, M. A. and Gregory, J., "The effect of rapid mixing on the break-up and re-formation of flocs", Journal of Chemical Technology and Biotechnology, vol. 79, No. 7, pp. 782-788 (2004).
出國參加國際會議報告
台灣科技大學化工系 劉志成 教授 2008.06.12
一 會議背景:
界面科學於污染控制(Interfaces Against Pollution)是起源於 1997 年荷蘭瓦尼亨 農業大學(Wageningen Agricultural University)主辦的國際研討會,歷經四屆歐洲的主 辦,本次第五屆輪由日本幾個大學,包括東京科學大學筑波大學京都大學等聯合主 辦。本人是第一次參加,乃考慮旅途短,而且京都是文化古都,本人蒙國科會經費 補助參加。因為地緣關係,再加上為增長研究生國際經驗的考慮,此行亦伴同博士 班學生 Warmadewanthi 前往,她由本實驗室經費補助機票款,以及註冊費生活費 等,並正式報名參加,也是難得經驗。
二 會議內容:
本次研討會台灣共有五人報名參加,分別代表為中山大學楊金鐘教授,及嘉南 藥理科技大學盧明俊與廖志祥教授,人數最多是日本,其他還包括韓國、美國、法 國、加拿大、澳大利亞、荷蘭、俄羅斯、越南、中國、阿根廷、英國等。本人 5 月 30 日傍晚抵達,5 月 3 1 日進行文化之旅,參訪金閤寺清水寺等。會議首日是 6 月 1 日,傍晚是開幕典禮,傍晚大會演講是荷蘭瓦尼亨農業大學 Dr Koopal 以腐植質 在界面之角色之研究,他以宏觀角度強調研究的重要,以及相關科技的潛在價值與 極限,非常有力的內容,且十分具創意。接著是大會安排兩場主題演講,晚上參加 酒會認識朋友,並共進晚餐。本次會議的特色之一是連續三天上午均為主題演講,
總數約二十篇,但是其中許多演講者多著重自己新近突破,眼光與課題狹窄,不容 易充分理解,再加上界面科學於污染控制是很廣泛的議題,所以對本人實在嫌吃 力,亦覺得學習有限。因為材料科學,化學,空氣污染的學門似乎都加入,廣度 夠,卻顯得鬆散。第二天上午聽主題演講,下午為論文宣讀,晚上為大會晚宴,現 場許多各國年輕研究生,談笑風生,真是豐富輕鬆的一晚!
第三天下午博士班學生 Warmadewanthi 報告”半導體研磨廢水與含氟廢水共同 處理”,十分清楚,本人則報告”沉澱浮除水中磷酸”,尚稱順利週三(06/04)上午則 打包返台北。整個三天議程結束好像一轉眼工夫,本人與學生均有始有終,從頭到 尾積極聽講討論,只在晚間才安排聚餐及逛街。
三 會議心得:
這一次是大會安排有缺失的國際研討會,議程至大會前一週才確定,會議進行 不緊湊,討論議題無法聚焦,相較其他專業領域經常性國際學術交流的經驗,本人 有點失望,本人長久執行國科會浮除程序之計畫,亦有論文發表,卻很難找到同行 研究學者切磋,實在受益有限。
Precipitation flotation of phosphate from water Warmadewanthi, Ching-Jung Chang and J. C. Liu*
Department of Chemical Engineering,
National Taiwan University of Science and Technology 43 Keelung Road, Section 4, Taipei 106, Taiwan
Abstract
The removal of phosphate from water was by precipitation flotation was examined. Calcium chloride (CaCl2) was added to induce precipitates. Effects of both molar ratio ([Ca2+]: [HPO42-
]) and pH on precipitation were examined, and experimental results were compared with those from equilibrium modeling by PHREEQC. Molar ratio and pH were the key parameters in determining the residual phosphate concentration, and monetite (CaHPO4), hydroxyapatite (Ca5(PO4)3OH), and amorphous calcium phosphate precipitates (Ca3(PO4)2.xH2O) were found to be the dominant solid species. Dispersed air flotation was utilized for the removal of precipitates from water using various collectors. Anionic collector, sodium dodecylsulfate (SDS) and sodium oleate (NaOl) could become adsorbed onto solid surfaces and facilitated the flotation removal of calcium phosphate precipitates. Neither nonionic collector Brij35 nor cationic cetyltrimethyl ammonium bromide (CTAB) were effective collector. Effects concentration of collector on flotation reactions were studied as well. The ionic strength and the presence of anions affected the removal efficiency of calcium phosphate precipitate when SDS was the collector.
However, they can be overcome by increasing the concentration of SDS or by adding SOl.
Keywords: Calcium phosphate; Collector; Flotation; Oleate; Phosphate; Wastewater.
1. Introduction
Phosphorus is commonly found in municipal wastewater with concentration ranging
from 4 to 12 mg/l [1]. It can be removed by biological processes and chemical
precipitation processes. Higher concentrations of phosphate are also present in landfill
leachate and wastewaters from some conventional industries, such as fertilizer and
electroplating. It is noted that both semiconductor and optoelectronic industries produce
significant amount of wastewater that contains very high concentration of phosphate,
since phosphoric acid (H3PO4) is extensively used in etching unit in semiconductor and
thin-film transistor liquid crystal display (TFT-LCD) fabrication [2]. According to a
survey conducted by Taiwan EPA, average phosphate concentration in optoelectronic
wastewater is 297 mg/l with a maximum of 1505 mg/l, while it averages 63 mg/l with a
maximum of 220 mg/l in semiconductor wastewater. Practical experiences among some
electronic and optoelectronic plants in Taiwan show that biological processes may not be
suitable in treating high-phosphate wastewater, owing to the complex operation and
limited efficiency. Thus, the existing wastewater treatment process usually consists of
chemical precipitation using calcium salts, followed by biological process. Even so, the
treated effluent still contains phosphate in the range of 50 – 70 mg/l and is a major
environmental concern. It is imperative to investigate more effective methods in removing
high concentration of phosphate from wastewater.
Chemical precipitation processes by using aluminum, iron, and calcium salts have long
been utilized to remove phosphate from water. The multivalent metal ions used most
commonly are aluminum and iron [1]. Phosphate is removed either through formation of
aluminum phosphate precipitates or via adsorption onto aluminum hydroxide [3].
However, the disadvantage is that the sludge cannot be reclaimed and reused due to
complex constituents [4]. Calcium can react with phosphate to form precipitates, such as,
in order of decreasing solubility, hydroxypatite (HAP, Ca5(PO4)3OH), tricalcium
phosphate (TCP, Ca3(PO4)2), octacalcium phosphate (OCP, Ca8(HPO4)2(PO4)4.5 H2O),
monetite (CaHPO4), brushite (CaHPO4. 2 H2O), and amorphous calcium phosphate
(ACP, Ca3(PO4)2.xH2O), depending on pH and molar ratio of calcium to phosphate [5].
There are several advantages in using calcium salts for precipitation of phosphate; namely
less amount of sludge, high removal efficiency, and reusable sludge [4]. However, the
calcium phosphate precipitates are difficult to settle, and polymers are required as
flocculant aids [1]. It is needed to further develop solid/liquid separation technologies.
Mineral flotation is a well-established commercial technique. The sparingly soluble
calcium minerals such as calcite, fluorite, and apatite are largely concentrated by flotation
processes. For effective separation, selective adsorption of the collectors at mineral/water
interfaces is essential, so that surface of minerals is made hydrophobic and flotation can
be obtained [6]. One of the most widely used collectors is sodium oleate (SOl) that
separates by flotation apatite preferentially from a solution containing dolomite [7 - 10].
Therefore, the mechanism of the adsorption of SOl on apatite has been extensively studied,
mainly because of the important role of adsorption in flotation [6, 11]. It has to be pointed
out that the species and ion composition in the aqueous phase affect the flotation
separation behaviors significantly. On the premise that the differences in solution
chemistry between apatite ore suspension and wastewater containing calcium phosphate
precipitates can be well delineated, dispersed air flotation (DiAF) appears to be an
excellent alternative technique for treating the phosphate-containing wastewater, knowing
that flotation processes posses advantages of small footprint, flexible operation, and high
efficiency.. The major objectives of the current work were to study the feasibility of
utilizing DiAF in precipitation flotation of phosphate-containing wastewater, and to
investigate important parameters in the design and operation of DiAF.
2. Materials and Methods
Stock solutions of synthetic wastewater and calcium chloride were made by dissolving
potassium dihydrogen phosphate (KH2PO4, Merck) and calcium chloride dehydrate
(CaCl2.2H2O, Merck), respectively in water. Measured amount of Ca2+ solution was
added to 1 liter of phosphate-containing water (100 mg/l) to obtain different molar ratio of
calcium to phosphate, [Ca2+]/[HPO42-]. The pH of the solution was adjusted at fixed value
with a potentiometric automatic titrator (KEM, AT-400) and mixed for 10 min to allow the
precipitation reaction to proceed to completion. The suspended solid (SS) concentration of
the wastewater suspension was first measured, and residual phosphate concentration was
determined by ion chromatography (Dionex, DX-100) equipped with a column (IonPac
AS4A-SC) and an auto sampler (Spectra AS1000) after filtration (MFS, 0.2 μ m).
Calcium phosphate precipitates were vacuum- dried at 50 oC for 24 hours and
characterized by X-ray diffraction (Philips, MP 710). Meanwhile, the wastewater
suspension was added with collector and transferred to the flotation column for flotation
experiments. A bench-scale, batch-type apparatus was used in the flotation experiments
[12]. Flotation time was then kept at 10 min since kinetic study showed negligible
increment in flotation efficiency with longer flotation time. When the reaction was
completed, the phosphate concentration and the suspended solid concentration in the
remaining solution were measured. Zeta potential was measured by a zeta meter (Malvern,
Zetamaster). Average diameter of particle was found to be 7 - 9 μm as measured by a
particle sizer (Malvern 2600C). PHREEQC was used for modeling of equilibrium
speciation of calcium and phosphate. This model is developed by the US Geological
Survey for assessment of equilibrium chemical speciation in solution [13]. It has a
significant advantage that allows users to create personal thermodynamic database in
which possible solid phases can be included with their characteristic values of solubility
product (Ksp), enthalpy, and stoichiometric coefficient.
3. Results and discussion
Experimental residual phosphate concentrations as compared with model
predictions are shown in Fig. 1. Molar ratio, [Ca2+]/[HPO42-], of 1:1 resulted in residual
phosphate concentration of 35 mg/l at pH 10, and decreased to 30 mg/l as pH became
higher. Higher removal efficiency of phosphate was found at molar ratio of 2:1, and
residual phosphate of < 3 mg/l was obtained at molar ratio of 3:1 at pH > 9.0. It is in
agreement with literature that calcium phosphate precipitation is almost complete for pH
> 9.8 [14]. It was found that residual phosphate concentrations at pH > 6 were all higher
than model predictions regardless of molar ratio. Chemical equilibrium between calcium
and phosphate at molar ratio of 2:1 or 3:1, as predicted by PHREEQC, shows that HAP
starts to form at pH > 5.0, and HAP is the dominant species for pH > 6.5. However, it has
been indicated that precipitation reaction of HAP is very slow, and a number of species,
such as amorphous calcium phosphate (ACP), octacalcium phosphate (OCP) and brushite,
act as precursors to the precipitation of HAP; With time these species will be transformed
to HAP [15, 16]. The dominant precipitates formed, depending upon pH, are in fact
brushite, monetite, and ACP, while predicted equilibrium solubility is much lower since it
is based on HAP [15]. This is the reason for the differences between experimental results
and model predictions of residual phosphate in lower pH range. It is confirmed in the
XRD analysis (Fig. 2) in which monetite, ACP, and HAP are found in calcium phosphate
precipitates formed at molar ratio of 3;1 and at pH 10.0.
Effects of collector type and concentration on the removal of phosphate and calcium
phosphate precipitates are illustrated in Figs. 3A and 3b. For initial phosphate
concentration of 100mg/l, pH of 10.0, and [Ca2+]/[HPO42-] of 3, it was found that residual
phosphate concentrations were all below 0.5 mg/l, while the removal efficiency of
suspended solid increased with SDS concentration and reached 98 % at SDS
concentration of 50 mg/l. It increased as SDS concentration increased further. When SOl
was used, it was found that higher concentration (100 mg/l) was needed to obtain 97 %
removal of suspended solid, and that was the maximum efficiency regardless of SOl
dosage. Data not shown here indicated that neither cationic collector, cetyltrimethyl
ammonium bromide (CTAB), nor nonionic collectors, Triton X-100 and Brij 35, could
induce effective flotation removal of suspended solid. This is in agreement with literature
that anionic collectors are extensively used for phosphate flotation [6 - 10].
Zeta potentials of precipitates as affected by collector concentration are shown in Figs.
4A and 4B. The surface of calcium phosphate precipitates were positively charged at pH
10.0, probably because of the presence of excess Ca2+. The positive zeta potential
decreased slightly with increasing SDS concentration, and became negative only at SDS
concentration of 250 mg/l. The decrease was caused by the charge neutralization as SDS
was adsorbed on solid surfaces [17]. It is probable for Ca2+ to react with SDS and form
Ca(DS)2 precipitate whose solubility product is 10-9.7. Since the remaining Ca2+
concentration is 1.55 x 10-3 M, Ca(DS)2 will form as SDS concentration exceeds 100
mg/l/. Fig. 4B shows that zeta potential abruptly decreased in the presence of SOl, and
charge reversal was found at low concentration of SOl. It can be explained by the
adsorption of SOl. Monolayer chemisorption is predominant and occurs by the reaction of
carboxylate head group with surface calcium sites of apatite surface when oleate
concentration is low [6]. The adsorption of SOl on apatite could become bilayer, and
surface calcium oleate precipitation will occur under high adsorption density; and result in
significant decrease in zeta potential [18]. Flotation of hydrophilic apatite requires the
adsorption of surfactant to render the mineral surface hydrophobic, and flotation reaction
is controlled by the formation and adsorption of calcium oleate in the interfacial region
[11].
Effects of ionic strength on the removal of phosphate and calcium phosphate
precipitates are illustrated in Figs. 5A and 5B. Residual phosphate concentration
increased gradually as sodium nitrate (NaNO3) concentration increased, due to the
decreased activity of phosphate ion. It is noted that the flotation removal of calcium
phosphate precipitates decreased significantly with increasing ionic strength when SDS
(50 mg/l) was used as collector. Removal of suspended solid decreased to 70 % at 0.05 M
of NaNO3, and it was completely inhibited at 0.1 M of NaNO3. Possible explanation is
that SDS is adsorbed on solid surface via electrostatic interactions, which is “screened”
and becomes weaker as ionic strength increases [19]. The competition between nitrate and
phosphate for adsorption sites might exacerbate the condition. However, flotation of
calcium precipitates was not affected by ionic strength when SOl was the collector (Fig.
5B). It is noted that excess Ca2+ in the solution will increase adsorption of oleate collector
onto apatite, and precipitates on the mineral surface [6]. The predominant chemisorption
of oleate renders it not significantly affected by ionic strength [18]. In addition,
precipitated calcium oleate is hydrophobic and its effect is not affected by ionic strength
either [6]. The hindered flotation of calcium phosphate precipitates using SDS as collector
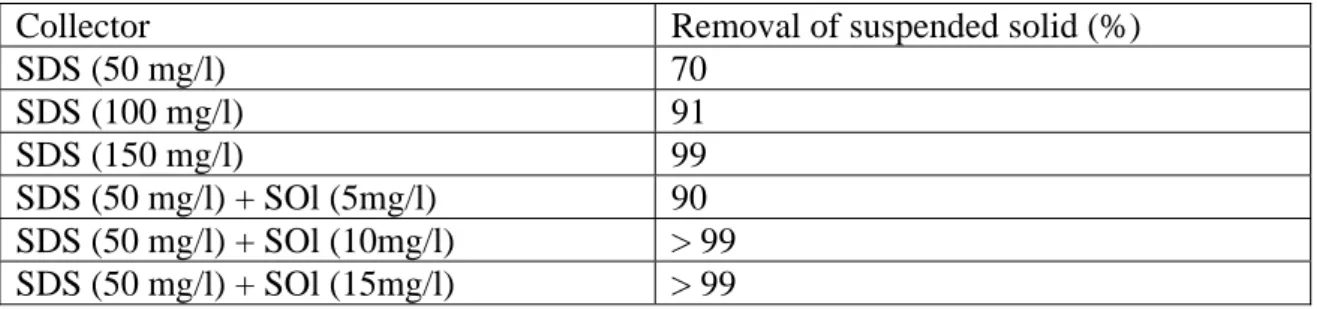
under 0.05 M of NaNO3 can be overcome by increasing SDS concentration or combined
use of SDS and SOl (Table 1). Removal efficiency of suspended solid was improved from
70 % to 91 % when SDS concentration was doubled to 100 mg/l, and it became > 99 % as
SDS concentration increased to > 150 mg/l. On the other hand, removal efficiency of
suspended solid increased to 90 % when 5 mg/l of SOl was added to the suspension, and
it reached over 99 % at SOl concentration of 10 mg/l. This may be due to the synergistic
advantages of using surfactant mixtures [6].
Since mixed acids are commonly used in etching unit in both semiconductor and
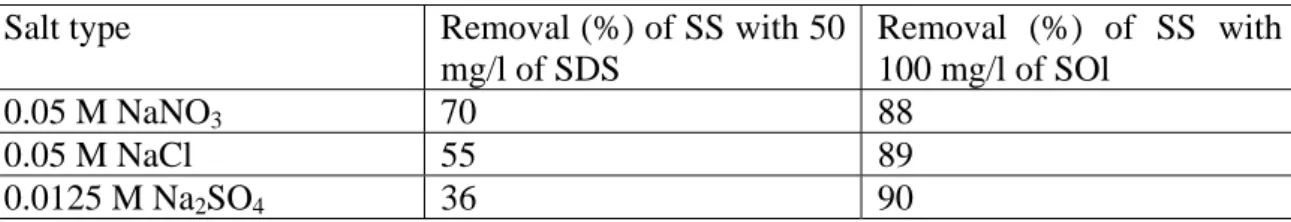
optoelectronic industries, anion interference was studied by using 0.05 M of NaNO3, 0.05
M of NaCl, and 0.0125 M of Na2SO4 as background electrolyte of identical ionic strength,
respectively. It was found that flotation removal of calcium precipitates, using SDS as
collector, decreased from 70 % to 55 % when NaNO3 was replaced by NaCl, and to 36 %
in the case by Na2SO4 (Table 2). This is in agreement with literature that anion with
higher valency has stronger effect on flotation efficiency [20]. It is because both SO42- and
Cl- could potentially be specifically adsorbed onto solid surfaces and interferes with the
adsorption of SDS. Again, flotation was not affected by anions when SOl was the
collector. It could be reasoned that the chemisorption of SOl onto calcium phosphate
precipitates was not affected by anions.
Application of precipitation flotation in treating industrial wastewater with high
concentration of phosphate seemed feasible from the study. Very low residual phosphate
concentration could be obtained as long as molar ratio and pH are properly controlled.
The separation of calcium phosphate precipitates could be induced by anionic collectors,
even under situations of high ionic strength or presence of interfering anions. Advantages
of the process include reusable sludge, flexible operation, and small footprint. However,
some issues need to be examined in further study. For example, to minimize excess Ca2+
in reacting with SOl, and to investigate the selective separation of PO43- and F-, a common
constituent in semiconductor and optoelectronic wastewater.
4. Conclusions
The current study demonstrated that calcium chloride was effective for removal of
phosphate from water. When molar ratio, [Ca2+]/[HPO42-], was 3:1, residual phosphate
concentration of < 3 mg/l was obtained at pH > 9.0. Though affected by molar ration and
pH, major precipitates are found to be monetite, ACP, and HAP. Anionic collectors, SDS
and SOl, were effective in flotation removal of calcium phosphate precipitates from water.
It was proposed that SDS was adsorbed onto solid surfaces through electrostatic
interactions and were significantly affected by increased ionic strength and anion
interferences. The chemisorption of SOl onto solid surfaces made the flotation efficiency
not much affected by changes in ionic strength and presence of anions, such as sulfate and
chloride.
![Fig. 1 3 4 5 6 7 8 9 10 11 120102030405060708090100I = 0.005 M[HPO42-] = 100 mg/LTemp = 25 oC](https://thumb-ap.123doks.com/thumbv2/9libinfo/9128294.412405/41.892.209.767.253.739/fig-i-m-hpo-mg-ltemp-oc.webp)
