C LINICAL I NVESTIGATION
Risk Factors of Recurrence After Curative Resection of Hepatocellular Carcinoma in Taiwan
Shih-Wei Lai, MD, Kuan-Fu Liao, MD, Chung-Yi Lin, MD, Chi-Hung Huang, MD and Yi-Ying Lin, MD
Abstract: Introduction: The purpose of this study was to explore the potential risk factors of hepatocellular carcinoma (HCC) recurrence after curative resection of primary HCC. Methods: This was a hospital- based retrospective cohort study. The authors analyzed the medical records of all the subjects with HCC initially treated by hepatic resection at a medical center in Taiwan from 1995 to 2006. In all, 222 subjects were enrolled in this study. The total observational period was 3 years. Results: There were 172 men (77.5%) and 50 women (22.5%).
The mean age was 57.0⫾ 13.7 years (range, 15–79 years). Among 222 subjects, the overall recurrence rates were 28.8% (64/222), 42.3%
(94/222) and 47.7% (106/222) at 1, 2 and 3 years, respectively.
Multivariate logistic regression analysis exhibited that tumor sizeⱖ5 cm [odds ratio (OR)⫽ 2.31, 95% confidence interval (CI) ⫽ 1.27–4.17], liver cirrhosis (OR⫽ 2.11, 95% CI ⫽ 1.18–3.79) and preoperative aspartate aminotransferase levelⱖ34 IU/L (OR ⫽ 2.02, 95% CI ⫽ 1.01–4.04) were independent risk factors of HCC recurrence. Conclusion: Patients who have larger tumor size, liver cirrhosis and higher preoperative aspartate aminotransferase level should be carefully followed up because they are at high risk of HCC recurrence postoperatively.
Key Indexing Terms: Aspartate aminotransferase; Cirrhosis; Hepato- cellular carcinoma; Recurrence; Tumor size. [Am J Med Sci 2011;
341(4):301–304.]
T o date, treatment modalities for hepatocellular carcinoma (HCC) include surgical resection, percutaneous radiofre- quency ablation, percutaneous ethanol injection, transarterial embolization and liver transplantation. However, HCC recur- rence is frequently detected after ablation therapy for primary HCC. The cumulative recurrence rates after ablation therapy are around 37.0% to 59.7% at 1 year and 71.6% to 76.5% at 3 years, respectively,1,2depending on the underlying causes, the initial treatment modalities, the follow-up duration and the population studied.
Although the real pathogenesis of HCC recurrence after ablation therapy remains unclear, the risk factors of HCC recurrence include the larger tumor size, the smaller ablative/
resection margin, the presence of vascular invasion, multiple HCC nodules, histologic classification, pretreatment high se- rum alpha-fetoprotein (AFP) level, pretreatment low platelet count, pretreatment low serum albumin level and visceral fat accumulation.
1–10Therefore, close observation to detect any early recurrence sign in patients with these mentioned risk factors is needed.
HCC ranked the second leading cause of cancer death in Taiwan in 2009.
11It accounted for approximately 7809 deaths in 2007, 7651 deaths in 2008 and 7759 death in 2009.
11At present, although some studies have reported the risk factors of HCC in Taiwan,
2–5for establishing the newer evidence, we conducted this retrospective cohort study to explore the poten- tial risk factors of HCC recurrence.
MATERIALS AND METHODS Study Population and Inclusion Criteria
This was a hospital-based retrospective cohort study. We analyzed the medical records of all the subjects diagnosed as having initial HCC at a medical center located at Taichung city in Taiwan from 1995 to 2006. The institutional review board of this medical center approved this retrospective study.
The inclusion criteria for this study were as follows: (i) subjects who underwent hepatic resection of primary tumors and pathologic reports confirmed as having HCC; (ii) subjects who did not have extrahepatic metastasis or vessel invasion based on imaging; and (iii) subjects who were considered as having complete curative response 1 month after hepatic resec- tion of primary tumors.
5,12All subjects underwent computed tomography or abdominal ultrasonography at 1 month after hepatic resection of primary tumors to assess the therapeutic effects. No focal lesion in the liver or in the other organs was regarded as complete curative response. In all, 222 subjects meeting the inclusion criteria were enrolled in this study. The total observational period was 3 years.
Serum Data
The preoperative serum data including aspartate amino- transferase (AST), alanine aminotransferase, albumin, total
From the Department of Internal Medicine (K-FL, C-YL,C-HH,Y-YL), Taichung Tzu Chi General Hospital, Taichung County, Taiwan; and De- partment of Family Medicine (S-WL), China Medical University Hospital, Taichung city, Taiwan.
Submitted June 21, 2010; accepted in revised form September 30, 2010.
The authors disclose no conflicts of interest.
Correspondence: Shih-Wei Lai, MD, Department of Family Medicine, China Medical University Hospital, No 2, Yuh-Der Road, Taichung city, 404, Taiwan (E-mail: wei@mail.cmuh.org.tw).
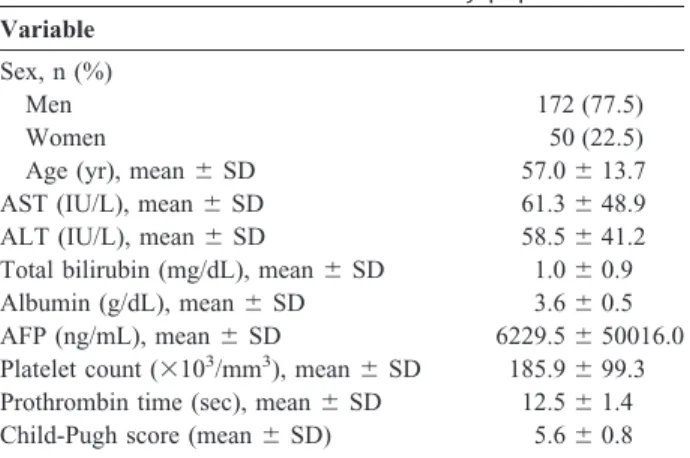
TABLE 1. Basic characteristics of the study population Variable
Sex, n (%)
Men 172 (77.5)
Women 50 (22.5)
Age (yr), mean⫾ SD 57.0⫾ 13.7
AST (IU/L), mean⫾ SD 61.3⫾ 48.9
ALT (IU/L), mean⫾ SD 58.5⫾ 41.2
Total bilirubin (mg/dL), mean⫾ SD 1.0⫾ 0.9
Albumin (g/dL), mean⫾ SD 3.6⫾ 0.5
AFP (ng/mL), mean⫾ SD 6229.5⫾ 50016.0
Platelet count (⫻103/mm3), mean⫾ SD 185.9⫾ 99.3 Prothrombin time (sec), mean⫾ SD 12.5⫾ 1.4 Child-Pugh score (mean⫾ SD) 5.6⫾ 0.8
AST, aspartate aminotransferase; ALT, alanine aminotransferase;
AFP, alpha-fetoprotein; SD, standard deviation.
The American Journal of the Medical Sciences • Volume 341, Number 4, April 2011
301
bilirubin, AFP, platelet count, prothrombin time, hepatitis B virus surface antigen and hepatitis C antibody were collected.
Child–Pugh score, HCC Pathological grade
13and Cancer of the Liver Italian Program score
14were also measured. Liver cir- rhosis was diagnosed by pathologic finding. Ascites was de- tected by abdominal ultrasonography. All subjects underwent routine abdominal ultrasonography, liver function tests and serum AFP at 3-month intervals after hepatic resection of primary tumors. When suspicious findings on ultrasonography or abnormal serum data were detected, computed tomography was arranged to confirm recurrent HCC.
Statistical Analysis
Statistical analysis was performed by SPSS (Taiwan Version 12.0; Sinter Information, Taipei, Taiwan). The Student
t test was performed for continuous variables, and the
2test was performed for qualitative variables. The relative risks were estimated by adjusted odds ratio (OR) and 95% confidence interval (CI) using a multivariate logistic regression model.
Cumulative recurrence rates were calculated by the Kaplan–
Meier method during follow-up period, and differences were compared by log-lank test. A P-value of ⬍0.05 was considered statistically significant.
RESULTS
Basic Characteristics of the Study Population
The basic characteristics were shown in Table 1. There were 172 men (77.5%) and 50 women (22.5%). The mean age was 57.0 ⫾ 13.7 years (range, 15–79 years).
Related Factors of HCC Recurrence by Univariate Analysis
Among 222 subjects, the overall recurrence rates were 28.8% (64/222), 42.3% (94/222) and 47.7% (106/222) at 1, 2 and 3 years, respectively. By using the Student t test and
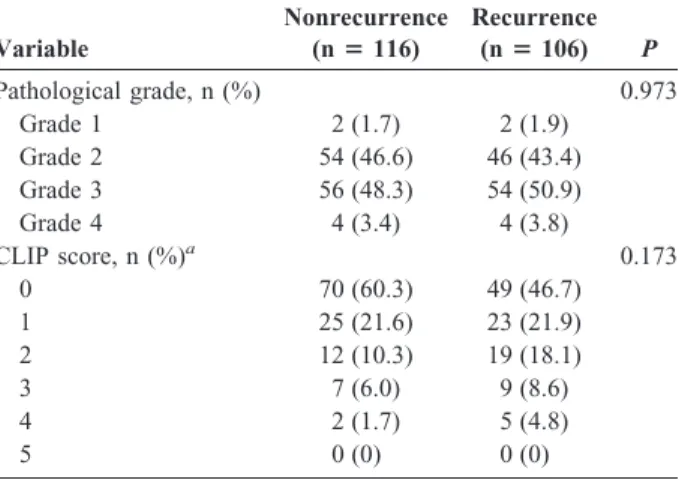
2test, the statistically related factors of HCC recurrence were found to be preoperative AST level (P ⫽ 0.014), preoperative AFP level (P ⫽ 0.041), liver cirrhosis (P ⫽ 0.012) and tumor size (P ⫽ 0.009; Table 2).
TABLE 2. Factors related to recurrence of HCC after hepatic resection by univariate analysis
Variable
Nonrecurrence (nⴝ 116)
Recurrence (nⴝ 106) P Age (yr), mean⫾ SD 56.9⫾ 13.6 57.1⫾ 13.8 0.891 Prothrombin time (sec),
mean⫾ SD 12.5⫾ 1.4 12.5⫾ 1.3 0.837
Child-Pugh score
(mean⫾ SD) 5.5⫾ 0.9 5.6⫾ 0.8 0.504
Sex, n (%) 0.547
Men 88 (75.9) 84 (79.2)
Women 28 (24.1) 22 (20.8)
AST (IU/L), n (%)a 0.014
⬍34 34 (29.3) 16 (15.4)
ⱖ34 82 (70.7) 88 (84.6)
ALT (IU/L), n (%)a 0.972
⬍40 46 (39.7) 41 (39.4)
ⱖ40 70 (60.3) 63 (60.6)
Total bilirubin (mg/dL), n (%)a
0.400
⬍1.3 96 (83.5) 91 (87.5)
ⱖ1.3 19 (16.5) 13 (12.5)
Albumin (g/dL), n (%)a 0.071
ⱖ3.8 58 (50.4) 39 (38.2)
⬍3.8 57 (49.6) 63 (61.8)
AFP (ng/mL), n (%)a 0.041
⬍9 52 (45.6) 33 (32.0)
ⱖ9 62 (54.4) 70 (68.0)
Platelet count, n (%)a 0.618
ⱖ100,000/mm3 100 (86.2) 88 (83.8)
⬍100,000/mm3 16 (13.8) 17 (16.2)
Hepatitis status, n (%) 0.202
HBsAg (⫺) and
anti-HCV (⫺) 23 (19.8) 15 (14.2)
HBsAg (⫹) and
anti-HCV (⫺) 53 (45.7) 40 (37.7)
HBsAg (⫺) and
anti-HCV (⫹) 33 (28.4) 40 (37.7)
HBsAg (⫹) and
anti-HCV (⫹) 7 (6.0) 11 (10.4)
Ascites, n (%) 0.427
No 112 (96.6) 100 (94.3)
Yes 4 (3.4) 6 (5.7)
Liver cirrhosis, n (%) 0.012
No 61 (52.6) 38 (35.8)
Yes 55 (47.4) 68 (64.2)
Tumor nodule, n (%) 0.215
Solitary 103 (88.8) 88 (83.0)
ⱖ2 13 (11.2) 18 (17.0)
Tumor size (cm), n (%) 0.009
⬍5 81 (69.8) 56 (52.8)
ⱖ5 35 (30.2) 50 (47.2)
Section margin (cm), n (%)a
0.565
ⱖ1 40 (48.2) 37 (52.9)
⬍1 43 (51.8) 33 (47.1)
Variable
Nonrecurrence (nⴝ 116)
Recurrence (nⴝ 106) P
Pathological grade, n (%) 0.973
Grade 1 2 (1.7) 2 (1.9)
Grade 2 54 (46.6) 46 (43.4)
Grade 3 56 (48.3) 54 (50.9)
Grade 4 4 (3.4) 4 (3.8)
CLIP score, n (%)a 0.173
0 70 (60.3) 49 (46.7)
1 25 (21.6) 23 (21.9)
2 12 (10.3) 19 (18.1)
3 7 (6.0) 9 (8.6)
4 2 (1.7) 5 (4.8)
5 0 (0) 0 (0)
aImprecise summation of total subjects was because of missing data.
AST, aspartate aminotransferase; ALT, alanine aminotransferase;
AFP, alpha-fetoprotein; HBsAg, hepatitis B virus surface antigen;
HCV, hepatitis C virus; CLIP, Cancer of the Liver Italian Program; SD, standard deviation.
Lai et al
302
Volume 341, Number 4, April 2011Risk Factors of HCC Recurrence by Multivariate Logistic Regression
Only the statistically related factors identified in univar- iate analysis were further analyzed. After controlling for the other covariates, multivariate logistic regression analysis ex- hibited that the OR of HCC recurrence was 2.31 (95% CI ⫽ 1.27– 4.17, P ⫽ 0.006) for subjects with tumor size ⱖ5 cm, compared with subjects with tumor size ⬍5 cm. The OR of HCC recurrence was 2.11 (95% CI ⫽ 1.18–3.79, P ⫽ 0.012) for subjects with liver cirrhosis, compared with subjects with- out liver cirrhosis. The OR of HCC recurrence was 2.02 (95%
CI ⫽ 1.01–4.04, P ⫽ 0.047) for subjects with preoperative AST level ⱖ34 IU/L, compared with subjects with preoperative AST level ⬍34 IU/L (Table 3).
Cumulative Recurrence Rates of HCC
We further analyzed the cumulative recurrence rates of HCC by tumor size, liver cirrhosis and preoperative AST level.
The recurrence rates for subjects with tumor size ⱖ5 cm and ⬍5 cm were 43.5% and 19.7% at 1 year (P ⬍ 0.0001), 52.9% and 35.8% at 2 years (P ⫽ 0.0016) and 58.8% and 40.9% at 3 years (P ⫽ 0.0012), respectively. The recurrence rates for subjects with liver cirrhosis and without cirrhosis were 31.7% and 25.3% at 1 year (P ⫽ 0.3994), 49.6% and 33.3% at 2 years (P ⫽ 0.0377) and 55.3% and 38.4% at 3 years (P ⫽ 0.0273), respectively. The recurrence rates for subjects with preoperative AST level ⱖ34 IU/L and ⬍34 IU/L were 33.5% and 12.0% at 1 year (P ⫽ 0.005), 47.1% and 24.0% at 2 years (P ⫽ 0.0048) 51.8% and 32.0% at 3 years (P ⫽ 0.0111), respectively.
DISCUSSION
Although not novel, our study made a major finding that larger tumor size, liver cirrhosis and higher preoperative AST level are independent risk factors of HCC recurrence. In this study, we found that a 2.3-fold risk of HCC recurrence in subjects with tumor size ⱖ5 cm is revealed when compared with tumor size ⬍5 cm. In the study by Jwo et al,
3they found tumor size greater than 5 cm was also a risk factor of HCC recurrence. In the study by Lam et al,
6they found tumor size
⬎2.5 cm was the only independent risk factor of local recur- rence. To the best of our knowledge, there is not a consensus about what size of tumor is risky for HCC recurrence, but we think the larger the tumor, the riskier for HCC recurrence.
In this study, we found that a 2.1-fold risk of HCC recurrence in subjects with liver cirrhosis is revealed when compared with subjects without liver cirrhosis. The former studies have also proven that liver cirrhosis is a risk factor of HCC recurrence.
15,16In the study by Poon et al,
17they found
liver cirrhosis is the only significant risk factor for late recur- rence ( ⬎1 year; risk ratio ⫽ 2.378, P ⫽ 0.018). Cirrhosis is known as a precancerous lesion. The previous studies have reported that the high risk for developing HCC in cirrhotic liver was related to high hepatocellular proliferation, presumably by increased rate of random mutations and promotion.
18,19In this study, the risk of HCC recurrence was increased by 2-fold in subjects with preoperative AST level ⱖ34 IU/L, when compared with subjects with preoperative AST level ⬍34 IU/L. In the study by Kubo et al,
20the risk of HCC recurrence was increased by 1.7-fold in subjects with AST level ⬎40 IU/L (95% CI ⫽ 1.05–2.84, P ⫽ 0.03). In the study by Fuke et al,
21the risk of HCC distant recurrence was increased by 4.3-fold in subjects with AST level ⬎60 IU/L (95% CI ⫽ 1.918–9.721,
P⫽ 0.0004). These above findings further confirm that the underlying inflammatory state of liver has greater effect on HCC recurrence.
20,21Some limitations should be mentioned. First, this was a population-based study. These data could not be representative of all people in Taiwan. Second, the follow-up period was short. Some potential risk factors might not be revealed during the short time. Third, the prognostic importance of microscopic vascular invasion was not included in this study. A long-term prospective study with a more representative group of subjects is needed to confirm our data.
CONCLUSION
This study has shown that tumor size ⱖ5 cm, liver cirrhosis and preoperative AST level ⱖ34 IU/L are the risk factors of HCC recurrence. That is, patients who have larger tumor size, liver cirrhosis and higher preoperative AST level should receive close surveillance because they are at high risk of HCC recurrence postoperatively. We hope that this study can provide the basic information about HCC recurrence in Taiwan.
REFERENCES
1. Ikeda K, Saitoh S, Tsubota A, et al. Risk factors for tumor recurrence and prognosis after curative resection of hepatocellular carcinoma.
Cancer 1993;71:19 –25.
2. Chen MF, Hwang TL, Jeng LB, et al. Postoperative recurrence of hepatocellular carcinoma. Two hundred five consecutive patients who underwent hepatic resection in 15 years. Arch Surg 1994;129:738 – 42.
3. Jwo SC, Chiu JH, Chau GY, et al. Risk factors linked to tumor recurrence of human hepatocellular carcinoma after hepatic resection.
Hepatology 1992;16:1367–71.
4. Yu HC, Cheng JS, Lai KH, et al. Factors for early tumor recurrence of single small hepatocellular carcinoma after percutaneous radiofre- quency ablation therapy. World J Gastroenterol 2005;11:1439 – 44.
5. Huang YH, Wu JC, Chen CH, et al. Comparison of recurrence after hepatic resection in patients with hepatitis B vs. hepatitis C-related small hepatocellular carcinoma in hepatitis B virus endemic area. Liver Int 2005;25:236 – 41.
6. Lam VW, Ng KK, Chok KS, et al. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J Am Coll Surg 2008;207:20 –9.
7. Ibrahim S, Roychowdhury A, Hean TK. Risk factors for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. Am J Surg 2007;194:17–22.
8. Okuwaki Y, Nakazawa T, Shibuya A, et al. Intrahepatic distant recurrence after radiofrequency ablation for a single small hepato- cellular carcinoma: risk factors and patterns. J Gastroenterol 2008;
43:71– 8.
9. Yamanaka Y, Shiraki K, Miyashita K, et al. Risk factors for the TABLE 3. Factors related to HCC recurrence by multivariate
analysis
Variable
Odds ratio (95% CI) Tumor size (cm) (ⱖ5 vs. ⬍5) 2.31 (1.27–4.17)a Liver cirrhosis (yes vs. no) 2.11 (1.18–3.79)b AST (IU/L) (ⱖ34 vs. ⬍34) 2.02 (1.01–4.04)b
AFP (ng/mL) (ⱖ9 vs. ⬍9) 1.59 (0.89–2.85)
aP⬍ 0.01.
bP⬍ 0.05.
AST, aspartate aminotransferase; AFP, alpha-fetoprotein; CI, con- fidence interval.
Risk Factors of Hepatocellular Carcinoma Recurrence
© 2011 Lippincott Williams & Wilkins
303
recurrence of hepatocellular carcinoma after radiofrequency ablation of hepatocellular carcinoma in patients with hepatitis C. World J Gastro- enterol 2005;11:2174 – 8.
10. Ohki T, Tateishi R, Shiina S, et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after cu- rative treatment in patients with suspected NASH. Gut 2009;58:839 – 44.
11. Department of Health, Taiwan. Main Causes of Death in 2009. Avail- able at: http://www.doh.gov.tw/CHT2006/DM/DM2_2.aspx?now_fod_
list_no⫽11122&class_no⫽440&level_no⫽3. Accessed August 1, 2010.
12. Kuzuya T, Katano Y, Kumada T, et al. Efficacy of antiviral therapy with lamivudine after initial treatment for hepatitis B virus-related hepatocellular carcinoma. J Gastroenterol Hepatol 2007;22:1929 –35.
13. Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48900 necropsies. Cancer 1954;7:462–503.
14. Cancer of the Liver Italian Program (CLIP) investigators: a new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology 1998;28:751–5.
15. Ishii H, Okada S, Nose H, et al. Predictive factors for recurrence after percutaneous ethanol injection for solitary hepatocellular carcinoma.
Hepatogastroenterology 1996;43:938 – 43.
16. Hanazaki K, Kajikawa S, Shimozawa N, et al. Survival and recur- rence after hepatic resection of 386 consecutive patients with hepato- cellular carcinoma. J Am Coll Surg 2000;191:381– 8.
17. Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 2000;89:500 –7.
18. Tarao K, Ohkawa S, Shimizu A, et al. Significance of hepatocel- lular proliferation in the development of hepatocellular carcinoma from anti-hepatitis C virus-positive cirrhotic patients. Cancer 1994;
73:1149 –54.
19. Rua S, Comino A, Fruttero A, et al. Flow cytometric DNA analysis of cirrhotic liver cells in patients with hepatocellular carcinoma can provide a new prognostic factor. Cancer 1996;78:1195–202.
20. Kubo S, Hirohashi K, Tanaka H, et al. Risk factors for recurrence after resection of hepatitis C virus-related hepatocellular carcinoma.
World J Surg 2000;24:1559 – 65.
21. Fuke H, Sugimoto K, Shiraki K, et al. Predictive factors for distant recurrence of HCV-related hepatocellular carcinoma after radiofre- quency ablation combined with chemoembolization. Aliment Pharma- col Ther 2008;27:1253– 60.
Lai et al