Title: A Review of Potential Harmful Interactions between Anticoagulant/Antiplatelet Agents and Chinese Herbal Medicines
Hsin-Hui Tsai
1,2, Hsiang-Wen Lin
1,2,3*, Ying-Hung Lu
1, Yi-Ling Chen
1, Gail B. Mahady
41. School of Pharmacy and Graduate Institute, College of Pharmacy, China Medical University, Taichung, Taiwan,
2. Department of Pharmacy, China Medical University Hospital, Taichung, Taiwan,
3. Department of Pharmacy Administration, College of Pharmacy, University of Illinois at Chicago, Chicago IL, USA,
4. Department of Pharmacy Practice, College of Pharmacy, PAHO/WHO Collaborating Centre for Traditional Medicine, University of Illinois at Chicago, Chicago IL, USA
*Corresponding author Hsiang-Wen Lin, PhD,
School of Pharmacy and Graduate Institute, College of Pharmacy, China Medical University
No. 91 Hsueh-Shih Road, Taichung, Taiwan 40402, R.O.C.
hsiangwl@mail.cmu.edu.tw, hsiangwl@yahoo.com
Tel: 886-4-22053366 ext 5151 Fax: 886-4-22078083
Funding: This study was fully supported by the Committee on Chinese Medicine and Pharmacy, Department of Health, Executive Yuan, Taiwan, R.O.C. (grant number CCMP99-RD-016) and partially supported by National Science Council (grant number NSC 99-2320-B-039 -031 -MY3).
The funders have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests: The authors have declared that no competing interest exists.
Short title: DRUG INTERACTIONS OF CHINESE HERBAL MEDICINES
Abstract
Background: The risks attributed to drug-herb interactions, even when known, are often ignored or
underestimated, especially for those involving anti-clotting drugs and Chinese medicines. The aim of this study was to structurally search and evaluate the existing evidence-based data associated with potential drug interactions between anticoagulant/antiplatelet drugs and Chinese herbal medicines (CHMs) and evaluate the documented mechanisms, consequences, and/or severity of interactions.
Methodology and Findings: Information related to anticoagulant/antiplatelet drug-CHM
interactions was retrieved from eight interaction-based textbooks, four web resources and available primary biomedical literature. The primary literature searches were conducted in English and/or Chinese from January 2000 through December 2011 using the secondary databases (e.g., PubMed, Airiti Library, China Journal full-text database). The search terms included the corresponding medical subject headings and key words. Herbs or natural products not used as a single entity CHM or in Chinese Medicinal Prescriptions were excluded from further review. The corresponding mechanisms and severity ratings of interactions were retrieved using MicroMedex®, Lexicomp®
and Natural Medicines Comprehensive Database®. Finally, we found 90 single entity CHMs contributed to 306 documented drug-CHM interactions. A total of 194 (63.4%) interactions were verified for its evidence describing possible mechanisms and severity. Of them, 155 interactions (79.9%) were attributable to pharmacodynamic interactions, and almost all were rated as moderate to severe interactions. The major consequences of these interactions were increased bleeding risks due to the additive anticoagulant or antiplatelet effects of the CHMs, specifically danshen, dong quai, ginger, ginkgo, licorice, and turmeric.
Conclusions/Significance: Conventional anticoagulants and antiplatelet drugs were documented to
have harmful interactions with some commonly used single entity CHMs. For those patients who
are taking conventional anti-clotting medications with CHMs for cardiovascular or cerebrovascular
diseases, the potential risks of increased bleeding due to drug-CHM interactions should not be
ignored.
Introduction
Ischemic heart disease and stroke are the two primary causes of death worldwide, accounting for 24% of all deaths reported in 2008 [1]. Anticoagulants and antiplatelet drugs are important standard therapies used to prevent clot formation in the treatment and prevention of cardiovascular and cerebrovascular diseases [2]. However, most anticoagulant and antiplatelet agents have drug interactions with a variety of other medications, foods, and dietary supplements [3,4]. A systematic review published in 2008 reported that medications with anticoagulant or antiplatelet activity are most likely to have interactions with herbal medicines [5]. Specifically, those patients given anticoagulant agents are at a higher risk of suffering from potentially harmful drug interactions, compared to other cardiovascular drugs, when co-administered with herbal medicines [6].
The use of complementary and alternative medicine (CAM), including herbal medicines, is widespread and has increased worldwide over the last decade [7,8]. A systematic review of CAM found that the prevalence of CAM use varied widely from 9% to 65% [9]. Nearly 40% of patients with cardiovascular disease or stroke had used CAM therapies concomitantly with their prescribed medications [10,11]. Traditional Chinese Medicine (TCM) is one of the dominant forms of CAM for many Asian populations [7,12,13], including those who migrated to western countries. A substantial percentage of Asian patients were likely to use TCM in combination with the
conventional medicines [14-17]. For instance, 13% of patients using anticoagulant or antiplatelet agents were also prescribed concentrated CHMs concomitantly in a Taiwanese population-based study [17].
To date, there is very limited documented information about potential prescription drug interactions with CHMs. Considerable proportions of clinical practitioners or patients underreport their use of natural health products, including CHMs [18]. The majority of physicians and
professional trainees have limited training on herbal adverse events, toxicities, and drug interactions
[19]. The lack of training and education may be associated with a reduced recognition of potential
drug-herb interactions, specifically their occurrence and associated adverse events. While patients
taking anticoagulant/antiplatelet drugs are more likely to be exposed to potential drug-herb
interactions (i.e., increased risks of bleeding [20]), the aim of this study was to systematically review the available published evidence-based data and biomedical reports associated with drug interactions between anticoagulant/antiplatelet drugs and CHMs, and further to evaluate the documented mechanisms, consequences, and/or severity of interactions.
Materials and Methods
Evidence retrieval and literature search
The evidence regarding “harmful” interactions between anticoagulant/antiplatelet drugs and herbal medications used in TCM (in terms of Chinese herbal medicine [CHM]) was the main focus and extracted from eight interaction-based textbooks [21-28], four web resources [29-32], and available primary biomedical literature sources. In this review, we defined CHM as the same definition derived from the web site of National Center for Complementary and Alternative Medicine and originally cited from Chinese Materia Medica [33]. The search of primary articles was conducted in four English and three Chinese secondary databases, including MEDLINE, PubMed, EMBASE, Cochrane Library, Airiti Library, Index to Taiwan Periodical Literature System, and the China Journal full-text database. The search terms in these databases included the corresponding medical subject headings (MeSH terms) and key words as follows: ‘herb drug interactions’; ‘anticoagulants’
OR ‘antiplatelet drugs’ OR ‘warfarin’ OR ‘heparin’ OR ‘aspirin’ OR ‘clopidogrel’ OR
‘dipyridamole’ OR ‘ticlopidine’ AND ‘Traditional Chinese Medicine’ OR ‘Chinese medication’
OR ‘herbal medicine’ OR ‘phytotherapy’ AND ‘interaction.’ The searches were restricted to either the English or Chinese language during 2000 to 2011 (from January 2000 through December 2011).
The articles were selected based on the title and abstract. The selected articles were retrieved
independently by two authors (YLC, YHL), and then validated by the other two authors (HHT,
HWL). Specifically, all retrieved literature were selected regardless of types of study, i.e., animal
studies, clinical trials, observational studies, or review articles. Those in vitro studies or literature
without interaction reports, or corresponding studies about pure compounds (but not for single
entity CHMs), were excluded.
Evidence extraction and review
Initially, any level of evidence-based interactions, as long as it was retrieved from the
aforementioned books, web sites, or primary literature, between the anticoagulant/antiplatelet agents and any natural products or herbs (i.e., either being classified as CHMs or not) was first extracted and documented. However, animal-derived medications used in TCM were not included.
Then, we used a standardized data abstraction checklist on Excel® spreadsheet to extract all relevant data, including common name, scientific name and/or binomial source, plant parts, dose, route of administration, drug name, consequence of interactions, and evidence resources, etc, if they were available. Data for all anticoagulant/antiplatelet agents was extracted regardless of belonging to a drug class or an individual drug. The anticoagulants were composed of heparin and warfarin while antiplatelet agents included aspirin, clopidogrel, dipyridamole and ticlopidine. All relevant data in the literature was extracted and compiled by two of the authors (YLC, YHL), and then validated by the other two authors (HHT, HWL). To cross-validate the retrieved and reviewed information, we conducted the focus group review and discussions. A focus group included five experienced pharmacists practicing in either Western medicine or Chinese medicine on a daily basis in Taiwanese hospitals. Any disagreements about classifications and information conflict were resolved by consensus. Specifically, all of the members in the focus group were trained in both Western and Chinese medicines in a School of Pharmacy and/or their master graduate programs.
They all have more than 15 years of experience practicing in TCM pharmacy or clinical pharmacy services in hospitals that provide medical services with both Western and TCM medicines. None of these members were authors.
Data management and analysis
Next, the following review and analysis particularly focused on those single entity CHMs
commonly listed in Chinese Medicine books, including the Taiwan Herbal Pharmacopoeia (THP)
[34], Chinese Medicinal Herbs Preparation [35], and Chinese Materia Medica [36]. Thus, those
natural products or herbs that were not documented in the aforementioned books and/or any combination of Chinese Medicinal Prescriptions, which were listed in some ancient TCM books, were excluded for further analyses. Moreover, those single entity CHMs included for further review were verified by two experienced pharmacists who were members of the focus group.
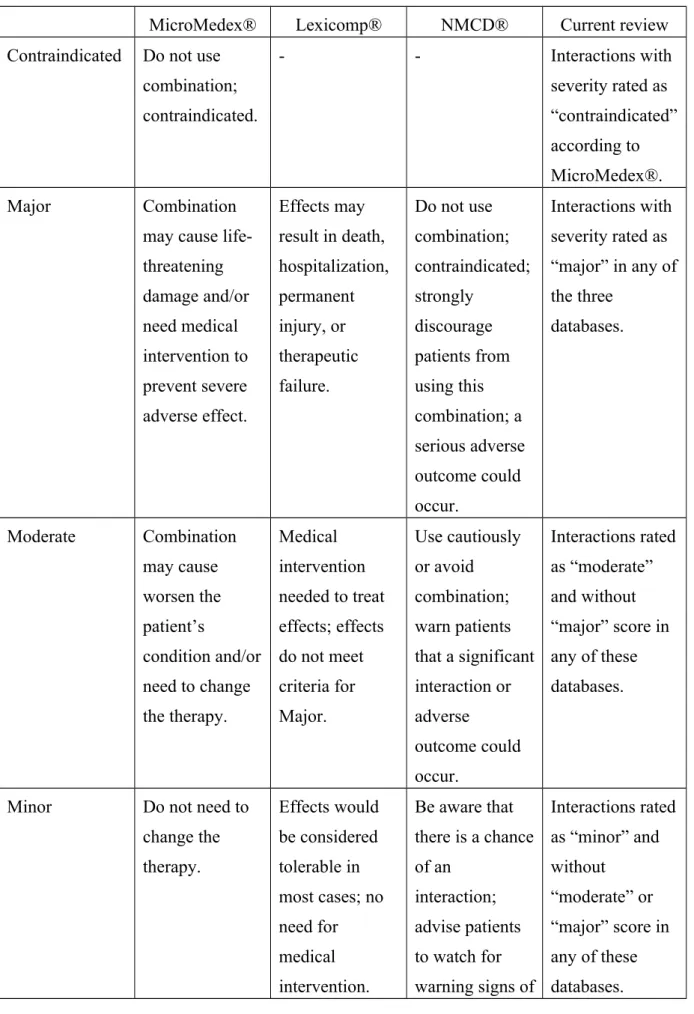
To obtain proof about clinical significance of interactions, we identified and verified the severity ratings and possible mechanisms of each interaction using three well-known interactions databases (MicroMedex® [37], Lexi-Interact in Lexicomp® [38], or “Natural Product/Drug Interaction Checker” in Natural Medicines Comprehensive Database® (NMCD®) [39]). Because of the
various definitions of severity ratings among these three databases, we compiled the rating schemes and categorized the severity of each interaction into six types in this review: “contraindicated,”
“major,” “moderate,” “minor,” “no interaction,” and “no available information for the item.” The comparisons of severity rating definitions in different databases with this review were listed in Table 1. Accordingly, the mechanisms for interactions were also categorized into pharmacokinetics, pharmacodynamics, pharmacokinetics plus pharmacodynamics, and unknown based upon the records from these three databases. All of the related data was compiled and managed using the Excel® spreadsheets. Last, the descriptive analyses were performed to explore the frequency and
proportion of the retrieved evidence associated with the interaction numbers, the corresponding types of retrieved mechanisms and severity ratings of interactions.
Result
Literature search and evidence retrieval
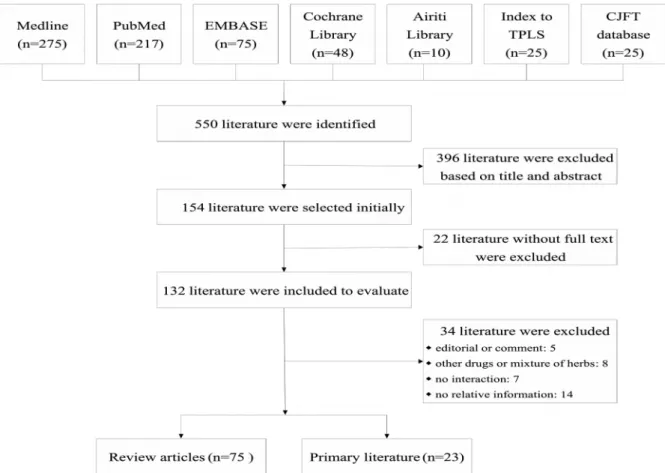
A total of 154 articles were selected from the initial 550 retrieved literature reports based on
their relevance to the purpose of review. After thorough evaluation and analysis, 98 articles with
full text (75 review articles [5,6,40-112], and 23 primary studies, i.e., 4 animal studies [113-116], 4
clinical trials [117-120], and 15 observational studies [20,121-134]) were included for further
evaluation (Figure 1). The summaries of retrieved primary literature are listed in Table 2, while the
summaries of retrieved review articles are listed in Appendix 1. The retrieved data with missing
information concerning the common name, scientific name, plant source, plant parts, and dose for the natural products/herbs or CHM were excluded from Table 2 and Appendix 1, but may be accessed upon specific request to the authors.
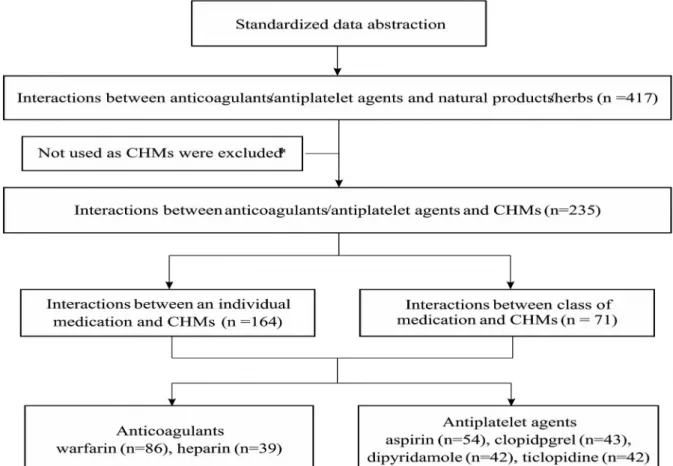
Initially, there were 417 interactions in total obtained from the aforementioned evidence-based resources. Of them, 235 interactions between anticoagulant/antiplatelet agents and specific single CHMs were extracted for further evaluation. After expanding the interactions to individual medications from the different drug classes, a total of 306 interactions between distinct
anticoagulant/antiplatelet agents and single entity CHMs were identified. Warfarin and aspirin accounted for the majority of documented, evidenced-based interactions with single entity CHMs (Figure 2). Overall, 90 distinct single entity CHMs contributed to the 306 documented interactions.
Severity rating and mechanisms upon availability in three databases
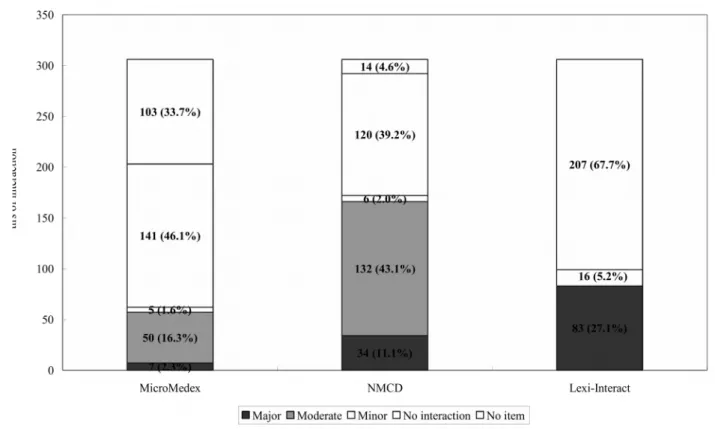
Of the 306 interactions between distinct anticoagulant/antiplatelet agents and single entity CHMs, 203 (66.3%), 292 (95.4%), and 99 (32.3%) drug-CHM interactions were retrieved with some relevant information about interactions from MicroMedex®, NMCD®, and Lexicomp®, respectively. However, only 62 (20.2%), 172 (56.2%), and 83 (27.1%) of these interactions in MicroMedex®, NMCD®, and Lexicomp®, respectively, contained identifiable severity ratings and
interaction mechanisms (Figure 3). Overall, a total of 194 (63.4%) interactions were found to have severity ratings and mechanisms in at least one of these databases. While 50 interactions with information in MicroMedex® (80.6%) were categorized as the moderate interactions, 132
interactions documented in NMCD® (76.7%) were classified as moderate. However, all of the 83 documented interactions in Lexicomp® were found to have major drug-CHM interactions (Figure 3). Consequently, of all 194 interactions being identified with the severity rating in any of the three databases, only 29 (15.0%) were found in all of the three databases, 65 (33.5%) could be identified in two databases, while more than half (100, 51.5%) were documented in only one database.
Of those identifiable mechanisms for interactions, the majority (155, 79.9%) were attributable
to pharmacodynamic-related mechanisms. 8.3% were attributed to pharmacokinetic-related
mechanisms and 11.3% were the results of both pharmacodynamic and pharmacokinetic mechanisms. Most of the pharmacodynamic-related interactions were due to the additive anticoagulant/antiplatelet effect of the single entity CHM. For example, some CHMs, i.e., clove (Eugenia caryophyllata), cat's claw (Uncaria tomentosa), and ginger (Zingiber officinale), were reported to inhibit cyclooxygenase activity, platelet aggregation, and thromboxane synthetase activity, respectively [37,39]. Most of these potential interactions have not been documented in clinical practice. In contrast, a few documented single entity CHMs with pharmacodynamic-related interactions causing decreased anticoagulant effects were reported in the literature. For example, alfalfa (Medicago sativa) and green tea (Camellia sinensis) may antagonize the anticoagulant effects of warfarin due to the presence of vitamin K in these products [37,39]. As for the documented pharmacokinetic-related interactions, the majority were due to the inhibition of
warfarin or clopidogrel metabolism via the Cytochrome P450 (CYP) pathway, including CYP 1A2, 3A4, and 2C9. For example, dandelion (Taraxacum officinale), bitter orange (Citrus aurantium), and milk thistle (Silybum marianum) were reported to inhibit CYP1A2, 3A4, and 2C9, respectively.
Therefore, these herbal products may inhibit warfarin or clopidogrel catabolism, and result in an increase in their anticoagulation/antiplatelet activities [39]. Similarly, ginkgo (Ginkgo biloba), red clover (Trifolium pratense), and Siberian ginseng (Eleutherococcus senticosus) were documented to inhibit all of these three CYP enzymes, thus similar effects on the anticoagulation action of warfarin or clopidogrel may be expected [37,39].
Consequences of drug–CHM Interactions
Of the 90 interactions that involve a single entity CHM, the majority would result in an increased
risk of bleeding when used in combination with anticoagulants or antiplatelet agents (Table 3). For
instance, commonly used CHMs including danshen (Salvia miltiorrhiza), dong quai (Angelica
sinensis), ginger (Zingiber officinale), and licorice (Glycyrrhizae uralensis) were documented to
have major interactions with anticoagulants or antiplatelet drugs. The combinations causing major
interactions may cause life-threatening damage and/or severe adverse events. Thus, we strongly
discourage patients and clinicians from using these combinations [37-39]. Specifically, celery, a functional food and classified as a CHM in the Chinese Materia Medica, is reported to increase bleeding in Philp’s book and a few review articles and was then verified in the Lexicomp® record [26,81,82,85,94]. There were no published primary data that describe the rationale or potential mechanism of this interaction. Therefore, interpretation of the data related to celery should be cautious.
Furthermore, a few reports showed that single entity CHMs may decrease the effectiveness of anticoagulant/antiplatelet agents. Agrimony (Agrimonia eupatoria), myrrh (Commiphora myrrha), and St. John's wort (Hypericum perforatum) were documented to result in a decreased effectiveness of warfarin [21,22,24,25,72,135], while pepper (Piper nigrum) and cannabis (Cannabis sativa) were reported to reduce the antiplatelet efficacy of aspirin [28]. Nevertheless, we also noted the
conflicting consequences of interactions reported in the different evidence resources. Some evidence suggested that alfalfa, Asian ginseng (Panax ginseng), green tea, and Siberian ginseng may reduce the anticoagulant effects of anticoagulant/antiplatelet agents [5,37,73,74], while some published data suggested that these single entity CHMs might increase the risk of bleeding episodes if they were used concurrently with anticoagulants and antiplatelet drugs [38,39,73].
Discussion
In this review, the potential interactions between anticoagulant/antiplatelet drugs and single entity CHMs were analyzed and evaluated based on retrievable and published evidence. We found 90 commonly used single entity CHMs (such as danshen, dong quai, ginger, and licorice) were involved in 306 evidence-based drug interactions with anticoagulant and/or antiplatelet drugs.
Warfarin and aspirin were the two drugs reported to have the largest numbers of documented interactions with the greatest number of single entity CHMs. Most of these interactions were moderate to severe and attributable to pharmacodynamic mechanisms. The majority of these interactions were found to increase the risks of bleeding.
In the United States and Singapore, 10-20% of patients used herbal therapies or CHMs when
using prescribed anticoagulant or antiplatelet medications for cardiovascular diseases or stroke [10,11]. A survey also showed that one in five patients in Malaysia who took anticoagulant or antiplatelet drugs, also concurrently used herbal therapies [20]. While the use of TCM is prevalent among Asian populations [12,13], there is also an increasing trend of using TCM in Western countries. Thus, it is necessary that clinicians be aware of these drug-CHMs interactions in order to effectively manage those patients who are using anticoagulant/antiplatelet drugs with the listed CHMs.
In this review, the majority of documented interactions were rated as “major or severe” in scale. However, the actual consequences of concurrent use might be changed depending on the used medications, dose levels, binomial source, plant parts, and/or route of administration of CHMs, in addition to the patients’ characteristics (such as genetic differences and dietary habits), their use patterns, and combinations of single CHMs and/or Chinese Medicinal Prescriptions (based on TCM theory). As for the safety and effectiveness of using anticoagulants and antiplatelets, health care providers need to be more judicious in counseling their patients regarding the possibility of interactions between these drugs and herbal remedies or CHMs. If concurrent use is unavoidable, then health care providers should closely monitor patients for potential adverse events associated with these possible interactions (e.g., increased international normalized ratio, bleeding).
In fact, the interactions between herbal remedies or CHMs and drugs are often more complex due to the multiple ingredients found in all single entity CHMs [87,94] and their binomial source, plant parts, dose, quality, preparation and/or route of administration [54]. Again, the likelihood of drug-CHM interactions may be different when using combinations of several single entity CHMs or those Chinese Medicinal Prescriptions. In particular, Chinese Medicinal Prescriptions (combined TCM medicines based on the theory of TCM) are sometimes the first line of therapy in Asian countries [136,137]. For instance, Jia Wei Hiaxo Yao San is commonly used for anxiety, irritability, and depression, and is one of the most frequently used Chinese Medicinal Prescriptions in Taiwan.
This well known ancient Chinese herbal formula is generally used for liver Qi stagnation but
contains at least ten distinct entity of CHMs [137], including dong quai, ginger, and licorice, which
are documented to have major interactions with anticoagulant or antiplatelet drugs [37-39].
Therefore, those patients using Jia Wei Hiaxo Yao San might encounter higher bleeding risks due to the occurrence of significant major interactions. In this case, health care professionals could more aggressively take appropriate actions to prevent them from causing serious life threatening adverse events in their patients if they were aware of such potential risks.
In this review, we also found that the major documented mechanisms of interactions between anticoagulant/antiplatelet drugs and CHMs were attributable to their pharmacodynamic interactions, especially due to additive anticoagulant or antiplatelet effects. Many of the commonly used CHMs such as clove, danshen, ginger, and licorice are documented to have intrinsic anticoagulant or antiplatelet properties [85,138]. However, these blood-activating or stasis-resolving CHMs (e.g., danshen, and dong quai) are widely used in TCM for the treatment of cardiovascular or
cerebrovascular diseases. Although some clinical studies have been conducted to investigate the effects of these CHMs on stroke or coronary diseases [139,140], further large scale, rigorous clinical trials are needed to confirm the benefits and risks of using these CHMs. With limited robust evidence and clinical trials supporting the use of CHMs, it is crucial for physicians and pharmacy practitioners to educate their patients on why they need to disclose their concurrent use of
conventional medications and CAM, including CHMs, especially for those patients who are taking anticoagulant or antiplatelet drugs.
Currently, there is no single comprehensive database to identify the facts of drug-CHM interactions, levels of evidence sufficiency, corresponding mechanism, and/or clinical significance.
Instead, we utilized three commonly used natural products or drug interaction-related databases to identify and verify the information needed and retrieved this information regarding their mechanism and severity rating. The results of this review show that more than half of documented drug
interactions retrieved from books, web sites, or primary literature could be identified in NMCD®,
and less of them could be retrieved in MicroMedex® or Lexicomp®. This finding is consistent with
the results of a previous study which was conducted in 2008 to evaluate the commonly used dietary
supplement databases [141]. Of these databases, NMCD® had been recognized as the most
appropriate database used to answer questions (including drug interactions) about dietary
supplements [141]. In addition, we also found that only a few interactions could be identified in all of the tertiary literature (MicroMedex®, Drug interaction facts) and there existed variations in the severity rating of interactions [142]. This finding is also in agreement with Abarca’s evaluation, which concluded that only a few major drug-drug interactions were listed in the four available interaction compendium and with very little agreement among them [142]. Thus, further rigorous qualitative studies that include opinions from a variety of experts on CHMs and drug interactions are needed to provide more consistent and robust information about the levels of clinical
significance and mechanisms and combinations to be avoided to prevent drug-CHM interactions from occurring.
Nevertheless, our review has a number of limitations. First, there exists discordance in the nomenclature of Chinese medicine and herbs, as well as in both of the English and Chinese resources that need to be addressed. In particular, some evidence was retrieved from the English resources showing that an inconsistency of homonyms, synonyms, or translation may exist.
Furthermore, only a small portion of the literature listed the common names of the natural products or herbal remedies, and the majority of them lacked any information on the scientific names and/or binomial source. Therefore, we need to be cautious when interpreting the findings on the natural products, herbs and CHMs per se. Second, the majority of literature reports did not specify which parts of natural products/herbs and CHMs (e.g., roots, leaves, seeds, or flowers) were used, on what doses, or which routes of administration were taken. In fact, the active ingredients and doses of CHMs may vary by situation and person based on TCM theory. Usually, the kinds and parts of CHMs and their preparation procedures are chosen based upon the stages of patients’ disease status or condition. Lastly, only those interactions with evidence-based data retrieved from MicroMedex®, NMCD®, or Lexicomp®, were further reviewed for the corresponding mechanisms and rating of
severity in this study. Thus, the interpretations might be subjective to information obtained from the
retrieved database(s), and validated by the reviewers and members in the focus group. However, we
have tried our best to screen and highlight the interactions using the best available evidence and
consistent approach, although not all reports or studies were published or relevant documented interactions analyzed.
Conclusions
Conventional anticoagulants and antiplatelet drugs were documented to have harmful interactions with some commonly used single entity CHMs. Concurrent use of some herbal remedies may increase or reduce the pharmacologic effects of anticoagulant and antiplatelet drugs with moderate or severe consequences. For those patients who are taking conventional anti-clotting medications with CHMs for cardiovascular or cerebrovascular diseases, the potential risks of increased bleeding due to drug-CHM interactions should not be ignored. This review provides some guidance to health care professionals as to how to recognize the potential risks of interactions between
anticoagulants/antiplatelets and single entity CHMs. Moreover, it is critical for all health care professionals to be able to initiate and effectively communicate and discuss the proper use of CHM- related products with conventional medications (i.e., anticoagulant or antiplatelet agents for
cardiovascular or cerebrovascular diseases) because many patients may not voluntarily disclose their CHM-related use to their physicians.
Acknowledgements
The authors would like to express their gratitude to Chiu-Lin Tsai for his insights and comments initially on the identification of CHMs, Po-Ming Huang and Shan-Chieh Wu for their assistance with the review and manuscript, Jun-Fon Wang and Po-Ming Huang for their help on data management, the other reviewers for their insights of drug-CHM interaction information, as well as Dan Lu, Matthias C. Lu and Jenna Beall for their comments on the manuscript.
Author Contributions
HHT and HWL participated in designing the review. YHL and YLC searched the databases and
retrieved the articles. YHL and YLC extracted the data, while HHT and HWL validated it. Finally,
HHT managed the data, while HHT and HWL wrote the manuscript. GBM reviewed and revised
the manuscript. All authors read and approved the final manuscript.
References
1. World Health Organization (WHO) (2008). The 10 leading causes of death by broad income group. http://www.who.int/mediacentre/factsheets/fs310/en/. Accessed May 25, 2012.
2. Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, et al. (2011) Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association.
Stroke 42: 227-276.
3. Holbrook AM, Pereira JA, Labiris R, McDonald H, Douketis JD, et al. (2005) Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 165: 1095-1106.
4. Dunn SP, Macaulay TE (2011) Drug-Drug Interactions Associated with Antiplatelet Therapy. Cardiovasc Hematol Agents Med Chem 9: 231.
5. Ulbricht C, Chao W, Costa D, Rusie-Seamon E, Weissner W, et al. (2008) Clinical evidence of herb-drug interactions: a systematic review by the natural standard research
collaboration. Curr Drug Metab 9: 1063-1120.
6. Izzo AA, Di Carlo G, Borrelli F, Ernst E (2005) Cardiovascular pharmacotherapy and herbal medicines: the risk of drug interaction. Int J Cardiol 98: 1-14.
7. Su D, Li L (2011) Trends in the use of complementary and alternative medicine in the United States: 2002-2007. J Health Care Poor Underserved 22: 296-310.
8. Rossler W, Lauber C, Angst J, Haker H, Gamma A, et al. (2007) The use of complementary and alternative medicine in the general population: Results from a longitudinal community study. Psychol Med 37: 73-84.
9. Ernst E (2000) Prevalence of use of complementary/alternative medicine: a systematic review. Bull World Health Organ 78: 252-257.
10. Yeh GY, Davis RB, Phillips RS (2006) Use of complementary therapies in patients with cardiovascular disease. Am J Cardiol 98: 673-680.
11. Lee GBW, Charn TC, Chew ZH, Ng TP (2004) Complementary and alternative medicine
use in patients with chronic diseases in primary care is associated with perceived quality of
care and cultural beliefs. Fam Pract 21: 654-660.
12. Chang LC, Huang N, Chou YJ, Lee CH, Kao FY, et al. (2008) Utilization patterns of Chinese medicine and Western medicine under the National Health Insurance Program in Taiwan, a population-based study from 1997 to 2003. BMC Health Serv Res 8: 170.
13. Lim MK, Sadarangani P, Chan HL, Heng JY (2005) Complementary and alternative medicine use in multiracial Singapore. Complement Ther Med 13: 16-24.
14. Lai D, Chappell N (2007) Use of Traditional Chinese Medicine by older Chinese immigrants in Canada. Fam Pract 24: 56-64.
15. Chen FN, Lee CPD, Hsieh CL, Lin CC, Chiang HM, et al. (2008) Survey on Adult Behavior and Medication of Chinese and Western Medicine-Based on the Outpatients of a Medical Center in Mid-Taiwan. J Int Chin West Med 10: 25-34.
16. Rochelle TL, Marks DF (2010) Medical pluralism of the Chinese in London: an exploratory study. Br J Health Psychol 15: 715-728.
17. Tsai HH, Lin HW, Chien CR, Li TC (2012) Concurrent use of antiplatelets, anticoagulants, or digoxin with Chinese medications: a population-based cohort study. Eur J Clin
Pharmacol DOI 10.1007/s00228-012-1359-6.
18. Alherbish A, Charrois TL, Ackman ML, Tsuyuki RT, Ezekowitz JA (2011) The prevalence of natural health product use in patients with acute cardiovascular disease. PLoS One 6:
e19623.
19. Suchard JR, Suchard MA, Steinfeldt JL (2004) Physician knowledge of herbal toxicities and adverse herb-drug interactions. Eur J Emerg Med 11: 193-197.
20. Saw JT, Bahari MB, Ang HH, Lim YH (2006) Potential drug-herb interaction with antiplatelet/anticoagulant drugs. Complement Ther Clin Pract 12: 236-241.
21. Mahady GB (2001) Botanical dietary supplements: Quality, safety and efficacy. Lisse, The Netherlands: Swets & Zeitlinger Publishers.
22. Cassileth BC, Yeung KS (2003) Herb-drug interactions in oncology. Hamilton: London BC Decker Inc.
23. Ulbricht CE (2005) Natural standard herbs & supplement reference: Evidence-based clinical
review. St. Louis, Mo.: Mosby/Elsevier.
24. Tatro DS (2011) Drug interaction facts. Saint Louis: Wolters Kluwer Health/Facts &
Comparisons.
25. Stargrove MB, Treasure J, McKee DL (2008) Herb, nutrient, and drug interactions: Clinical implications and therapeutic strategies. St. Louis: Mosby Elsevier.
26. Philp RB (2004) Herbal-drug interactions and adverse effects: An evidence-based quick reference guide. USA: McGraw-Hill Professional.
27. Jennes F, Flaws B (2007) Herb toxicities & drug interactions: A formula approach. Boulder:
Blue Poppy Press.
28. Williamson E, Driver S, Baxter K (2009) Stockley's herbal medicines interactions. London:
Pharmaceutical Press.
29. Committee on Chinese Medicine and Pharmacy, Department of Health, Executive Yuan.
Abstract of yearbook. http://www.ccmp.gov.tw/en/research/result.asp?relno=51&level=C.
Accessed March 10, 2012.
30. Department of Health, Executive Yuan, R.O.C. (TAIWAN). Government research bulletin.
http://grbsearch.stpi.narl.org.tw/GRB/. Accessed March 15, 2012.
31. Office of Dietary Supplements. Dietary Supplement Fact Sheets.
http://ods.od.nih.gov/factsheets/list-all/. Accessed March 19, 2012.
32. National Center for Complementary and Alternative Medicine. Herbs at a Glance.
http://nccam.nih.gov/health/herbsataglance.htm. Accessed March 20, 2012.
33. National Center for Complementary and Alternative Medicine (2010) Traditional Chinese Medicine: An Introduction. http://nccam.nih.gov/health/whatiscam/chinesemed.htm.
Accessed March 1, 2012.
34. Committee on Chinese Medicine and Pharmacy (2004) Taiwan Herbal Pharmacopoeia.
Taipei: Department of Health, Executive Yuan.
35. Chang HC, Tsai KU (2003) Chinese Medicinal Herbs Preparation. Taichung: China Medical University.
36. State Administration of Traditional Chinese Medicine (1999) Chinese Materia Medica.
Shanghai: Shanghai scientific and Technical Publishers.
37. MICROMEDEX® 1.0 (Healthcare Series). Interaction Database.
http://www.thomsonhc.com/hcs/librarian/ND_T/HCS/ND_PR/Main/CS/A304ED/DUPLIC ATIONSHIELDSYNC/95C0EB/ND_PG/PRIH/ND_B/HCS/ND_P/Main/PFActionId/hcs.In teractions.FindDrugInteractions. Accessed April 15, 2012.
38. Lexicomp. Lexi-Interact Online (2012).
http://www.uptodate.com/crlsql/interact/frameset.jsp. Accessed April 18, 2012.
39. Natural Medicines Comprehensive Database. Natural Product/Drug Interaction Checker.
http://naturaldatabase.therapeuticresearch.com/nd/Search.aspx?
s=ND&cs=&pt=7&rli=1&sh=. Accessed April 20, 2012.
40. Awang DV, Fugh-Berman A (2002) Herbal interactions with cardiovascular drugs. J Cardiovasc Nurs 16: 64-70.
41. Bone KM (2008) Potential interaction of Ginkgo biloba leaf with antiplatelet or anticoagulant drugs: what is the evidence? Mol Nutr Food Res 52: 764-771.
42. Borrelli F, Izzo AA (2009) Herb-drug interactions with St John's wort (Hypericum perforatum): an update on clinical observations. AAPS J 11: 710-727.
43. Brazier NC, Levine MA (2003) Drug-herb interaction among commonly used conventional medicines: a compendium for health care professionals. Am J Ther 10: 163-169.
44. Bressler R (2005) Herb-drug interactions. Interactions between saw palmetto and prescription medications. Geriatrics 60: 32.
45. Bressler R (2005) Herb-drug interactions: interactions between ginseng and prescription medications. Geriatrics 60: 16-17.
46. Bressler R (2005) Herb-drug interactions. St. John's wort and prescription medications.
Geriatrics 60: 21-23.
47. Bressler R (2005) Herb-drug interactions: interactions between Ginkgo biloba and prescription medications. Geriatrics 60: 30-33
48. Butterweck V, Derendorf H (2008) Potential of pharmacokinetic profiling for detecting
herbal interactions with drugs. Clin Pharmacokinet 47: 383-397.
49. Chan E, Tan M, Xin J, Sudarsanam S, Johnson DE (2010) Interactions between traditional Chinese medicines and Western therapeutics. Curr Opin Drug Discov Devel 13: 50-65.
50. Chavez ML, Jordan MA, Chavez PI (2006) Evidence-based drug-herbal interactions. Life Sci 78: 2146-2157.
51. Choi YH, Chin YW, Kim YG (2011) Herb-drug interactions: focus on metabolic enzymes and transporters. Arch Pharm Res 34: 1843-1863.
52. Cohen PA, Ernst E (2010) Safety of herbal supplements: a guide for cardiologists.
Cardiovasc Ther 28: 246-253.
53. Colalto C (2010) Herbal interactions on absorption of drugs: Mechanisms of action and clinical risk assessment. Pharmacol Res 62: 207-227.
54. Coxeter PD, McLachlan AJ, Duke CC, Roufogalis BD (2004) Herb-drug interactions: an evidence based approach. Curr Med Chem 11: 1513-1525.
55. De Smet PAGM, Floor-Schreudering A, Bouvy ML, Wensing M (2008) Clinical risk management of interactions between natural products and drugs. Curr Drug Metab 9: 1055- 1062.
56. Di Carlo G, Borrelli F, Ernst E, Izzo AA (2001) St John's wort: Prozac from the plant kingdom. Trends Pharmacol Sci 22: 292-297.
57. Di YM, Li CG, Xue CC, Zhou S-F (2008) Clinical drugs that interact with St. John's wort and implication in drug development. Curr Pharm Des 14: 1723-1742.
58. Ernst E (2000) Herb-drug interactions: potentially important but woefully under-researched.
Eur J Clin Pharmacol 56: 523-524.
59. Ernst E (2004) Risks of herbal medicinal products. Pharmacoepidemiol Drug Saf 13: 767- 771.
60. Fugh-Berman A (2000) Herb-drug interactions. Lancet 355: 134-138.
61. Fugh-Berman A, Ernst E (2001) Herb-drug interactions: review and assessment of report reliability. Br J Clin Pharmacol 52: 587-595.
62. Gardiner P, Phillips R, Shaughnessy AF (2008) Herbal and dietary supplement--drug
interactions in patients with chronic illnesses. Am Fam Physician 77: 73-78.
63. Greenblatt DJ, von Moltke LL (2005) Interaction of warfarin with drugs, natural substances, and foods. J Clin Pharmacol 45: 127-132.
64. Greeson JM, Sanford B, Monti DA (2001) St. John's wort (Hypericum perforatum): a review of the current pharmacological, toxicological, and clinical literature.
Psychopharmacology (Berl) 153: 402-414.
65. Haller CA (2006) Clinical approach to adverse events and interactions related to herbal and dietary supplements. Clin Toxicol (Phila) 44: 605-610.
66. Holcomb SS (2009) Common herb-drug interactions: what you should know. Nurse Pract 34: 21-29.
67. Hu Z, Yang X, Ho PCL, Chan SY, Heng PWS, et al. (2005) Herb-drug interactions: a literature review. Drugs 65: 1239-1282.
68. Huang S-M, Lesko LJ (2004) Drug-drug, drug-dietary supplement, and drug-citrus fruit and other food interactions: what have we learned? J Clin Pharmacol 44: 559-569.
69. Izzo AA (2005) Herb-drug interactions: an overview of the clinical evidence. Fundam Clin Pharmacol 19: 1-16.
70. Izzo AA, Ernst E (2001) Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs 61: 2163-2175.
71. Izzo AA, Ernst E (2009) Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs 69: 1777-1798.
72. Javed F, Golagani A, Sharp H (2008) Potential effects of herbal medicines and nutritional supplements on coagulation in ENT practice. J Laryngol Otol 122: 116-119.
73. Ko RJ (2004) A U.S. perspective on the adverse reactions from traditional Chinese medicines. J Chin Med Assoc 67: 109-116.
74. Kuhn MA (2002) Herbal remedies: drug-herb interactions. Crit Care Nurse 22: 22-28, 30, 32.
75. Madabushi R, Frank B, Drewelow B, Derendorf H, Butterweck V (2006) Hyperforin in St.
John's wort drug interactions. Eur J Clin Pharmacol 62: 225-233.
76. Markowitz JS, DeVane CL (2001) The emerging recognition of herb-drug interactions with
a focus on St. John's wort (Hypericum perforatum). Psychopharmacol Bull 35: 53-64.
77. McFadden R, Peterson N (2011) Interactions between drugs and four common medicinal herbs. Nurs Stand 25: 65-68.
78. Mills E, Wu P, Johnston BC, Gallicano K, Clarke M, et al. (2005) Natural health product- drug interactions: a systematic review of clinical trials. Ther Drug Monit 27: 549-557.
79. Norred CL, Brinker F (2001) Potential coagulation effects of preoperative complementary and alternative medicines. Altern Ther Health Med 7: 58-67.
80. Nutescu E, Chuatrisorn I, Hellenbart E (2011) Drug and dietary interactions of warfarin and novel oral anticoagulants: An update. J Thromb Thrombolysis 31: 326-343.
81. Nutescu EA, Shapiro NL, Ibrahim S, West P (2006) Warfarin and its interactions with foods, herbs and other dietary supplements. Expert Opin Drug Saf 5: 433-451.
82. Ohnishi N, Yokoyama T (2004) Interactions between medicines and functional foods or dietary supplements. Keio J Med 53: 137-150.
83. Pal D, Mitra AK (2006) MDR- and CYP3A4-mediated drug-herbal interactions. Life Sci 78: 2131-2145.
84. Poppenga RH (2002) Herbal medicine: potential for intoxication and interactions with conventional drugs. Clin Tech Small Anim Pract 17: 6-18.
85. Samuels N (2005) Herbal remedies and anticoagulant therapy. Thromb Haemost 93: 3-7.
86. Singh YN (2005) Potential for interaction of kava and St. John's wort with drugs. J Ethnopharmacol 100: 108-113.
87. Skalli S, Zaid A, Soulaymani R (2007) Drug interactions with herbal medicines. Ther Drug Monit 29: 679-686.
88. Tachjian A, Maria V, Jahangir A (2010) Use of herbal products and potential interactions in patients with cardiovascular diseases. J Am Coll Cardiol 55: 515-525.
89. Tirona RG, Bailey DG (2006) Herbal product-drug interactions mediated by induction. Br J Clin Pharmacol 61: 677-681.
90. Tomlinson B, Hu M, Lee VWY (2008) In vivo assessment of herb-drug interactions:
possible utility of a pharmacogenetic approach? Mol Nutr Food Res 52: 799-809.
91. Ueng YF, Chen RM (2004) The role of cytochrome P450 in herb-drug interactions. Curr Pharmacogenomics Person Med 2: 209-218.
92. Venkataramanan R, Komoroski B, Strom S (2006) In vitro and in vivo assessment of herb drug interactions. Life Sci 78: 2105-2115.
93. Whitten DL, Myers SP, Hawrelak JA, Wohlmuth H (2006) The effect of St John's wort extracts on CYP3A: a systematic review of prospective clinical trials. Br J Clin Pharmacol 62: 512-526.
94. Williamson EM (2003) Drug interactions between herbal and prescription medicines. Drug Saf 26: 1075-1092.
95. Williamson EM (2005) Interactions between herbal and conventional medicines. Expert Opin Drug Saf 4: 355-378.
96. Wittkowsky AK (2008) Dietary supplements, herbs and oral anticoagulants: the nature of the evidence. J Thromb Thrombolysis 25: 72-77.
97. Woodward KN (2005) The potential impact of the use of homeopathic and herbal remedies on monitoring the safety of prescription products. Hum Exp Toxicol 24: 219-233.
98. Yang XX, Hu ZP, Duan W, Zhu YZ, Zhou SF (2006) Drug-herb interactions: Eliminating toxicity with hard drug design. Curr Pharm Des 12: 4649-4664.
99. Zhou L, Zuo Z, Chow MS (2005) Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 45: 1345-1359.
100. Zhou S, Chan E, Pan S-Q, Huang M, Lee EJD (2004) Pharmacokinetic interactions of drugs with St John's wort. J Psychopharmacol 18: 262-276.
101. Zhou S, Gao Y, Jiang W, Huang M, Xu A, et al. (2003) Interactions of herbs with cytochrome P450. Drug Metab Rev 35: 35-98.
102. Zhou S-F, Zhou Z-W, Li C-G, Chen X, Yu X, et al. (2007) Identification of drugs that interact with herbs in drug development. Drug Discov Today 12: 664-673.
103. Mao XQ, Xie HT, Zhou HH (2007) MDR-and CYP3A4-mediated drug-herbal Interactions.
Chinese Journal of Clinical Pharmacology and Therapeutics 12: 728-734.
104. Wang TY (2011) Outcomes Influenced by Drug Interactions. The Journal of Long Term
Care 15: 24-32.
105. Wang YY (2003) Interactions between warfarin and other anticoagulant agents or herbal medicine. The Journal of Taiwan Pharmacy 19: 5-10.
106. Li JY, Shih HJ, Tsai ML (2008) Interactions between warfarin and chinese herbal medicine.
The Journal of Taiwan Pharmacy 24: 135-142.
107. Lin JJ (2003) Interactions between Herbal and Conventional Therapies: Focus on Ginseng and Ginkgo Biloba. Show Chwan Medical Journal 4: 1-4.
108. Chen FP, Zhong MX, Huang XZ (2011) The whole aspects of interaction between
traditional Chinese medicine and western medicine. Family Medicine & Primary Medical Care 26: 168-176.
109. Chen JF, Hu YJ, Miao CY (2007) Advancement of pharmacokinetic studies on Chinese herbal medicine-synthetic drug interactions. Clin J Clin Pharmacol Ther 12: 1348-1353.
110. Peng B, Li JR, He R (2010) The research status of herb-drug interactions. World Science and Technology-Modernization of Traditional Chinese Medicine. doi: 10.3969/j.issn.1674- 3849.2010.02.024.
111. Tsai CH, Lu CY (2009) Analysis on the interaction of warfarinand ginseng. Taipei Journal of Chinese Medicine 15: 98-109.
112. Lai YC, Chen YL, Lai JS (2007) Toxicity of Chinese Herbal Medicine and drug-herb Interactions. The Chung Shan Medical Journal 18: 343-357.
113. Kuo YH, Lin YL, Don MJ, Chen RM, Ueng YF (2006) Induction of cytochrome P450- dependent monooxygenase by extracts of the medicinal herb Salvia miltiorrhiza. J Pharm Pharmacol 58: 521-527.
114. Li Y, Wang N (2010) Antithrombotic effects of Danggui, Honghua and potential drug interaction with clopidogrel. J Ethnopharmacol 128: 623-628.
115. Makino T, Wakushima H, Okamoto T, Okukubo Y, Deguchi Y, et al. (2002)
Pharmacokinetic interactions between warfarin and kangen-karyu, a Chinese traditional herbal medicine, and their synergistic action. J Ethnopharmacol 82: 35-40.
116. Wu WWP, Yeung JHK (2010) Inhibition of warfarin hydroxylation by major tanshinones of
Danshen (Salvia miltiorrhiza) in the rat in vitro and in vivo. Phytomedicine 17: 219-226.
117. Abdul MI, Jiang X, Williams KM, Day RO, Roufogalis BD, et al. (2010) Pharmacokinetic and pharmacodynamic interactions of echinacea and policosanol with warfarin in healthy subjects. Br J Clin Pharmacol 69: 508-515.
118. Jiang X, Blair EYL, McLachlan AJ (2006) Investigation of the effects of herbal medicines on warfarin response in healthy subjects: a population pharmacokinetic-pharmacodynamic modeling approach. J Clin Pharmacol 46: 1370-1378.
119. Jiang X, Williams KM, Liauw WS, Ammit AJ, Roufogalis BD, et al. (2004) Effect of St John's wort and ginseng on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol 57: 592-599.
120. Yuan CS, Wei G, Dey L, Karrison T, Nahlik L, et al. (2004) Brief communication:
American ginseng reduces warfarin's effect in healthy patients: a randomized, controlled Trial. Ann Intern Med 141: 23-27.
121. Blalock SJ, Gregory PJ, Patel RA, Norton LL, Callahan LF, et al. (2009) Factors associated with potential medication-herb/natural product interactions in a rural community. Altern Ther Health Med 15: 26-34.
122. Bush TM, Rayburn KS, Holloway SW, Sanchez-Yamamoto DS, Allen BL, et al. (2007) Adverse interactions between herbal and dietary substances and prescription medications: a clinical survey. Altern Ther Health Med 13: 30-35.
123. Canter PH, Ernst E (2004) Herbal supplement use by persons aged over 50 years in Britain:
frequently used herbs, concomitant use of herbs, nutritional supplements and prescription drugs, rate of informing doctors and potential for negative interactions. Drugs Aging 21:
597-605.
124. Chan ALF, Leung HWC, Wu J-W, Chien T-W (2011) Risk of hemorrhage associated with co-prescriptions for Ginkgo biloba and antiplatelet or anticoagulant drugs. J Altern
Complement Med 17: 513-517.
125. Chan H-T, So L-T, Li S-W, Siu C-W, Lau C-P, et al. (2011) Effect of herbal consumption
on time in therapeutic range of warfarin therapy in patients with atrial fibrillation. J Cardiovasc Pharmacol 58: 87-90.
126. Charrois TL, Hill RL, Vu D, Foster BC, Boon HS, et al. (2007) Community identification of natural health product-drug interactions. Ann Pharmacother 41: 1124-1129.
127. Clauson KA, Santamarina ML, Rutledge JC (2008) Clinically relevant safety issues associated with St. John's wort product labels. BMC Complement Altern Med 8: 42.
128. Dergal JM, Gold JL, Laxer DA, Lee MSW, Binns MA, et al. (2002) Potential interactions between herbal medicines and conventional drug therapies used by older adults attending a memory clinic. Drugs Aging 19: 879-886.
129. Goldman RD, Rogovik AL, Lai D, Vohra S (2008) Potential interactions of drug-natural health products and natural health products-natural health products among children. J Pediatr 152: 521-526.
130. Rogers EA, Gough JE, Brewer KL (2001) Are emergency department patients at risk for herb-drug interactions? Acad Emerg Med 8: 932-934.
131. Segal R, Pilote L (2006) Warfarin interaction with Matricaria chamomilla. CMAJ 174:
1281-1282.
132. Shalansky S, Lynd L, Richardson K, Ingaszewski A, Kerr C (2007) Risk of warfarin-related bleeding events and supratherapeutic international normalized ratios associated with
complementary and alternative medicine: a longitudinal analysis. Pharmacotherapy 27:
1237-1247.
133. Su Q, Li Y (2010) Interaction between warfarin and the herbal product shengmai-yin: A case report of intracerebral hematoma. Yonsei Med J 51: 793-796.
134. Lai JN (2010) Causal inference of bleeding complications in patients with warfarin treatment in Taiwan - a eleven year follow up study. Yearbook of Chinese Medicine and Pharmacy 28: 291-388.
135. Al Faraj S (2005) Antagonism of the anticoagulant effect of warfarin caused by the use of
Commiphora molmol as a herbal medication: a case report. Ann Trop Med Parasitol 99:
219-220.
136. Yamashita H, Tsukayama H, Sugishita C (2002) Popularity of complementary and alternative medicine in Japan: a telephone survey. Complement Ther Med 10: 84-93.
137. Hsieh SC, Lai JN, Lee CF, Hu FC, Tseng WL, et al. (2008) The prescribing of Chinese herbal products in Taiwan: a cross-sectional analysis of the national health insurance reimbursement database. Pharmacoepidemiol Drug Saf 17: 609-619.
138. Mousa SA (2010) Antithrombotic effects of naturally derived products on coagulation and platelet function. Methods Mol Biol 663: 229-240.
139. Xu G, Zhao W, Zhou Z, Zhang R, Zhu W, et al. (2009) Danshen extracts decrease blood C reactive protein and prevent ischemic stroke recurrence: A controlled pilot study. Phytother Res 23: 1721-1725.
140. Zhu YF, Luo HM, Deng ZL, Fu DY, Yao W, et al. (2012) Effects of the Chinese patent medicine, Honghua injection, on platelet glycoprotein Tib/Ufa receptors in patients with acute coronary syndrome: A randomized controlled trial. Zhong Xi Yi Jie He Xue Bao 10:
318-323.
141. Clauson KA, Peak AS, Marsh WA, DiScala S, Bellinger RR (2008) Clinical decision support tools: focus on dietary supplement databases. Altern Ther Health Med 14: 36-40.
142. Abarca J, Malone DC, Armstrong EP, Grizzle AJ, Hansten PD, et al. (2004) Concordance of
severity ratings provided in four drug interaction compendia. J Am Pharm Assoc 44: 136-
141.
Figure 1. Flow chart of primary literature search.
TPLS: Taiwan Periodical Literature System; CJFT: China Journals Full-text.
Figure 2. Retrieved findings of interactions between anticoagulant/antiplatelet agents and single Chinese herbal medicines.
* Natural products or herbs which were not identified in the Taiwan Herbal Pharmacopoeia, Chinese Medicinal Herbs Preparation, or Chinese Materia Medica were excluded.
CHMs: Chinese herbal medicines.
Figure 3. Documented severity ratings of interactions between distinct
anticoagulant/antiplatelet agents and single CHM using three interaction databases.
The total interactions between distinct anticoagulant/antiplatelet agents and single CHMs were 306.
The classification of “No interaction” meant that there is no interaction between the medication and the single CHMs, while “No item” meant that there was no available information about the single CHMs in the database.
CHMs: Chinese herbal medicines.
Table 1. The comparisons of severity rating definitions in different databases with current review
MicroMedex® Lexicomp® NMCD® Current review
Contraindicated Do not use combination;
contraindicated.
- - Interactions with
severity rated as
“contraindicated”
according to MicroMedex®.
Major Combination
may cause life- threatening damage and/or need medical intervention to prevent severe adverse effect.
Effects may result in death, hospitalization, permanent injury, or therapeutic failure.
Do not use combination;
contraindicated;
strongly discourage patients from using this combination; a serious adverse outcome could occur.
Interactions with severity rated as
“major” in any of the three
databases.
Moderate Combination may cause worsen the patient’s
condition and/or need to change the therapy.
Medical intervention needed to treat effects; effects do not meet criteria for Major.
Use cautiously or avoid combination;
warn patients that a significant interaction or adverse
outcome could occur.
Interactions rated as “moderate”
and without
“major” score in any of these databases.
Minor Do not need to
change the therapy.
Effects would be considered tolerable in most cases; no need for medical intervention.
Be aware that there is a chance of an
interaction;
advise patients to watch for warning signs of
Interactions rated as “minor” and without
“moderate” or
“major” score in
any of these
databases.
a potential interaction.
No interaction - - - There was no
documented interaction between the medication and the single entity CHM
No available documented information for the item
- - - There was no
available information about the single entity CHM in these databases.
NMCD: Natural Medicines Comprehensive Database.
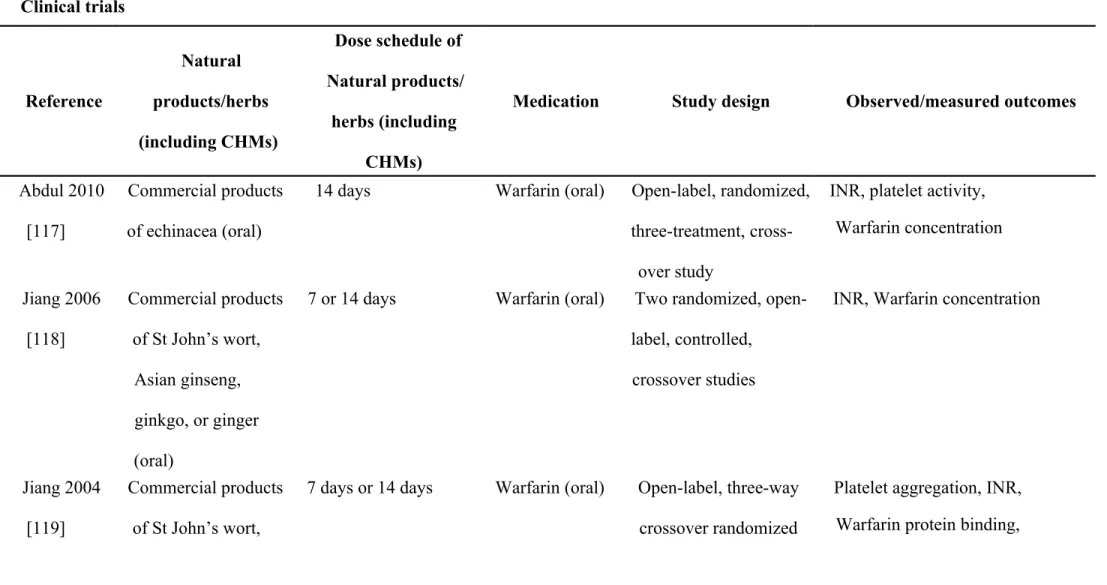
Table 2. Summary of the included primary literature and the retrieved relevant information about natural products or herbs (including CHMs)
Clinical trials
Reference
Natural products/herbs (including CHMs)
Dose schedule of Natural products/
herbs (including CHMs)
Medication Study design Observed/measured outcomes
Abdul 2010 [117]
Commercial products of echinacea (oral)
14 days Warfarin (oral) Open-label, randomized, three-treatment, cross-
over study
INR, platelet activity, Warfarin concentration
Jiang 2006 [118]
Commercial products of St John’s wort,
Asian ginseng, ginkgo, or ginger (oral)
7 or 14 days Warfarin (oral) Two randomized, open- label, controlled, crossover studies
INR, Warfarin concentration
Jiang 2004 [119]
Commercial products of St John’s wort,
7 days or 14 days Warfarin (oral) Open-label, three-way crossover randomized
Platelet aggregation, INR,
Warfarin protein binding,
ginseng (oral) study Warfarin concentration Yuan 2004
[120]
Root of American ginseng (oral)
3 weeks Warfarin (oral) Randomized, double- blind, placebo-
controlled trial.
INR, Warfarin concentration
Animal studies
Reference
Natural products/herbs (including CHMs)
Preparation of Natural products/herbs (including CHMs)
Medication Animal model Observed/measured outcomes
Kuo 2006 [113]
Dried roots of danshen
Ethyl acetate extracts 7-
Hydroxywarfarin
Mice The effect on CYP
Li 2010 [114]
Dried fruits of
danggui and honghua
Aqueous extracts Clopidogrel Rat Bleeding time and hematology
parameters Makino
2002 [115]
Commercial product of Kangen-Karyu
Aqueous extracts Warfarin Rat Plasma concentration, prothrombin
time, bleeding time Wu 2010
[116]
Commercial product of danshen
Ethyl acetate extracts Warfarin Rat Pharmacokinetics parameters
Observational studies
Reference
Natural products/herbs (including CHMs)
Medication Study design
Population (number of
participants) Observed/measured outcomes
Blalock 2009 [121]
Herbs and natural products
Conventional medication
Population-based epidemiological
study
Data from Johnston County Osteoarthritis Project (n=2523)
Prevalence of exposure to potential interactions
Bush 2007 [122]
Herbal and dietary substances
General Survey Outpatients from clinics
(n=804)
The rate of potential and observed adverse herb-drug interactions Canter 2004
[123]
Herbal and nutritional supplements
General Self-completed
survey
Elderly outpatients (n=271)
Associated risks of negative interactions with prescription drugs Chan 2011
[124]
Ginkgo biloba Antiplatelet or anticoagulant agents
Retrospective population based
study
Combination users of ginkgo and antiplatelet or anticoagulant agents (n=3579 or 15397)
The prevalence and frequency of co- prescription, the relative risk of
hemorrhage associated with combination
Chan 2011 [125]
Herbs Warfarin Survey Patients with
nonvalvular atrial fibrillation (n=250)
Effect of concomitant herbal intake on anticoagulation control
Charrois Natural health General Survey Community pharmacists Community pharmacists' knowledge
2007 [126] products (n=132) of interactions Clauson
2008 [127]
St. John's wort General Evaluation of label - Identification of labeling of 8
clinically relevant safety issues Dergal 2002
[128]
Herbal medicines Prescription and over- the-counter drugs
Survey Older adults (≥ 65 years) attending a memory clinic (n=195)
The frequency of potential interactions between herbal medicines and conventional drug therapies.
Goldman 2008 [129]
Natural health products
General Survey Parents and patients 0 to
18 years at a large pediatric emergency department (n=1804)
The frequency of concurrent use and potential interactions
Rogers 2001 [130]
Herbs General Survey Emergency department
patients with heart disease, diabetes, psychiatric disorders,
and/or hypertension (n=944)
Prevalence and occurrence of sever herb-drug interactions.
Saw 2006 Herbs Antiplatelet/anticoagula Cross-sectional Patients from Assessing the use of herbal
[20] nt drugs survey cardiology, neurology, infectious and
nephrology wards (n=250)
medicines in medical patients that may interact with antiplatelet or anticoagulant therapy
Segal 2006 [131]
Matricaria chamomilla
Warfarin Case report 70-year-old
woman
-
Shalansky 2007 [132]
Complementary and alternative medicine
Warfarin Prospective,
longitudinal study
Adults prescribed warfarin for an expected
duration of at least 4 months (n=171)
Risk factors for bleeding events and supratherapeutic INR
Su 2010 [133]
Shengmai-yin Warfarin Case report A 71-year-old man -
Lai 2010 [134]
Chinese herbal medicines
Warfarin Case-crossover
study
Warfarin users from database (n=4211)
Causal inference of potential interactions
CYP: cytochrome P450; VKORC1: vitamin K epoxide reductase complex subunit 1; INR: international normalized ratio
Table 3. Documented interactions between anticoagulant/antiplatelet drugs and Chinese herbal medicine,
awhich might increase the risks of bleeding.
Severity rating
bCHM documented to increase bleeding risks of the six anticoagulant/antiplatelet drugs
CHM documented to increase bleeding risks of at least one of the six anticoagulant/antiplatelet drugs Major Celery, Chamomile, Danshen, Dong
quai, Evening primrose, Fenugreek, Garlic, Ginger, Ginkgo, Horse chestnut, Licorice, Red clover, Reishi,
cTurmeric, Willow
Cat's claw
Moderate Andrographis, Bogbean, Cayenne, Clove, Flaxseed, Kudzu, Onion, Saw palmetto
Aloe vera, Asafetida, Bitter orange, Blackcurrant seed, Burdock, Cassia, Cinchona, Coltsfoot, Da huang, Eucalyptus, Lycium, Milk thistle, Nutmeg, Peony, Royal jelly, Safflower, Soybean, Valerian, Yarrow
No available information for the item
Artichoke, Astragalus, Dandelion,
dGlehnia, Kelp, Lavender,
Meadowsweet,
eRed yeast rice,
fSkullcap
American ginseng, Angelica,
fArtemesia, Black cohosh,
fChrysanthemum flower, Corydalis yanhuso, Dang shen, Erigeron, Geum japonicum, Hawthorn,
eHorseradish, Jamaica quassia, Japanese brome, Lovage, Magnolia bark, Motherwort, Plantain, Pubescent angelica, Rue, Shiitake, Sweet melilot, Tamarind,
eWood ear mushrooms
a
Chinese herbal medicine were identified according to Taiwan Herbal Pharmacopoeia,[34]
Chinese Medicinal Herbs Preparation,[35] or Chinese Materia Medica.[36]
b
The severity rating was categorized according to MicroMedex®, Lexicomp® and Natural Medicines Comprehensive Database®. Interactions with severity rated as major in any of the three databases were included in the “major” type, while interactions rated as moderate and without major score in any database were included in the “moderate” type. “No available documented information for the item” meant that there was no available information about the single CHM in these databases.
c
Interaction with heparin is lack of information about severity rating.
d
Interaction with clopidogrel or warfarin is moderate.
e
Interaction with aspirin is moderate.
f