Chemical mechanical planarization (CMP) is widely recognized as the most promising method to achieve global planarization for ultralarge-scale integrated (ULSI) multilevel interconnection.1Al- though CMP for interlayer or intermetal dielectrics (ILD or IMD) has been used and studied extensively, metal CMP, especially the Al CMP process is relatively new and less understood due to the diffi- culties and the complicated electrochemical nature of Al.2Ideally, in a metal CMP process, the metal being polished should dissolve fast enough to support an acceptable removal rate. On the other hand, the passive etching rate of the metal should be slow enough in the ab- sence of polishing action to avoid dishing defects.3Thus, one of the key issues for the metal film CMP is to develop a slurry that meets these requirements. It is no doubt that the electrochemical properties of metal/slurry system appear to play an important role in metal CMP. Recently, several investigators have reported the development of in situ electrochemical techniques to evaluate polishing character- istics of copper3-6and tungsten.7-8DC open-circuit potential (OCP) and anodic potentiodynamic polarization measurements were the pri- mary techniques involved.
Phosphoric acid base slurry composed mainly of phosphoric acid, hydrogen peroxide, and alumina abrasive, developed by Yu et al.,10 was claimed to be able to achieve high removal rate and good pla- narity of Al CMP. Phosphoric acid is widely used in electropolishing for Al.11A number of studies have revealed that the oxide grown on aluminum in phosphoric acid was of porous type.12,13The porous film was mainly composed of aluminum phosphate and aluminum hydroxide, which was slightly soluble in this electrolyte.
In a study by Tseng et al.,9who used hydrogen peroxide as an oxidant, the removal rate data indicated that there existed a strong correlation between the Al-CMP removal process and the chemical reaction with phosphoric acid based slurry, while mechanical abra- sion by the abrasive particles plays only a minor role during Al CMP.
The specific chemical and mechanical reactions, however, were not fully explored. In this study, therefore, further investigation into the electrochemical behavior in the presence of abrasion during Al CMP is attempted to clarify the removal mechanism involved in Al CMP.
Experimental
Specimens and slurries preparations.—Commercial pure alu- minum (99.7%) sheets with a chemical composition shown in Table I was used. The specimens were cut to a dimension of 1 3 1 3 0.2 cm and were abraded on one surface with SiC paper to a finish of no.
800. A copper wire was connected to each specimen for electric con- duction and then mounted with epoxy with the abraded surface exposed.
All electrolytes were prepared from ACS reagent-grade chemi- cals and deionized (DI) water. The electrolyte was prepared 24 h
before testing. Citric acid was added for particle suspension in the slurry. Potassium hydroxide was used to adjust the electrolyte to pH 4. a-Al2O3with a particle size of 50 nm was added (5 wt %) to the electrolyte just prior to each test. The composition of the test slurry is shown in Table II. The slurry temperature was controlled at 25 6 28C during static and polishing state tests.
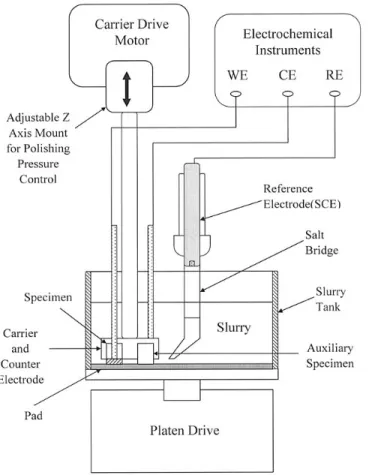
Polisher setup.—A novel polisher simulating the polishing mechanism of CMP was designed in this study. The system consists of an 8 in. platen and a 3 in. carrier. The platen was bonded with slur- ry holder to avoid splash of slurry during the polishing process. The platen speed could be varied with a rotating speed in the range 0–150 rpm. The down force between the specimen carrier and the platen could be varied from 1 to 10 psi. A Rodel Politex polish pad was affixed to the platen for polishing. During the polishing process, auxiliary specimens also made of Al specimens were added to the carrier for weight balance. The main features of the polisher layout and electrochemical setup are drawn schematically in Fig. 1. All electrochemical tests were performed in this polisher.
Electrochemical tests.—The potentiodynamic polarization tests were conducted in stagnant slurry and in the conditions while the specimens were held in the flowing slurry and subjected to contact pressure. The potential scan rate of 1 mV/s from active to noble potential was used. The instruments employed were an EG&G model 362 electrochemical system coupled with a Yokogawa LR4110 re- corder to monitor the potential and current during experiment. A sat- urated calomel electrode (SCE) was used as the reference electrode, and the carrier made of AISI 304 stainless steel acted as the counter electrode.
Electrochemical Behavior of Aluminum during Chemical Mechanical Polishing in Phosphoric Acid Base Slurry
Hong-Shi Kuo and Wen-Ta Tsai
Department of Materials Science and Engineering, National Cheng Kung University, Tainan, Taiwan
The electrochemical behavior of aluminum in chemical mechanical polishing (CMP) slurry was investigated using potentiody- namic polarization measurement and electrochemical impedance spectroscopy. The slurry used was mainly composed of phos- phoric acid, citric acid, and 50 nm Al2O3particles. The effects of slurry flow and contact pressure on the electrochemical behav- ior of Al were explored. The roles of chemical and mechanical interaction on the removal rate of Al during CMP were also attempt- ed. The results showed that the corrosion potential decreased with increasing contact pressure and platen rotating speed. The dis- solution rate, which was in reversing proportion to the polarization resistance, also increased with increasing the contact pressure and platen rotating speed. The repeated passivation, film breakdown due to mechanical abrasion, and metal dissolution steps took place during the simulating CMP process. The passive film formed in the testing slurry consisted of Al2O3, Al(OH)3, and AlPO4.
© 2000 The Electrochemical Society. S0013-4651(99)03-073-6. All rights reserved.
Manuscript submitted March 17, 1999; revised manuscript received September 8, 1999.
Table I. Chemical composition of aluminum used.
Al Si Fe Cu Mn Mg Cr Zn Ti
wt % Balance 0.1 0.2 — — — — — 0.01
Table II. Composition of slurry used.
Chemicals Concentration
Phosphoric acid 5 vol %
Citric acid 0.5 M
0.05 mm alumina powder 5 wt %
Potassium hydroxide pH adjuster
Electrochemical impedance spectroscopy (EIS) measurements were performed with Gamry EIS 900 system. Impedance measure- ments were performed in the frequency range 100 K to 0.1 Hz at OCP. A small amplitude perturbation of 10 mV in a sine waveform was applied to ensure linearity for impedance analyis.
Surface analysis by X-ray photoelectron spectroscopy.—After CMP, the specimens were removed from the polisher, cleaned in DI water, and then analyzed with X-ray photoelectron spectroscopy (XPS). The XPS analyses were performed with a VG ESCA 210 instrument, with excitation by Mg Ka radiation (hn 5 1253.6 eV).
The binding energies were calibrated against the binding energy of C (1s) (hn 5 284.6 eV).
Results and Discussion
Polarization behavior.—The potentiodynamic polarization curves for Al immersed in H3PO4-citric acid solution at pH 4 with 5 wt % abrasive Al2O3particle are shown in Fig. 2. In stagnant slur- ry, the corrosion potential is 2760 mV (SCE) and the corrosion (metal dissolution) current density is only 10.79 mA/cm2. The cor- rosion current density was obtained using Tafel’s extrapolation tech- nique. By extrapolation of the linear region of the cathodic polariza- tion curve (about 2100 to 2200 mV below corrosion potential) to the corrosion potential, the corrosion current density was deter- mined. The polarization curve 1 in Fig. 2 revealed that there existed a wide passive range in stagnant slurry. The passive current density was less than 70 mA/cm2. This result demonstrates that Al could be easily passivated in the slurry in stagnant condition. By holding the platen of the polisher at a rotating speed of 100 rpm and keeping the specimen suspended in the slurry without contacting the pad, the polarization curve of Al was depicted as curve 2 in Fig. 2. Under such a circumstance, the corrosion potential (2890 mV) was shifted toward the negative direction as compared with that in stagnant slur- ry. The corrosion current was increased to 40 mA/cm2. A passive range was still observed with the passive current density about
100mA/cm2. Curve 3 in Fig. 2 shows the polarization curve of Al in the testing slurry with a platen rotating speed of 100 rpm and with a download of 5 psi on the specimen against the polishing pad. The corrosion potential was further reduced while the corrosion current density increased to 610 mA/cm2as could be seen from curve 3 in Fig. 2. A plateau current density in the order of several thousand microamperes per square centimeter was found.
The results shown in Fig. 2 indicate that both fluid velocity and contact pressure affected the electrochemical behavior of Al in Al2O3 containing acidic slurry. Under load-free condition, the decrease in corrosion potential and the increase in passive current density in a flowing slurry was attributed to the erosion effect of Al2O3particles on the Al surface. It is known that Al is easily passivated in phos- phoric acid solution.12,13The passive film could be destroyed by the impingement of Al2O3particles, resulting in the creation of fresh Al metal surface.17 Thus, the specimen surface became active and a higher dissolution rate (four times higher than that in stagnant slur- ry) was seen. Since the repassivation rate of Al was quite high,20-22 the mechanical effect was compensated and gave rise to a plateau current density of 100 mA/cm2in anodic potential region. When 5 psi load was applied and under platen rotating condition, passivation could not be maintained. The chemomechanical effect gave rise to a much higher dissolution rate (610 mA/cm2, 60 times higher than in stagnant slurry). Similar results have been reported by Ford et al.20 They found that the rate of dissolution of Al-7 wt % Mg alloy rotat- ing disk electrode in sulfate/chloride solution was increased by sev- eral orders of magnitude when the high-impedance oxide film was mechanically removed. The high plateau anodic current density was the limiting current density of Al-Mg alloy in the flowing slurry and under pressure conditions.
Using Faraday’s law, the corrosion current density (610mA/cm2) of Al in the testing slurry, with 100 rpm of platen rotating speed and under 5 psi pressure, was equivalent to a dissolution rate of 126 Å/min. Comparing with the removal rate of about 700 Å/min reported by Tseng et al.9in the same slurry as used in this study, the dissolution rate only contributed 18% of the total removal rate. The fact that the chemical reaction rate (or dissolution rate) was much less than the removal rate of CMP was also observed for W 8and Cu.19 Kneer et al.8pointed out that the chemical oxidation or dissolution of tungsten was not the primary removal mechanism in CMP. On the other hand, in the study on the determination of CMP removal rate of Al in DI water with 5 wt % Al2O3 slurry, Tseng and co-workers9 Figure 1. Schematic diagram showing the apparatus used.
Figure 2. Effects of contact pressure and platen rotating speed on potentio- dynamic polarization curves of aluminum in phosphoric acid–based slurry.
found that the removal rate of Al was about 96 Å/min. In the absence of phosphoric acid and citric acid, the removal rate might be consid- ered mainly due to mechanical effect. Comparing with the removal rate of 700 Å/min in phosphoric-base slurry, the mechanical polish- ing only contributed 13% of the CMP removal rate. In other words, the chemo-mechanical removal rate was much higher than the sum of the respective dissolution and mechanical wear rates. Clearly, there existed a synergistic interaction between electrochemical and mechanical effects.
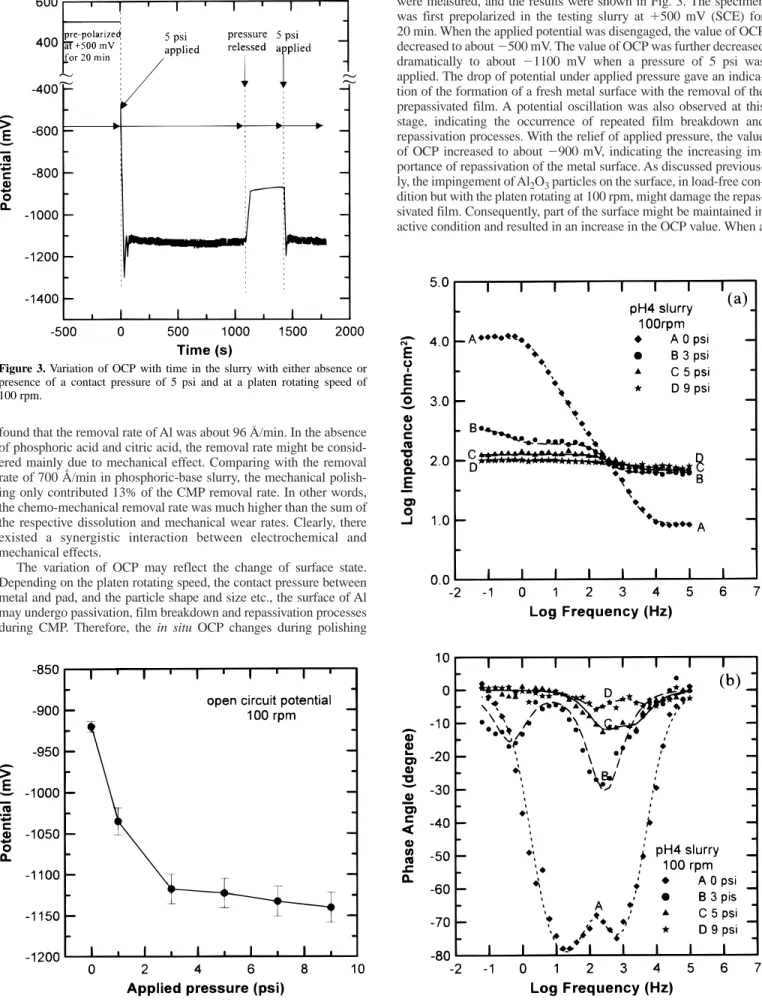
The variation of OCP may reflect the change of surface state.
Depending on the platen rotating speed, the contact pressure between metal and pad, and the particle shape and size etc., the surface of Al may undergo passivation, film breakdown and repassivation processes during CMP. Therefore, the in situ OCP changes during polishing
were measured, and the results were shown in Fig. 3. The specimen was first prepolarized in the testing slurry at 1500 mV (SCE) for 20 min. When the applied potential was disengaged, the value of OCP decreased to about 2500 mV. The value of OCP was further decreased dramatically to about 21100 mV when a pressure of 5 psi was applied. The drop of potential under applied pressure gave an indica- tion of the formation of a fresh metal surface with the removal of the prepassivated film. A potential oscillation was also observed at this stage, indicating the occurrence of repeated film breakdown and repassivation processes. With the relief of applied pressure, the value of OCP increased to about 2900 mV, indicating the increasing im- portance of repassivation of the metal surface. As discussed previous- ly, the impingement of Al2O3particles on the surface, in load-free con- dition but with the platen rotating at 100 rpm, might damage the repas- sivated film. Consequently, part of the surface might be maintained in active condition and resulted in an increase in the OCP value. When a
Figure 3. Variation of OCP with time in the slurry with either absence or presence of a contact pressure of 5 psi and at a platen rotating speed of 100 rpm.
Figure 4. Effect of contact pressure on OCP for aluminum in the slurry at a platen rotating speed of 100 rpm.
Figure 5. Effect of contact pressure on the Bode plots for aluminum in the slurry at a platen rotating speed of 100 rpm.
pressure of 5 psi was applied again, the value of OCP resumed that of a fresh Al surface (21100 mV). The changes of OCP with pressure shown in Fig. 3 coincided with the corrosion potentials determined in polarization measurements as revealed by curves 2 and 3 in Fig. 2.
The fact that an oscillation of potential occurred when a contact pressure existed between the specimen and the pad indicated that repeated repassivation and film breakdown took place during CMP.
Since Al is easily passivated in the phosphoric base acid, the extent of film breakdown would be dependent on the magnitude of applied pressure. The effect of applied pressure on the change of OCP was thus investigated with the results depicted in Fig. 4. The mean value of OCP decreased with increasing pressure up to 3 psi and then reached a constant value near 21100 mV. The results shown in Fig. 4 demonstrated the complicated chemo-mechanical effect on CMP.
EIS measurements.—The effects of contact pressure and platen rotating speed on the electrochemical behaviors of Al in Al2O3con- taining phosphoric acid base slurry were further evaluated using EIS.
Figure 5 shows the changes of Bode plots at different applied pres- sures. At load-free condition, the impedance measured at the high- frequency end was the solution resistance (Rs) with a value of 8.55V cm2. The impedance obtained at low frequency was the total imped- ance of the Al/slurry interface, which included the solution resis- tance, the charge-transfer impedance (Rct), and the passive film im-
pedance (Rf).24The equivalent circuit simulating the electrochemi- cal behavior of this system is depicted in Fig. 6, where Cdlis the dou- ble-layer capacitance and Cf is the passive film capacitance. The equivalent circuit has also been applied to the Al-Cu-Si alloy/5 wt % NH4Cl system.25The sum of Rctand Rfmay also be defined as the polarization resistance Rp. The impedance at different applied pres- sure are listed in Table III. The higher the polarization resistance, the lower the dissolution rate became. With no contact pressure applied between the specimen and the pad, as can be seen in Table III, the polarization resistance was 11,500 V cm2. When a pressure of 3 psi was applied, a substantial decrease in polarization resistance was found, as can be seen in Fig. 5. The decrease in polarization resis- tance was mainly associated with the damage of passive film by mechanical abrasion, which led to the formation of fresh surface. As the applied pressure was increased to 5 or 9 psi, the polarization resistance was further decreased. Consequently, the dissolution rate
Table III. Values of Rs, Rf1 Rct, and Rtotalfor aluminum in phosphoric acid base slurry determined from EIS at a platen speed of 100 rpm and at different contact pressures.
Rs Rp5 Rct1 Rf Rtotal
Pressure (psi) (V cm2) (V cm2) (V cm2)
0 8.55 11,503 11,512
3 60.8 1,1265 11,327
5 66.5 111,60 11,127
9 70.5 111,28 111,99
Table IV. Values of Rs, Rf1 Rct, and Rtotalfor aluminum in phosphoric acid base slurry determined from EIS at a contact pressure of 5 psi and at different platen speeds.
Rotating Rs Rp5 Rct1 Rf Rtotal
speed (rpm) (V cm2) (V cm2) (V cm2)
10 80.4 11,6481. 11,728
120 40.1 1,31701. 1,3210
100 66.5 111,59.7 11,126
Figure 6. Equivalent circuit diagram showing the electrochemical character- istics of aluminum during CMP in phosphoric acid–base slurry.
Figure 7. Effects of platen rotating speeds on the Bode plots for aluminum in the slurry at a contact pressure of 5 psi.
would increase as the pressure increased. EIS results were in good agreement with those obtained from polarization measurements. In- terestingly, it was found that the solution resistance increased as the applied pressure increased. Under applied pressure conditions, the pathway between the specimen and the pad for fluid flow became smaller. An increase in solution resistance was thus observed.
Both the impedance and phase angle vs. frequency plots revealed that two capacitance elements existed in the Al/slurry interface when no pressure was applied. Besides the double-layer capacitance, the second capacitance was derived from the existence of passive film.
When contact pressure was present, two peaks in the phase angle vs.
frequency plot were still found, indicating passive film still remained on the surface. In sulfate/chloride solution of pH 1, Ford et al.
reported that the time required for the repassivation of Al to form a monolayer of surface film was only a few miniseconds.20The pres- ence of surface film under applied pressure condition was due to the extremely high repassivation rate of Al in the present testing slurry.
The EIS results of Al in the testing slurry, at a contact pressure of 5 psi and at various platen-rotating speeds, are given in Table IV and
displayed in Fig. 7 in the form of Bode plots. From the impedance vs. frequency data alone (Fig. 7a), it was found that the platen rotat- ing speed greatly affected the impedance of dissolution reaction at the Al/slurry interface. Figure 7b shows that there were two capaci- tor responses which were also dependent on the platen rotating speed. The decrease in impedance at the low-frequency region with increasing rotating speed was attributed to the increasing difficulty in the repassivation of the Al surface. As could be seen in Fig. 7a, two orders of magnitude changes in impedance could occur as the rotating speed increased from zero to 100 rpm. The low impedance observed at a platen rotating speed of 100 rpm indicated that a rather high dissolution rate of Al took place. The solid lines in both Fig. 5 and 7 represent the fitting with the equivalent circuit of Fig. 6. The results show that the equivalent circuit demonstrated in Fig. 6 repre- sent fairly well the electrochemical nature of the Al/slurry system in the condition simulating the CMP process.
In the study of tungsten CMP, Kaufman et al.7proposed that a passive film would be formed on the metal surface in the slurry. The passive film could then be scratched by mechanical abrasion by solid
Figure 8. XPS for (a) Al (2p), (b) O (1s), and (c) P (2p) after CMP of alu- minum in the phosphoric acid base slurry.
particles. Dissolution of metal then would occur in the scratched area. The successive passivation, film damage, and dissolution processes were considered as the main mechanisms for metal re- moval and global planarization for CMP. The repeated dissolution, film formation, and film rupture processes have also been reported for the wear-corrosion18and stress-corrosion cracking23of stainless steels in aqueous environments.
Surface analysis.—As described previously, the formation of passive film plays an important role in CMP. XPS analysis was thus performed to characterize the film formed in the slurry. The spectra of Al and P were then analyzed. The results are shown in Fig. 8. As shown in Fig. 8a, the binding energies of 72.85 and 74.3 eV, respec- tively, represent the existence of metallic and oxidized aluminum.14 Figure 8b shows the XPS spectra of the O 1s region. The O 1s spec- tra could be fitted with three peaks: one at 533.4 eV corresponding to Al(OH)3,15one at 532.2 eV corresponding to O22in H2O,16and the third peak at 531.0 eV corresponding to Al2O3.14 Figure 8c shows the spectra for P 2p. The binding energy of 132.9 eV repre- sents the existence of AlPO4.14Table V shows the values of standard binding energies for various chemical species. Consequently, the passive film formed on the metal surface in the testing slurry might be thus composed of Al2O3, Al(OH)3, and AlPO4. The formation of AlPO4probably took place according to the following reactions
Al 1 H2PO42r AlPO41 2H11 3e [1]
The transformation of oxide to phosphate according to the following reaction was also possible
Al2O31 2H2PO421 2H1r 2AlPO41 3H2O [2]
The presence of Al2O3, Al(OH)3, and AlPO4 on the metal surface provided passivity for Al in CMP slurry.
Conclusions
The electrochemical behaviors of Al in 5 vol % H3PO41 0.5 M citric acid base slurry containing 5 wt % Al2O3and under various pol- ishing contact pressures and platen rotating speed, simulating the CMP condition, were investigated. The corrosion potential decreased with increasing contact pressure and platen rotating speed. The dis- solution rate, which was inversely proportional to the polarization resistance, also increased with increasing the contact pressure and platen rotating speed. The repeated processes of passivation, film breakdown, and metal dissolution took place during the simulating CMP process. The passive film formed on Al in the testing slurry con- sisted of Al2O3, Al(OH)3, and AlPO4.
Acknowledgment
The authors thank Dr. W. T. Tseng for valuable discussion, R. C.
Lee for XPS analysis, and are grateful for the support of this research by the National Science Council, Taiwan, under contract no. NSC 86-2216-E-006-045.
National Cheng Kung University assisted in meeting the publication costs of this article.
References
1. F. Malik and M. Hasan, Thin Solid Films, 270, 612 (1995).
2. M. A. Fury, D. L. Scherber, and M. A. Stell, MRS Bull., 61 (Nov 1995).
3. R. J. Gutmann, J. M. Steigerwald, L. You, D. T. Price, and J. Neirynck, Thin Solid Films, 270, 596 (1995).
4. J. M. Steigerwald, S. P. Muraka, R. J. Gutmann, and D. J. Duquette, J. Electrochem.
Soc., 141, 3512 (1994).
5. J. M. Steigerwald, D. J. Duquette, S. P. Muraka, and R. J. Gutmann, J. Electrochem.
Soc., 142, 2379 (1995).
6. R. Carpio, J. Farkas, and R. Jairath, Thin Solid Films, 266, 238 (1995).
7. F. B. Kaufman, D. B. Thompson, R. E. Broadie, M. A. Jaso, W. L. Guthrie, D. J.
Pearson, and M. B. Small, J. Electrochem. Soc., 138, 3460 (1991).
8. E. A. Kneer, C. Raghunath, V. Mathew, S. Raghavon, and J. S. Jeon, J. Elec- trochem. Soc., 144, 3041 (1997).
9. W. T. Tseng, J. Wu, and Y. S. Chang, Mater. Res. Soc. Symp. Proc., 477, 125 (1997).
10. C. C. Yu, T. T. Doan, and A. E. Laulusa, U.S. Pat. 5209816 (1993).
11. R. Vidal and A. C. West, J. Electrochem. Soc., 145, 4067 (1998).
12. A. A. Mazhar, F. E. Heakal, and K. M. Award, Thin Solid Films, 192, 193 (1990).
13. G. E. Thompson, R. C. Furneaux, G. C. Wood, and R. Hutchings, J. Electrochem.
Soc., 125, 1480 (1978).
14. D. Briggs and M. P. Seah, Practical Surface Analysis, 2nd ed., John-Wiley & Sons, New York (1994).
15. C. D. Wager, W. M. Riggs, L. E. Davis, J. F. Moulder, and G. E. Muilenber, Hand- book of X-Ray Photoelectron Spectroscopy, Perkin-Elmer, Eden Prairie, MN (1979).
16. W. P. Yang, D. Costa, and P. Marcus, J. Electrochem. Soc., 141, 111 (1994).
17. Y. Li, B. T. Burstein, and I. M. Hutchings, Wear, 181-183, 70 (1995).
18. H. Abd-El-Kader and S. M. El-Raghy, Corros. Sci., 26, 647 (1986).
19. C. A. Sainio, D. J. Duqette, J. Steigerwald, and S. P. Muraka, J. Electron. Mater., 25, 1593 (1996).
20. F. P. Ford, G. T. Burstein, and T. P. Hoar, J. Electrochem. Soc., 127, 1325 (1980).
21. T. Hagyard and W. B. Earl, J. Electrochem. Soc., 114, 694 (1967).
22. T. Hagyard and W. B. Earl, J. Electrochem. Soc., 115, 623 (1968).
23. R. C. Newman, in Corrosion Mechanisms in Theory and Practice, P. Marcus and J. Oudar, Editors, p. 313, Marcel Dekker, New York (1995).
24. G. W. Walter, Corros. Sci., 26, 681 (1986).
25. A. J. Griffin, Jr. and F. R. Brotzen, J. Electrochem. Soc., 141, 3473 (1994).
Table V. Al (2p), O (1s), and P (2p) binding energies for known aluminum compounds.
Region Species Binding energy (eV)
Al 2p Al0 72.85 (Ref. 14), 72.65 (Ref. 15) Al2O3 73.85 (Ref. 14), 74.7 (Ref. 15) Al(OH)3 74.3 (Ref. 14)
O 1s Al2O3 531.0 (Ref. 14), 531.6 (Ref. 15) Al(OH)3 533.4 (Ref. 15)
H2O 532.2 (Ref. 16), 533.1 (Ref. 14)
P 2p P0 130.2 (Ref. 14), 129.6 (Ref. 15)
AlPO4 132.9 (Ref. 14)