Communication
Synthesis and Crystal Structure of the Dicopper(II) Complex
[Cu2{Ph2P(==O)(o-C6H4)CO2}2(THF)2(H2O)2][BF4]2
by Oxidation of
[Cu{Ph2P(o-C6H4)C(==O)H}2(NCMe)][BF4] with Aqueous H2O2
Wen-Yann Yeha* ( ), Gene-Hsiang Leeb( ) and Shie-Ming Pengb( )a
Department of Chemistry, National Sun Yat-Sen University, Kaohsiung 804, Taiwan, R.O.C. b
Department of Chemistry, National Taiwan University, Taipei 106, Taiwan, R.O.C.
Treatment of [Cu(pcho)2(NCMe)][BF4] 1 (pcho = 2-(diphenylphosphino)benzaldehyde) with aque-ous H2O2in THF solvent affords [Cu2(dpb)2(THF)2(H2O)2] [BF4]22 (dpb = 2-(diphenylphosphinoxide)-benzoate) after crystallization from diethyl ether. This reaction involves oxidation of Cu(I) to Cu(II) ion, phosphine to phosphinoxide, and benzaldehyde to benzoate species. The crystal structure of 2 consists of two copper(II) atoms bridged by two carboxylate moieties of the dpb ligands. The coordination about each copper(II) atom is a distorted trigonal bipyramid.
Keywords: Cu(II) complex; 2-(Diphenylphosphinoxide)benzoate;
2-(Diphenylphosphino)benz-aldehyde.
Copper-catalyzed substrate oxygenation is an impor-tant biochemical process.1–3It may possess mononuclear, dinuclear, or trinuclear copper-active sites.4For instance, the mononuclear copper-active site was found in dopamine b-monooxygenase and quercetin 2,3-dioxygenase,5
and the dinuclear copper-active site was found in tyrosinases and catechol oxidases.6,7Recently, the reactions of an oxidant (eg., O2or H2O2) with a mononuclear copper complex to lead to products comprising [Cu2(m-O)2]n+ or isomeric [Cu2(O2)]n+ cores have attracted considerable attention aimed at understanding the mechanisms of oxidation catal-ysis in synthetic and biological systems.8–10We have pre-pared the trigonal complex [Cu(pcho)2(NCMe)][BF4] 111 (pcho = 2-(diphenylphosphino)benzaldehyde), where the copper(I) atom is bonded to an acetonitrile ligand and two phosphine groups of the pcho ligands. In this paper we wish
to report the reaction of 1 with H2O2to give a dinuclear copper(II) complex concomitant with oxidation of the pcho ligands.
Typically, compound 1 (220 mg, 0.30 mmol) was dis-solved in THF (15 mL) and an excess amount of aqueous H2O2(30%; 1 mL, 7.9 mmol) was added. The solution was stirred at ambient temperature for 12 h to result in a solu-tion color change from yellow to light blue. The solusolu-tion was topped with 30 mL of diethyl ether to afford crystals of the dicopper(II) complex [Cu2(dpb)2(THF)2(H2O)2] [BF4]2
212(dpb = 2-(diphenylphosphinoxide)benzoate) in 71% (120 mg, 0.107 mmol), while the known organic product 2-(diphenylphosphinoxide)benzaldehyde 313(51 mg, 0.17 mmol) was isolated from the mother liquid as a white pow-der (eq. 1). There was no reaction by stirring the THF solu-tion of 1 in air (20% O2) for three days. The formation of 2 Journal of the Chinese Chemical Society, 2006, 53, 271-274 271
Ph2 Cu Cu O O O O H2O O O OH2 O P P Ph2 O Cu P O H P O H MeNC Ph2 Ph2 1 H2O2(aq) THF I II II 2 2 O P (1) H Ph2 O + 3
apparently involves three oxidation steps, namely Cu(I) to Cu(II) ion, phosphine to phosphinoxide,14and benzalde-hyde to benzoate species. Since treating pcho molecules with aqueous H2O2in THF afforded 3 exclusively, the cop-per centers must play an important role in the transforma-tion of pcho to dpb ligands. It is plausible that a reactive bis(m-oxo)dicopper intermediate is formed,15
followed by oxygen-transfer to the formyl group to generate a carbox-ylate species. However, the details remain unclear at this stage.
Compound 2 forms light blue crystals, indicating the presence of d9Cu(II) atoms. Thus, the31P NMR shows no signal for the phosphinoxide group, likely due to the para-magnetic effects,16and the1H NMR spectrum shows sev-eral broad signals in the ranged 8.7-1.9 ppm. The X-band EPR spectrum measured on a powder sample at 77 K shows g||= 2.56 and g^= 2.18. The IR spectrum of 2 in a KBr pellet shows broad and intense bands at 1600 and 1368 cm–1, corresponding to thenas(COO–) andns(COO–) vibra-tion of the bridging carboxylate group within a dinuclear species.17
The structure of 218consists of discrete cations [Cu2 -(dpb)2(THF)2(H2O)2]2+and tetrafluoroborate counter an-ions. The ORTEP drawing for the cation is depicted in Fig. 1, and the selected bond distances and bond angles are col-lected in Table 1. There is a crystallographic center of sym-metry imposed on the complex. Each dbp ligand is
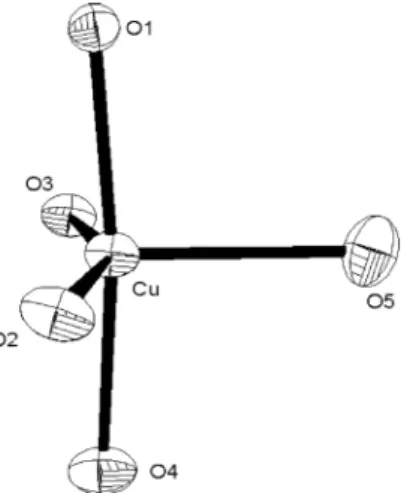
triden-tate, with the carboxylic group bridging two copper centers and the phosphinoxide groups-coordinated to one copper atom. The two copper atoms are separated by 4.46 Å. The coordination about the Cu(II) ion can be described as a dis-torted trigonal bipyramid (Fig. 2), where the equatorial sites are linked to a THF (O5), a phosphinoxide (O3), and a benzoate (O2) ligands, and the axial sites are linked to a water (O4) and another benzoate (O1) ligand. The Cu–O (1~4) distances are comparable, ranging from 1.925(1) Å through 1.959(2) Å, while the Cu–O5 distance to the THF
272 J. Chin. Chem. Soc., Vol. 53, No. 2, 2006 Yeh et al.
Fig. 1. Molecular structure of 2. The BF4–anions and hydrogen atoms have been artificially omitted for clarity.
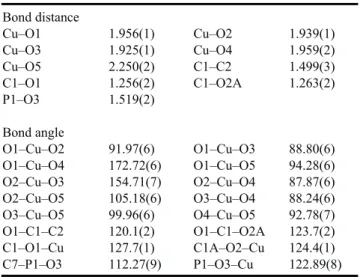
Table 1. Selected bond distances (Å) and bond angles (°) for compound 2 Bond distance Cu–O1 1.956(1) Cu–O2 1.939(1) Cu–O3 1.925(1) Cu–O4 1.959(2) Cu–O5 2.250(2) C1–C2 1.499(3) C1–O1 1.256(2) C1–O2A 1.263(2) P1–O3 1.519(2) Bond angle O1–Cu–O2 91.97(6) O1–Cu–O3 88.80(6) O1–Cu–O4 172.72(6) O1–Cu–O5 94.28(6) O2–Cu–O3 154.71(7) O2–Cu–O4 87.87(6) O2–Cu–O5 105.18(6) O3–Cu–O4 88.24(6) O3–Cu–O5 99.96(6) O4–Cu–O5 92.78(7) O1–C1–C2 120.1(2) O1–C1–O2A 123.7(2) C1–O1–Cu 127.7(1) C1A–O2–Cu 124.4(1) C7–P1–O3 112.27(9) P1–O3–Cu 122.89(8)
ligand is substantially longer, being 2.250(2) Å. The axial O1–Cu–O4 angle is 172.72(6)°. The three equatorial O–Cu–O angles sum to 359.9°, consistent with the small displacement of the copper atom from the plane (0.04 Å). However, the large, constrained O2–Cu–O3 bond angle (154.71(7)°) causes compression of the O2–Cu–O5 angle (105.18(6)°) and the O3–Cu–O5 angle (99.9(6)°) away from the idealized 120°, which should increase steric repul-sions to the THF ligand. Therefore, lengthening of the Cu–O5 bond can be rationalized in terms of steric effects to relax ligand interactions. Overall, the framework of 2 can be viewed as a fused pentacycle (Fig. 3), which is unique in dicopper(II) systems.
ACKNOWLEDGEMENT
We are grateful for support of this work by the
Na-tional Science Council of Taiwan.
Received July 28, 2005.
REFERENCES
1. Ibers, J. A.; Holm, R. H. Science (Washington, D.C.) 1980, 209, 223.
2. Demmin, T. R.; Swerdloff, M. D.; Rogi, M. M. J. Am.
Chem. Soc. 1981, 103, 5795.
3. Solomon, E. I.; Sundaram, U. M.; Machonkin, T. E. Chem. Rev. 1996, 96, 2563.
4. Itoh, S. In Comprehensive Coordination Chemistry II;
Elsevier: New York, 2004; Vol. 8, p 369. 5. Klinman, J. P. Chem. Rev. 1996, 96, 2541.
6. Solomon, E. I.; Chen, P.; Metz, M.; Lee, S.-K.; Palmer, A. E. Angew. Chem. Int. Ed. 2001, 40, 4570.
7. Gerdemann, C.; Eicken, C.; Krebs, B. Acc. Chem. Res. 2002, 35, 183.
8. Que, Jr. L.; Tolman, W. B. Angew. Chem. Int. Ed. 2002, 41, 1114.
9. Mirica, L. M.; Ottensaelder, X.; Stack, T. D. P. Chem. Rev.
2004, 104, 1013.
10. Lewis, E. A.; Tolman, W. B. Chem. Rev. 2004, 104, 1047 11. Yeh, W.-Y.; Liu, Y.-C.; Peng, S.-M.; Lee, G.-H. Inorg. Chim.
Acta 2005, 358, 1987.
12. Characterization of 2: MS FAB m/z: 855 ([Cu2(dpb)2(BF4)]+).
IR (KBr pellete): 3450, 3065, 1600, 1386, 1010 cm–1.1H
NMR (CD3CN):d 8.7 (br), 7.8 (br), 7.2 (br), 5.8 (br, Ph), 3.7
(br), 1.9 (br, THF) ppm. Anal. for C46H48B2Cu2F8O10P2:
Found C, 49.72; H, 4.23; Calcd. C, 49.18; H, 4.31%. 13. Ravindar, V.; Hemling, H.; Schumann, H.; Blum, J. Synth.
Commun. 1992, 22, 1453.
14. Hsu, M.-A.; Yeh, W.-Y.; Peng, S.-M. Lee, G.-H. J. Chin. Chem. Soc. 1994, 41, 441.
15. Aboelella, N. W.; York, J. T.; Reynolds, A. M.; Fujita, K.; Kinsinger, C. R.; Cramer, C. J.; Riordan, C. G.; Tolman, W. B. Chem. Commun. 2004, 1716.
16. Drago, R. S. Physical Methods in Chemistry; W. B. Saunders Co.: Philadelphia, U.S.A., 1977.
17. Youngme, S.; Chailuecha, C.; van Albada, G. A.; Pakawatchai, C.; Chaichit, N.; Reedijk, J. Inorg. Chim. Acta
2005, 358, 1068.
18. Crystal data of 2: Mf= 1123.48, triclinic, space group P`1, a
= 10.10736) Å, b = 11.2948(7) Å, c = 12.3737(8) Å,a =
67.534(1)°, b = 68.348(1)°, g = 75.725(1)°, V = 1204.1(1) Å3,
Z = 1,rcalcd= 1.549 g cm–3,m = 1.037 mm–1, F(000) = 574,q Synthesis of [Cu2{Ph2P(==O)(o-C6H4)CO2}2(THF)2(H2O)2][BF4]2 J. Chin. Chem. Soc., Vol. 53, No. 2, 2006 273
Fig. 2. The distorted trigonal bipyramidal coordina-tion about the copper atom of 2.
range 1.87–27.50°, 316 variables refined with 5497
inde-pendent reflections to final R indices (I > 2s(I)) of R1 =
0.0371 and wR2 = 0.0905, and GOF = 1.046. The
crystallo-graphic data have been deposited with the Cambridge Crys-tallographic Data Centre, CCDC No. 279033.