Improved Densification of Carbonyl Iron Compacts by the
Addition of Fine Alumina Powders
Y.C. LU and K.S. HWANG
An investigation of the effect of alumina particles on the sintering behavior of a carbonyl iron powder compact was carried out in this study. Two different-sized alumina, 0.05 and 0.4mm, were added to the iron compact at amounts up to 1.2 wt pct. When 0.4mm alumina particles were added, no sintering enhancement was observed. But, in contrast to previous results reported in literature, the addition of 0.1 to 0.2 wt pct of 0.05mm alumina particles was found to improve the densification. With 0.1 wt pct, the sintered density increased from 7.25 to 7.40 g/cm3after the compact was sintered at 1350
8C for 1 hour in hydrogen. Dilatometric curves showed that alumina impeded the early-stage sintering of iron in theaphase, but improved densification in thegphase at high temperatures. These results, along with microstructural analysis, suggested that alumina particles exhibit dual roles; their physical presence blocks the diffusion of iron atoms, thus causing inhibition of sintering, while their grain-boundary pinning effect prevents exaggerated grain growth of iron and helps densification. It follows that, depending upon the amount and size of the alumina powders, either an increase or decrease in the final sintered density can be obtained.
I. INTRODUCTION also showed inhibited sintering by the addition of inert oxides.[11] Other observations on the inhibited sintering of
CARBONYL
iron powders are frequently used inmak-copper, nickel, and cobalt were also noticed, as summarized ing powder injection–molded (PIM) compacts, which in a review article by Ashby et al.[12]
require high sintered densities. However, the density of the
Although most studies reported that inert oxides retarded compacts for these applications is usually not fully dense densification of metal powders, Imai and Miyazaki[13] dem-after sintering.[1,2,3] One of the main reasons for attaining
onstrated that the volume diffusion of silver increased such less-than-desired densification is that the exaggerated
slightly when it was mixed with 2 wt pct Al2O3. Lu et al.[14] grain growth usually occurs when iron transforms from the reported an improved sintered density of PIM iron compacts
aphase to theg phase.[4–7]The exaggerated grain growth
when aluminum stearate was added in the binder system. causes pore isolation from grain boundaries. Once these The stearate was found to form Al
2O3 during debinding pores are trapped inside the grain, they cannot be eliminated
and sintering and helped densification. Comparing these
within a practical timeframe. previous studies, the different results on the effects of
alu-To improve the sintered density of carbonyl iron compacts,
mina could be caused by the differences in the size and the the main challenge is to inhibit the grain growth, particularly amounts of alumina particles and/or the process (press-and-during the phase transformation. One of the common sinter molding or PIM). The purpose of this study was, thus approaches is to add inert dispersoids into the iron in the
to re-examine the effects of alumina on the sintering behavior hopes that they will impede the grain-boundary migration. of carbonyl iron powders. This study used only the press-However, most previous studies indicated that inert oxides
and-sinter process and concentrated on the effects of the caused an inhibition of sintering.[8–11]Corti and Cotterill[8]
amount and size of alumina powders. The amount of alumina mixed g alumina particles, which were smaller than 0.03
used ranged from 0.1 to 1.2 wt pct, and two sizes, 0.05 and
mm, with carbonyl iron powders by dry ball milling for 24 0.4 mm, were compared. Also, both wet and dry mixing hours. The powder mixture was then pressed and sintered.
techniques were employed to compare the effect of the uni-The amount of alumina employed ranged from 0.16 to 16.0 formity of the alumina distribution. The results showed that wt pct, after which a marked inhibition of densification was an improvement in sintered density can be obtained by add-observed. This study attributed the inhibition effect to the
ing a small amount of 0.05mm alumina particles. restricted diffusion of iron atoms due to the presence of
alumina particles on the interparticle iron-iron contacts. Singh and Houseman[9] and Singh[10]studied the effect of
II. EXPERIMENTAL PROCEDURE alumina, titania, and zirconia on the densification of carbonyl
Most carbonyl iron powders contain 0.5 to 1.0 wt pct iron powders between 1300 8C and 1490 8C. The particle
carbon, which is a critical element in influencing the sin-size of the three oxides varied from 0.005 to 0.040mm, and
tering behavior of iron. Thus, to avoid the complexity inher-the amount employed was between 0.5 and 2.0 wt pct. All
ent in analyzing the sintering results of this study, the iron three oxides inhibited densification. Studies on iron catalysts
powder used was a reduced grade (CIP-R-1430, ISP Corp., Wayne, NJ) which contained only 0.065 wt pct carbon. It had an average particle size of 7mm, and its characteristics Y.C. LU, Graduate Student, and K.S. HWANG, Professor, are with the are given in Table I.
Institute of Materials Science and Engineering, National Taiwan University,
Two types of alumina, 0.40mma-alumina and 0.05mm Taipei, Taiwan 106, Republic of China.
Table I. The Characteristics of Carbonyl Iron Powders Used in This Study
Powder designation CIP-R-1430 Surface area, m2/g 0.336 Pycnometer density, g/cm3 7.84 d50,mm 7 d90 15 d10 3.1 Carbon 0.065 pct Nitrogen 0.006 pct Oxygen 0.420 pct
Supplier ISP Corp. (Wayne, NJ)
of these alumina particles are different, both of them are considered to be chemically inert to iron. Thus, the results
and discussions presented in the following sections are Fig. 1—The sintered densities of iron compacts containing different related to their particle size alone. To attain a uniform oxide amounts of 0.05mm alumina.
distribution in the iron matrix, alumina powders were dis-persed in alcohol first and then mixed with iron powders to form a slurry, using a mortar and pestle. The slurry was dried, ground to 2325 mesh powders, and then pressed into 55 pct dense pellets, 12 mm in diameter and 5 mm in thickness, using the floating die technique. No lubricant was used. The green compacts were heated at a rate of 108C/ min and then isothermally sintered at 12008C and 13508C, respectively, for 1 hour in hydrogen.
To understand the effect of alumina on the sintering behav-ior of carbonyl iron powders, dilatometry analysis was employed to monitor the dimensional change of the compact during sintering. To examine the microstructural evolution, specimens were removed at different stages during heating. For optical microscopy analysis, sintered compacts were infiltrated with epoxy and cured prior to grinding and pol-ishing, so that the pore shape would not be distorted. For scanning electron microscopy (SEM) examination, speci-mens were immersed in liquid nitrogen first and then frac-tured. With this method, little plastic deformation occurred,
and the true internal structure of the compact, particularly Fig. 2—The sintered densities of iron compacts containing different
the neck morphology, was retained. amounts of 0.40mm alumina.
III. RESULTS A. Sintered Density
The sintered densities of specimens containing different amounts of 0.05mm alumina particles are shown in Figure 1. At 1200 8C, the density of carbonyl iron compacts increased from 6.55 to 6.65 g/cm3with a 0.1 wt pct alumina addition. As the temperature increased from 12008C to 1350 8C, the density also increased from 7.25 g/cm3of the pure iron to 7.40 g/cm3with 0.1 wt pct of alumina. In contrast, when 0.40 mm a-alumina was added, the density of the compact decreased at both 12008C and 1350 8C, as shown in Figure 2.
These results are different from those reported in Refer-ences 9 and 10, which showed decreased densities in all tests. The difference could be caused by the alumina or the mixing methods employed. To simulate the dry mixing method used by Singh and Houseman,[9,10]0.05mm alumina particles were mixed with carbonyl iron powders by dry
ball milling for 24 hours. Figure 3 shows that the sintered Fig. 3—The sintered densities of alumina-containing compacts prepared by the dry ball-milling method.
(a) (b)
(d ) (c)
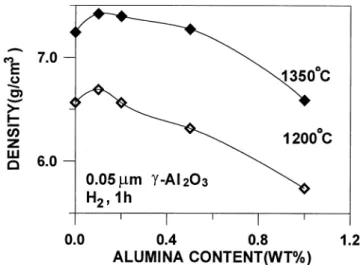
Fig. 4—The alumina particle distribution in (a) dry ball-milled powders containing 1 wt pct 0.05mm alumina, (b) wet-mixed powders containing 1 wt pct 0.05mm alumina, (c) wet-mixed powders containing 1 wt pct 0.4mm alumina, and (d ) as-received carbonyl iron powders.
increased, just as reported in Singh and Houseman’s stud-ies.[9,10] The pure iron compact also shows a decrease in sintered density with dry ball-milled powders. Figure 4 com-pares the morphology of the mixed powders, which con-tained 1 wt pct 0.05 mm aluimna particles. When the dry mixing technique was used, some alumina particles were embedded on the iron powder surface, and the iron particles became more irregular in shape, as shown in Figure 4(a). In contrast, the wet-mixed powder mixtures with 1 wt pct of 0.05 mm alumina and 0.4 mm alumina, as shown in Figures 4(b) and (c), respectively, illustrate that most alu-mina particles were uniformly distributed. The iron powder also retained its original size and shape, as shown in Fig-ure 4(d).
B. Dilatometry Analysis
Fig. 5—Dilatometer curves of iron compacts with and without alumina To better understand the effect of alumina particles on
additions. the sintering behavior of iron compacts, dilatometry tests
were performed on specimens with and without alumina additions. Figure 5 shows that the density of the pure iron
compact increased significantly with the increase in tempera- the phase transformation, and the final density was greater than that without the alumina addition. Similar inhibition ture in the a phase. But, the densification rate decreased
dramatically after the phase transformation, due to the exag- was observed during sintering in theaphase on the specimen containing 0.4mm alumina. However, no sintering improve-gerated grain growth. By adding 0.1 wt pct of 0.05 mm
alumina particles, the sintering rate in the a phase was ment was obtained in thegphase.
Figure 6 compares the effect of the amount of alumina impeded. However, enhanced sintering was observed after
(a) Fig. 6—The effect of the amount of 0.05mm alumina on the sintering
behavior of carbonyl iron compacts.
on the sintering behavior of iron compacts. As the amount of alumina increased from 0.1 to 0.5 wt pct, very little sintering was observed in theaphase, and the densification-rate change, which usually occurs at the phase transforma-tion, became even unnoticeable. Significant densification did not occur until 11008C. The final sintered density was lower than that of the pure iron compact.
C. Microstructure
(b) Since exaggerated grain growth is the most critical factor
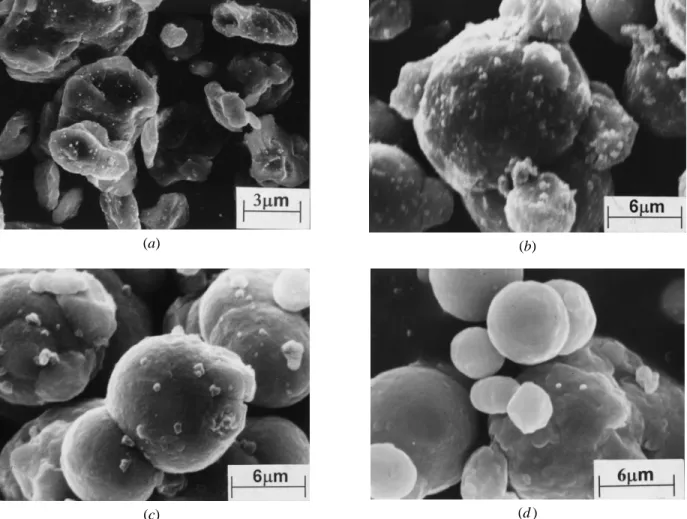
in influencing the final sintered density of carbonyl iron Fig. 7—The microstructure of pure iron compacts after being heated to (a) compacts, the evolution of the microstructure during heating 8908C, showing pores attached to the grain boundary (indicated by arrows);
and (b) 9508C, showing pores trapped inside the grains. was monitored. Figure 7(a) shows the microstructure of a
pure iron specimen heated to 8908C, in which most pores are still connected to the grain boundaries. As the temperature
increased to 9508C, significant grain growth occurred, leav- of the microstructure correspond to the sintering behavior of the compacts shown in Figure 5.
ing isolated pores inside the grain, as shown in Figure 7(b).
Figure 8(a) demonstrated that, when 0.1 wt pct of 0.4mm Figure 10(a) shows the microstructure of pure iron speci-mens sintered at 13508C for 1 hour in hydrogen. Consider-alumina was added, the compact that was heated to 9508C
showed slightly less grain growth than that of pure iron, able grain growth was observed, and the growth was in agreement with the results reported in previous literature.[4–7] shown in Figure 7(b), and most interparticle necks were
still clearly discernible. But, exaggerated grain growth and In contrast, compacts with either 0.4 or 0.05mm alumina additions, as shown in Figures 10(b) and (c), respectively, trapped pores were still apparent. Thus, similar to iron, the
densification rate was significantly impeded after the phase reveal smaller grain sizes.
To observe the morphology change of the alumina powder transformation, as shown in Figure 5. When 0.1 wt pct of
0.05mm alumina was added, the inhibition effect on grain in the iron matrix, 1 wt pct of 0.05 and 0.4 mm alumina, respectively, were mixed with iron powders. The mixed growth and neck growth became more significant, as shown
in Figure 8(b). This helped improve the shrinkage rate in powders were poured into an alumina boat and were sintered at 12008C and 1350 8C, respectively, for 1 hour in hydrogen. thegphase, as shown in Figure 5.
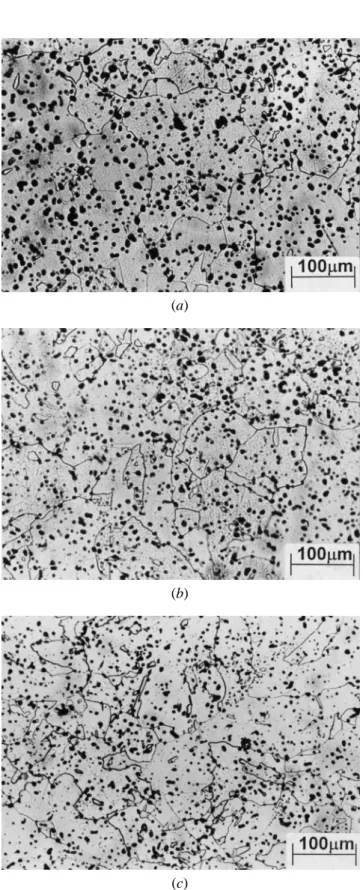
The specimens that had been heated to 9508C were also The lightly sintered compacts were fractured and then exam-ined under SEM. Compared to the alumina particle sizes fractured after being immersed in liquid nitrogen. Figure
9(a) shows that the fractured areas in pure iron compacts shown in Figures 4(b) and (c), Figure 11 illustrates that, after being heated to 1200 8C and 1350 8C, the alumina were quite large, and the interparticle necks were no longer
discernible. On the other hand, alumina-containing compacts particles coarsened, particularly the 0.05mm alumina. This coarsening phenomena was also reported in previous tended to fracture at the necks, particularly in the compact
that contained 0.1 wt pct of 0.05mm alumina, as shown in literature.[15,16,17]
The effects of alumina particle size on the sintering behav-Figure 9(b). This further confirmed that sintering was
hin-dered by alumina particles and that smaller necks were ior of carbonyl iron powder, as shown previously, can be summarized in Table III. With the same amount of alumina obtained. Figure 9(c) shows that the fracture surface of a
specimen containing 0.4mm alumina had a mixture of both (0.1 wt pct), finer particles are more effective in inhibiting sintering in theaphase. At high temperatures in thegphase, large transgranular and small neck areas. These evolutions
(a) (a)
(b) (b)
Fig. 8—The microstructure of alumina-containing iron compacts after being heated to 9508C (a) with 0.1 wt pct 0.4mm alumina and (b) with 0.1 wt pct 0.05mm alumina.
there is less exaggerated grain growth, less pore isolation from grain boundaries, and more pronounced alumina coars-ening, which are all beneficial for sintering. However, there is a limited range of the amount of alumina which aids in sintering, and only within that range can the final density be improved. When coarse alumina particles (0.4mm) are employed, no improvement in sintered density can be found.
IV. DISCUSSION
One of the benefits of adding inert dispersoids into iron
(c) compacts is to retard the grain growth, so that the pores will
Fig. 9—The fractured surface, as shown by arrows, of compacts that were remain attached to the grain boundaries and, thus, improve
heated to 9508C: (a) pure iron, (b) iron with 0.1 wt pct 0.05mm alumina, densification. The dispersoids, however, could block the
and (c) iron with 0.1 wt pct 0.4mm alumina. diffusion path of iron atoms and interfere with the mass flow
of iron atoms, particularly on the grain-boundary diffusion mechanism. The final sintered density is, thus, influenced
by these two counteracting effects: grain-boundary pinning present, as illustrated by the dilatometric curves shown in Figures 5 and 6. As the compact passed the phase-transfor-and diffusion blocking. Since the grain growth in pure iron
compacts was not significant in thea phase, as shown in mation temperature where exaggerated grain growth usually occurs, the grain-boundary pinning effect became distinct, Figure 7(a), the benefit of retarding the grain growth by
adding alumina was not apparent below 9128C. However, as demonstrated by comparing the microstructures shown in Figures 7(b) and Figure 8(b). These figures also show the diffusion-blocking effect was still effective. Thus,
Table II. The Percentage of the Surface Area of a 7mm Iron Powder, Which Is Covered by Alumina Particles at
Various Weight Fractions
Alumina Content, Wt Pct Alumina Particle
Size,mm 0.10 0.20 0.50 1.00 1.45 12.7 0.40 0.8 1.6 3.9 7.9 11.4 100 0.05 6.8 13.7 34.1 68.8 100 —
that there is an optimum alumina content at which the grain-boundary pinning effect overshadows the diffusion-blocking effect and results in an improved sintered density. Singh[10] studied a case in which 7mm iron powder was completely covered by 0.005 to 0.03mm aluminum oxides. No
densifica-(a) tion enhancement was observed, because the grain-boundary
diffusion mechanism of iron was completely blocked. The calculated amounts of alumina with which the 7 mm iron powder surfaces were completely covered were 0.11 wt pct for 0.005mm alumina and 0.86 wt pct for 0.03mm alumina, respectively. In this study, the amounts of dispersoids needed for such complete surface coverage were 1.45 wt pct and 12.7 wt pct for the 0.05 and 0.40 mm alumina powders, respectively. Thus, should there be a density improvement, the optimum amount of alumina should be less than these two quantities. The results of this study showed that, at 1350 8C, sintering was improved by adding 0.1 and 0.2 wt pct of 0.05 mm alumina particles. These two alumina quantities gave only 6.8 and 13.7 pct coverage on the iron powder surface, respectively. The percentages of the surface area of a 7mm iron powder covered by alumina of other contents employed in this study are listed in Table II.
Zener’s theory[18] suggests that finer alumina particles (b)
result in a stronger pinning effect and less grain growth. This implies that a higher sintered density might be obtained by using finer alumina. In reviewing previous literature, which addressed the effect of fine dispersoids on the sintering of carbonyl iron powders, it is noted that Singh and House-man[9]used 0.005 to 0.03mm alumina and that the minimum amount was 0.5 wt pct. Assuming that the additives were uniformly distributed on the iron powder surface, this would mean that all iron powder surfaces were covered when 0.005
mm alumina was used. For 0.03mm alumina additions, 58 pct of the surface area was covered. Corti and Cotterill[8]used alumina particles smaller than 0.03mm, and the minimum amount employed was about 0.16 wt pct. This implies that more than 19 pct of the iron powder surface was covered. It is very likely that too much alumina was used in these studies, and, thus, no enhanced sintering was observed.
Another factor that may explain why previous studies did
(c) not find improved densification is the different types of
carbonyl iron powders used. The frequently used types con-Fig. 10—The microstructure of compacts sintered at 13508C for 1 h: (a)
pure iron, (b) iron with 0.1 wt pct 0.4mm alumina, and (c) iron with 0.1 tain about 0.6 to 0.8 wt pct carbon, which is much higher
wt pct 0.05mm alumina. than the 0.065 pct of the reduced grade used in this study.
Some others contain a small amount of silica, which was used as a tumbling media during powder preparation.[19] Since both carbon and silica could inhibit the exaggerated As more dispersoids were added into the compact, the
grain-boundary pinning effect, which is beneficial for densi- grain growth, the beneficial effect of adding alumina could, thus, be overshadowed.
fication, became more apparent. However, with the addition
of more dispersoids, more diffusion paths are blocked, and In a previous study by Lu et al.,[14]it was found that an aluminum stearate addition improved the densification of the densification is retarded. It is, thus, reasonable to expect
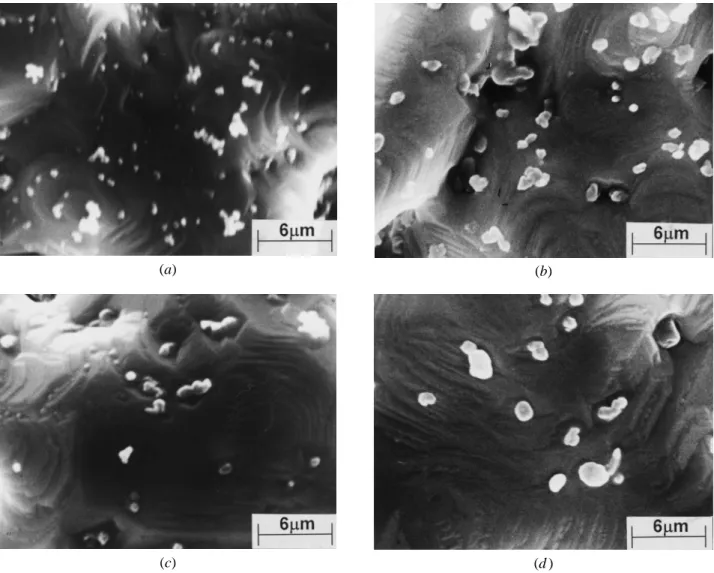
(a) (b)
(c) (d )
Fig. 11—The morphology changes of alumina particles at different temperatures: (a) 12008C, 0.05mm alumina; (b) 12008C, 0.40mm alumina; (c) 1350
8C, 0.05mm alumina; and (d ) 13508C, 0.40mm alumina.
Table III. The Effects of 0.1 Wt Pct 0.4mm and 0.05mm Alumina on the Sintering Behavior of Carbonyl Iron Powder Compacts
a-Phase Region
g-Phase Region Pore Isolation
Inhibition Degree of from Grain Degree of Alumina Final Grain Final Sintered Compact Type Effect Sintering Boundary Sintering Coarsening Size Density
Pure iron o o o o o o o
With 0.4mm alumina 1 2 2 1 1 2 2
With 0.05mm alumina 11 22 22 11 11 22 1
o: the status of pure iron;1: increased; 11: significantly increased; 2: decreased; and 22: significantly decreased.
carbonyl iron compacts that were prepared by both the pow- alumina particles alone can improve the densification of carbonyl iron compacts.
der injection molding and the die-compaction techniques.
Their results, from inductively coupled plasma analysis, Another factor that affects the sintering behavior of iron compacts is the coarsening of fine alumina particles. Figure indicated that some aluminum in the stearate was dissolved
in the iron matrix, while some other aluminum formed alu- 11 illustrates that, at high temperatures, coarsening of alu-mina occurred, particularly on the 0.05mm particles. This mina during debinding and sintering. Both of these
phenom-ena were attributed to the enhanced sintering; notably, reduces the blocking effect and contributes to the improved densification at high temperatures.
however, no experiment was performed to identify the
has been to retard the normal grain growth of the metal ACKNOWLEDGMENTS matrix during the final stage of sintering. However, for iron,
The authors are grateful for the support of this work by it provides an additional benefit of inhibiting the exaggerated
the National Science Council of the Republic of China under grain growth during the phase transformation at 912 8C.
Contract No. NSC86-2216-E-002-032. Thus, it is worthwhile to find out which effect contributes
more to the final enhanced densification. Another experi-ment was, thus, carried out by adding the same 0.05 mm
alumina particles to carbonyl nickel powders, which have REFERENCES
no allotropic transformation during heating. Although grain
refinement was observed, no sintering improvement was 1. H. Zhang and R.M. German: Int. J. Powder Metall., 1991, vol. 27 (3), pp. 249-54.
found at either 1200 8C or 1350 8C. This suggests that the
2. Y. Kiyota: U.S. Patent 5006164, 1991. main role of dispersoids in iron is not in inhibiting the normal
3. K. Hayashi and T.W. Lim: Mater. Trans. Jpn. Inst. Met., 1991, vol. grain growth, but more in retarding the exaggerated grain 32 (4), pp. 383-88.
growth during the phase transformation. 4. F.V. Lenel, G.S. Ansell, and J.R. Strife: in Modern Developments in
Powder Metallurgy, H.H. Hausner and W.E. Smith, eds., Metal Powder
Industries Federation, Princeton, NJ, 1974, vol. 6, pp. 275-92.
V. CONCLUSION 5. S.L. Fross: in Modern Developments in Powder Metallurgy, H.H. Hausner, ed., Plenum Press, New York, NY, 1966, vol. 2, pp. 3-11. 6. A.R. Poster and H.H. Hausner: in Modern Developments in Powder 1. In contrast to previous reports, the addition of 0.05mm
Metallurgy, H.H. Hausner, ed., Plenum Press, New York, NY, 1966, alumina to carbonyl iron powders increased the sintered vol. 2, pp. 26-44.
density when compacts were sintered at 1200 8C and 7. G. Cizeron and P. Lacombe: C.R. Acad. Bulg. Sci., 1955, vol. 241, pp. 409-10.
13508C for 1 hour in hydrogen. When larger-sized 0.4
8. C.W. Corti and P. Cotterill: Powder Metall. Int., 1974, vol. 6 (1), pp.
mm alumina was employed, no improvement in density
23-25. was found.
9. B.N. Singh and D.H. Houseman: Powder Metall. Int., 1977, vol. 3 2. Dilatometric analysis showed that fine 0.05mm alumina (1), pp. 26-29.
hindered the densification of iron compacts in the early 10. B.N. Singh: Powder Metall., 1972, vol. 15 (30), pp. 216-27. 11. J.M. Schultz: J. Catalysis, 1972, vol. 27, pp. 64-69. stage of sintering. However, it inhibited the exaggerated
12. M.F. Ashby, S. Bahk, J. Berk, and D. Turnbull: Progr. Mater. Sci., grain growth during the phase transformation and helped
1980, vol. 25, pp. 1-34.
densification at high temperatures. 13. Y. Imai and T. Miyazaki: Science Reports on the Research Institute, 3. The main contribution of adding fine alumina particles Tohoku University, Tohoku, 1962, vol. 14 (3), pp. 146-55.
14. Y.C. Lu, H.C. Chen, and K.S. Hwang: Advances in Powder Metallurgy is in inhibiting the exaggerated grain growth for iron
and Particulate Materials—1996, compiled by T.M. Cadle and K.S. during the phase transformation, not in retarding the
regu-Narasimhan, Metal Powder Industries Federation, Princeton, NJ, 1996, lar grain growth, which usually occurs at the final stage
vol. 3, pp. 11-143-11-150.
of the sintering. When carbonyl iron powder was replaced 15. A. Gatti: Powder Metall., 1962, vol. 5 (10), pp. 77-86.
by carbonyl nickel powder, which has no allotropic trans- 16. B.N. Singh, P. Cotterill, and M.B. Waldron: Powder Metall., 1969, vol. 12 (2), pp. 157-68.
formation during heating, no sintering improvement
17. R.E. Lawn, F.G. Wilson, and C.D. Desforges: Powder Metall., 1974, was observed.
vol. 17 (4), pp. 196-201.
4. The wet mixing technique gives a more-uniform alumina 18. C. Zener as quoted by C.S. Smith: Trans. AIME, 1942, vol. 175, pp. distribution and results in a higher sintered density than 15-51.
19. J. Japka: Int. J. Powder Metall., 1991, vol. 27 (2), pp. 107-14. that obtained by using the dry ball-milling method.