行政院國家科學委員會專題研究計畫成果報告
有機雜環化合物之光物理及光化學
Photophysics and Photochemistr y of Heterocyclic Compounds
計畫編號: NSC 87-2113-M-002-025 執行期限: 86 年 8 月 1 日至 87 年 7 月 31 日 主持人: 台灣大學化學系 何東英 中文摘要 研究有機雜環化合物之光化學反應 合成了苯乙烯口塞口分及苯乙烯口奎林等化合 物,研究光引發電子轉移反應所產生之陰 離子自由基之親核性加成反應 並且研究 與質子化有關之電子轉移反應。 關鍵字:光引發電子轉移、陰離子自由基、 親核性加成反應、質子化有關之電子轉 移。 Abstr actTo study the photochemistry and photo-physics of the heterocyclic compounds such as styrylthiophene and styrylquinolines. Reverse regioselective photoamination of 2-styrylthiophenes with ammonia in the presence of 1,4-dicyanobenzene (DCB) has been observed to give 1-amino-1-(
p-substitutedphenyl)-2-thienylethane as the only regioselective products. The
protonation dependent electron transfer of the styrylquinoline are also studied.
Keywor ds: photosensitized electron transfer,
radical cations, regioselective amination, protonation dependent electron transfer.
Introduction
Regioselectivity in photochemical reaction system is a topic of current interest.1 The regioselective photochemical amination reactions are useful tool to the synthesis of isoquinolines2 and aporphines3. The photoinduced electron transfer sensitized by electron deficient 1,4-dicyanobenzene (DCB) can produce cation radicals which has been identified by transient absorption study.4 In continuation to our interests in the selective reactions of the photoinduced cation radicals, a series of 2-styrythiophenes 1-5 are synthesized to study the photoamination reaction.
S X S X H2N + NH 3 CH3CN-C 6H6-H 2O X = OCH 3 (1,6 ); CH(CH 3)2 (2,7 ); CH 1-5 6-10 Scheme I hv / DCB
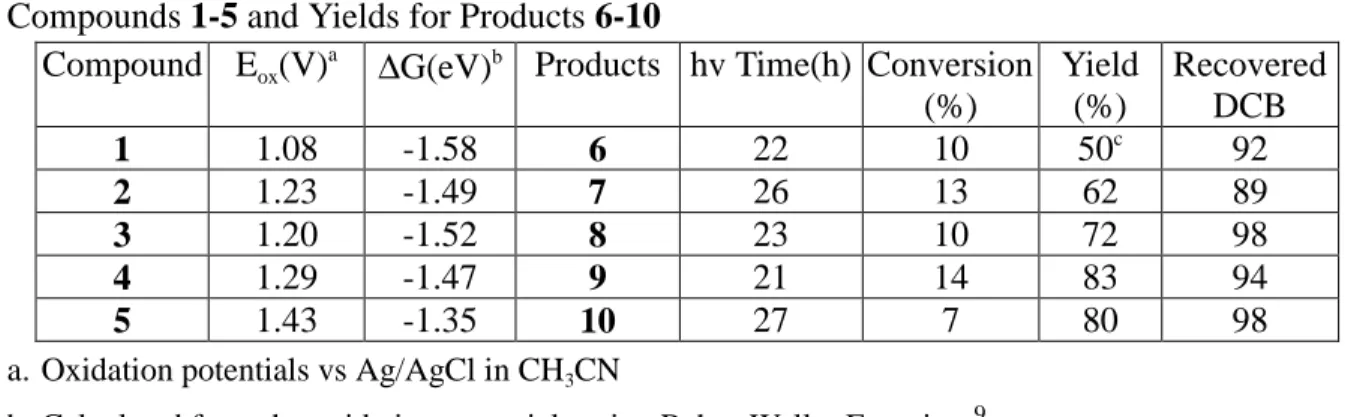
Table 1: Oxidation Potential (Eox) and Free Energy Changes of Electron Transfer (∆G) for
Compounds 1-5 and Yields for Products 6-10 Compound Eox(V)
a ∆
G(eV)b Products hv Time(h) Conversion
(%) Yield (%) Recovered DCB 1 1.08 -1.58 6 22 10 50c 92 2 1.23 -1.49 7 26 13 62 89 3 1.20 -1.52 8 23 10 72 98 4 1.29 -1.47 9 21 14 83 94 5 1.43 -1.35 10 27 7 80 98
a. Oxidation potentials vs Ag/AgCl in CH3CN
b. Calculated from the oxidation potentials using Rehm-Weller Equation.9 c. Isolated as the benzoyl amide.
Results
Direct irradiation of compounds 1-5 with ammonia at 300nm led to the trans-cis isomerization only. When compounds 1-5 were photolyzed with 1,4-dicyanobenzene (DCB) at 300nm in acetonitrile saturated with ammonia, 1-amino-1-(
p-substituted-phenyl)-2-thienyl ethanes (6-10) are isolated as the only regioselective products (Scheme I). By comparing the extinction coefficients and the concentration5 for 1-5 and DCB at 300nm, most (95%) incident light was absorbed by compounds 1-5. The DCB was recovered after irradiation. The isolated yields for the amine adducts 6-10 are based on the recovered starting material. (Table 1)5
Discussion
The negative values of the free energy changes for electron transfer (∆G) calculated from the Rehm and Weller6 equation are all negative which indicated that cation radicals of compounds 1-5 are formed from the electron transfer of the excited states of compounds 1-5 to DCB. The ammonia
attacks the cation radicals of compounds 1-5 to give the aminated cation radicals, which are deprotonated and undergo reduction by DCB anion radicals.
Regioselective nucleophilic addition of photosensitized reaction of compounds 1-5 is observed to afford 1-amino-1-(
p-substitutedphenyl)-2-thienyl ethanes (6-10) as the only regioselective products. The electron-donating effect of the thiophene group is capable of stabilizing and distributing the positive charge to the C-1 position of the cation radicals of compounds
1-5. This effect is stronger than the directing
effect of an alkoxy substituent reported by Yasuda et al.7
Protonation of 4’-(N,N-dimethyl-amino )-2-trans-styrylquinolines will quench the original charge transfer emission and two new emission bands appear. The higher energy fluorescence maximum is independent of solvent polarity and shows structured emission bands in a medium-polar solvent. The lower energy fluorescence maximum is structureless and shows a red-shift in a polar solvent. This is assigned as a charge transfer emission band
of single protonation in the quinoline ring. The relation between the neutral form, the monoprotonation form at the quinoline nitrogen (Q) and the doubly protonated form at both the doubly protonated form at both the aniline and quinoline nitrogen atom (D) can be summarized as Scheme II. The equilibrium between D and Q in the ground state strongly depends on the environment. Greater acidity will favor the formation of D. the equilibrium between D and Q may be determined by the basicity of the solvent medium.
The charge transfer behavior of 4’-
(N,N-dimethylamino)-2-trans-styryl-quinolines is strongly dependent on the acid concentration. The equilibrium between mono and doubly prontonation in the ground state for this styrylquinolines is sensitive to the acidity of the solvent. Excited state deprotonation of this styrylquinoline is observed in aprotic dichloromethane solvent. The excited state deprotonation process can be quenched by introducing protic solvent such as methanol the medium.
References
(1) Ho, T. I. and Chow, Y. L., In Supplement F2: The chemistry of amino, nitroso, nitro and related groups, Patai, S. and
Rappoport, Z., Eds.; John Wiley and Sons: Chichester, 1996; Part 2, Chapter 15.
(2) Yasuda, M.; Kubo, J.; Shima, K.
Heterocycles 1990, 31,1007-1010.
(3) Lewis, F. D.; Reddy, G. D.; Cohen, B. E.
Tetrahedron Letters. 1994, 35, 535.
(4) Takahashi, Y.; Nishioka, N.; Endoh, F.; Ikeda, H.; Miyashi, T. Tetrahedron Letters. 1996, 37, 1841-1844.
(5) The concentration of the compounds 1-5 and DCB are 5×10-3M and 2.5×10-2M respectively. Photolysis is using Rayonet Photochemical Reactor. All the isolated amine adducts gave satisfactory NMR and MS data, for example compound 9:
1 H-NMR (CDCl3) δ 3.07 (dd, J = 14.5, 8.0 Hz, 1H), 3.19 (dd, J = 14.5, 5.1 Hz, 1H), 4.19 (dd, J = 8.0, 5.1 Hz, 1H), 6.80 (d, J = 3.3 Hz, 1H), 6.91 (dd, J = 5.1, 3.3 Hz, 1H), 7.14 (dd, J = 5.1, 1.0 Hz, 1H), 7.2-7.4 (m, 5H); 13 C-NMR (CDCl3) D* D Q Q* S S* hv(A) hv(F) -H+ H+ hv(F) hv(F) hv(A) hv(A) H+ -H+ -H+ N N H H + + (D) N N H + (Q) Schem e II
δ 145, 141.3, 128.4, 127.2, 126.8, 126.4, 125.9, 123.9, 57.6, 40.3; EI-MS (70eV) m/z 204 (M++1, 62), 106 (C7H8N
+
, 100). (6) Rehm, D.; Weller, A. Isr. J. Chem. 1970,
8, 259-71.
(7) Yasuda, M.; Yamashita, T.; Shima, K.; Pac, C. J. Org. Chem. 1987, 52, 753-759.