For Review Only
BIDIRECTIONAL RELATION BETWEEN SCHIZOPHRENIA AND EPILEPSY: A POPULATION-BASED RETROSPECTIVE COHORT
STUDY
Journal: Epilepsia
Manuscript ID: EPI-00543-2011.R1
Manuscript Type: Full length original research paper Date Submitted by the
Author: n/a
Complete List of Authors: Chang, Yu-Tzu; China Medical University and Hospital, Division of pediatric neurology, department of pediatrics
Chen, Pei-Chun; China Medical University and Hospital, Management office for Health Data; China Medical University and Hospital, Department of Public Health
Tsai, I-Ju; China Medical University and Hospital, Management office for Health Data; China Medical University and Hospital, Department of Public Health
Sung, Fung-Chang; China Medical University and Hospital, Department of Public Health; China Medical University and Hospital, Management office for Health Data
Chin, Zheng-Nan; China Medical University and Hospital, Division of Pediatric Neurology, Department of Pediatrics
Kuo, Huang-Tsung; China Medical University and Hospital, Division of Pediatric Neurology, Department of Pediatrics
Tsai, Chang-Hai; China Medical University and Hospital, Division of Pediatric Neurology, Department of Pediatrics; Asia University, Department of Healthcare Administration
Chou, I-Ching; China Medical University and Hospital, Division of Pediatric Neurology, Department of Pediatrics; China Medical University, Graduate Institute of Integrated Medicine, College of Chinese Medicine
Key Words: bidirectional relation, epilepsy, incidence, schizophrenia
For Review Only
BIDIRECTIONAL RELATION BETWEEN SCHIZOPHRENIA AND EPILEPSY: A POPULATION-BASED RETROSPECTIVE
COHORT STUDY
Yu-Tzu Chang1, M.D., Pei-Chun Chen*2, 3, PhD, MSPH, I-Ju Tsai, MS2, 3 , Fung-Chang Sung, PhD, MSPH, Zheng-Nan Chin1, M.D., Huang-Tsung Kuo1, M.D., Ph.D., Chang-Hai Tsai1,4 , M.D., Ph.D. and I-Ching Chou*1,5, M.D.
1Division of Pediatric Neurology, Department of Pediatrics; 2
Management office for Health Data;
3
Department of Public Health, China Medical University and Hospital, Taichung,
Taiwan
4
Department of Healthcare Administration, Asia University, Taichung, Taiwan 5
Graduate Institute of Integrated Medicine, China Medical University College of
Chinese Medicine, Taichung, Taiwan
Reprints and Correspondence to: I-Ching Chou, M.D.
Associate Professor, Director and Attending Physician
1. Division of Pediatric Neurology, Department of Pediatrics, Children’s Medical Center, China Medical University Hospital
2. Graduate Institute of Integrated Medicine, College of Chinese Medicine, China Medical University, Taichung, Taiwan
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
Address: No.2, Yuh-Der Road, North District, Taichung 40447, TaiwanTaichung 40447, Taiwan
TEL: +886-4-22052121 extension 2066
FAX: +886-4-22032798
E-mail address: iching@mail.cmuh.org.tw
Cc e-mail: iching@mail.cmu.edu.tw
Co-correspondence to: Pei-Chun Chen, PhD MSPH
Department of Public Health, China Medical University and Hospital,
Address: No. 91, Hsueh-Shih Road, Taichung 40442, Taiwan
TEL: 886-4-2205-3366, ext 6119
FAX: 886-4-2205-4070
E-mail address: tw.mohd@gmail.com
Running title: SCHIZOPHRENIA AND EPILEPSY 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
Number of text pages: 28Numbers of words: 4687
Number of proposed pages of figures: 1/2
Number of proposed pages of tables: 1/2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
SUMMARY Purpose:
Schizophrenia and epilepsy may share a mutual susceptibility. This study examined
the bidirectional relation between the two disorders.
Methods:
We used claims data obtained from the Taiwan National Health Insurance database
to conduct retrospective cohort analyses. Analysis 1 compared 5195 patients with
incident schizophrenia diagnosed in1999-2008 with 20776 controls without the disease
randomly selected during the same period, frequency matched with sex and age. Analysis
2 comprised a similar method to compare 11527 patients with newly diagnosed epilepsy
with 46032 randomly selected sex- and age-matched controls. At the end of 2008,
analysis 1 measured the incidence and risk of developing epilepsy and analysis 2
measured the incidence and risk of developing schizophrenia.
Key findings:
In analysis 1, the incidence of epilepsy was higher in the schizophrenia cohort than
in the non-schizophrenia cohort (6.99 vs. 1.19 per 1,000 person-years) with an adjusted
hazard ratio [aHR] of 5.88 (95% confidence interval [CI] = 4.71-7.36) for schizophrenia
patients. In analysis 2, the incidence of schizophrenia was higher in the epilepsy cohort
than in the non-epilepsy comparison cohort (3.53 vs. 0.46 per 1,000 person-years) with
an aHR of 7.65 (95% CI = 6.04-9.69) for epilepsy patients. The effect of schizophrenia
on subsequent epilepsy was greater for women, but the association between epilepsy and
elevated incidence of schizophrenia was more pronounced in men.
Significance: 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
We found a strong bidirectional relation between schizophrenia and epilepsy. These
two conditions may share common causes. Further studies on the mechanism are
required.
Keywords: bidirectional relation , epilepsy, incidence, schizophrenia
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
INTRODUCTION
Epidemiological studies using clinical data and case-control designs have
demonstrated that psychosis is prevalent among patients with epilepsy (Lindsay, et al.
1979; Matsuura, et al. 2004; Mendez, et al. 1993; Perez & Trimble 1980; Slater, et al.
1963; Stefansson, et al. 1998). On the other hand, studies on psychiatric illness have also
shown a strong relationship between schizophrenia and epilepsy (Cascella, et al. 2009;
Qin, et al. 2005). These comorbid relationships imply a shared susceptibility between
schizophrenia and epilepsy, possibly due to genetic, environmental or neurobiological
factors (Cascella, et al. 2009).
Recent studies have found that psychiatric pathology could be a risk factor for the
development of epilepsy in children (Hesdorffer, et al. 2004; McAfee, et al. 2007). Both
psychosis and epilepsy are symptoms of an underlying neuropathological or
physiological dysfunction (Sachdev, 1998). Neurodevelopmental abnormalities such as
cortical dysgenesis or diffusive brain damage may cause both epilepsy and psychosis.
Besides, genetics may also play a role associated with both schizophrenia and epilepsy. A
case in point is the relationship between the Leucine-rich glioma inactivated 1(LGI1)
gene and the CNTNAP2 gene. LGI1 has been involved in neurodevelopment and
associated with both febrile seizures and schizophrenia (Cascella, et al. 2009, Scheel, et
al. 2002). CNTNAP2 is also a strong candidate gene for epilepsy and schizophrenia
(Friedman, et al. 2010). Both neuropathologic and genetic findings suggest that
neurodevelopment and its abnormalities may represent a framework associated with the
co-occurrence of epilepsy and schizophrenia.
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
A number of recent studies have demonstrated a bidirectional relationship between
depression, mood disorder and epilepsy (Forsgren, et al. 1990; Hesdorffer, et al. 2000;
Hesdorffer, et al. 2006; Kanner 2008b). However, to the best of our knowledge, no study
has investigated the bidirectional relationship between schizophrenia and epilepsy. In this
study, we used a representative data set to perform a two-way population-based
retrospective cohort study to determine the risk of developing schizophrenia among
patients with epilepsy and the risk of developing epilepsy among patients with
schizophrenia
METHODS Data source
In March 1995, the Taiwan Department of Health integrated 13 health insurance
schemes into a universal insurance program, and by 1999, approximately 99% of the 23
million people in Taiwan were enrolled in the National Health Insurance (NHI) program
(2009.06.05). 2010). The National Health Insurance system is a government-run program
that has contracted with more than 90% of health care facilities in Taiwan since it was
implemented in 1995 (Lu, 2003). Data used in this study were a sub-dataset of the
National Health Insurance Research Database (NHIRD), which contains longitudinal
claims data for a randomly selected cohort of one million people from all insured
beneficiaries. We used 3 different data files: registry of beneficiaries, inpatient claims,
and ambulatory care claims. These data files are linkable through an encrypted but unique
personal identification number and thus provide patient-level information on
demographic characteristics and medical history. The scrambled identifications secured
the patients confidentiality. This study is thus exempted from ethics review.
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
Study design and subjects
We conducted two analyses to evaluate the bidirectional relation between epilepsy
and schizophrenia. The same procedures were used in each analysis to select study
subjects (Figure 1). In analysis 1, we identified patients with schizophrenia (International
Classification of Diseases, Ninth Revision [ICD-9], Clinical Modification, code 295) and
a control group of individuals without schizophrenia. In analysis 2, we identified patients
with epilepsy (ICD-9 code 345) and a control group of individuals without the history of
epilepsy. In both sets of analyses, subjects in the patient groups were selected if they had
an initial ambulatory and/or inpatient claim for schizophrenia (analysis 1) or epilepsy
(analysis 2) during the study period 1999-2008. We defined the date of diagnosis as the
index date for each patient. The comparison groups comprised randomly selected age-
and sex-matched individuals without a history of schizophrenia (analysis 1) or epilepsy
(analysis 2) before 2008. The control to case ratio was 4 to 1. In analysis 1, we excluded
subjects who had been diagnosed with epilepsy before their diagnosis of schizophrenia to
ascertain the temporal association between schizophrenia and the subsequent occurrence
of epilepsy. Based on the same reason, patients with a diagnosis of schizophrenia before
diagnosis of epilepsy were excluded from analysis 2.
All subjects were followed from the index date until the onset of epilepsy for
analysis 1 or schizophrenia for analysis 2, or the end of follow-up by December 31, 2008,
or being censored for the termination of insurance coverage, loss to follow-up, or death.
Statistical analysis
SAS (version 9.1 for windows; SAS Institute Inc., Cary, NC, USA) was used for all
statistical analyses. For both sets of analyses, differences in demographic characteristics
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
between the patient group and the comparison group were examined using the Chi-square
test for categorical variables and the Wilcoxon two-sample test for continuous variables.
City districts and townships within which subjects were registered for insurance purposes
were grouped into 4 levels of urbanization based on population density (people/km2). Level 1 indicates the most urbanized area and level 4 indicates the least urbanized.
Baseline comparisons also included proportion of the elderly, proportion of agriculture
workers, and the number of physicians per 100,000 people in the residential area (Liu,
2006). For analysis 1, we assessed the overall and sex-specific incidence of epilepsy for
patients with schizophrenia and for subjects in the comparison group. To investigate the
risk of developing epilepsy associated with schizophrenia, we used Cox proportional
hazard regression models to estimate the hazard ratios (HRs) of developing epilepsy in
patients with schizophrenia compared with the comparison group. We adjusted for level
of urbanization in the multivariate models because there are regional differences in
accessibility of medical care in Taiwan and the descriptive analyses showed that
urbanization status was substantially associated with schizophrenia and epilepsy. We
used the same procedures for data analyses in analysis 2 as we did in analysis 1. In
analysis 2, the Cox proportional hazard regression models were used to assess HRs of
incidence of schizophrenia for patients with epilepsy compared with the non-epilepsy
comparison group. All significant levels were set at 0.05.
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
RESULTS
Analysis 1: schizophrenia and subsequent risk of epilepsy
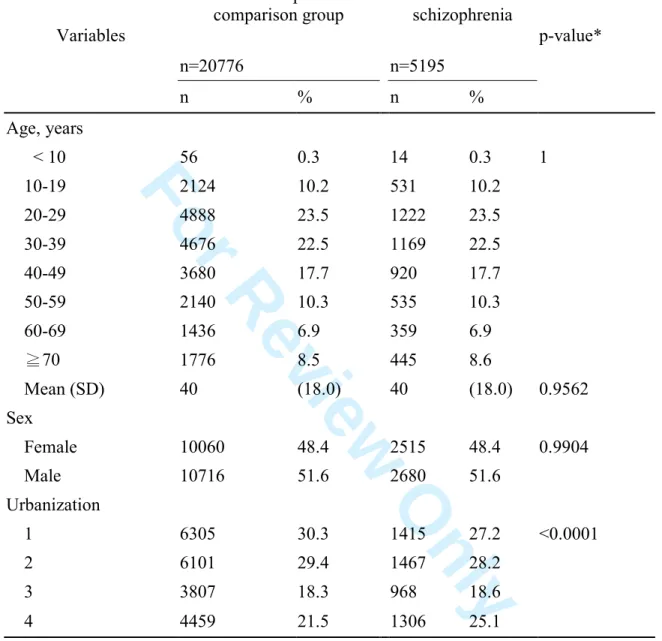
During 1999-2008, we identified 5195 patients with schizophrenia and 20776
subjects in the non- schizophrenia comparison group (Table 1). Almost half of patients
with schizophrenia were aged 20-39 years; and 48.4% were female patients. Age
distribution and the sex ratio in the comparison group were the same as those in the
schizophrenia group. Patients with schizophrenia were more likely to reside or work in
less urbanized areas than individuals in the comparison group (level 4, i.e., the least
urbanized, 25.1% vs. 21.5%).
The median follow-up was 5.2 years in the schizophrenia group and 5.5 years in the
comparison group. The incidence of epilepsy was higher in the schizophrenia group (6.99
vs. 1.19 per 1000 person-years) (Table 2). The Cox proportional hazard regression model
revealed that patients with schizophrenia were 5.88 times more likely to develop epilepsy
(95% CI = 4.71-7.36) after controlling for age, sex and urbanization level.
The incidence of epilepsy was higher in men than in women in both schizophrenia
and non-schizophrenia comparison group (Table 2). Schizophrenia was associated
increased incidences of epilepsy in both sexes, with a greater relative risk estimate for
women (adjusted HR=6.48, 95% CI=4.63-9.06) than for men (adjusted HR=5.42, 95%
CI=4.02-7.32). But the sex difference was not statistically significant (test for interaction
between sex and schizophrenia, p = 0.49).
Analysis 2: epilepsy and subsequent risk of schizophrenia
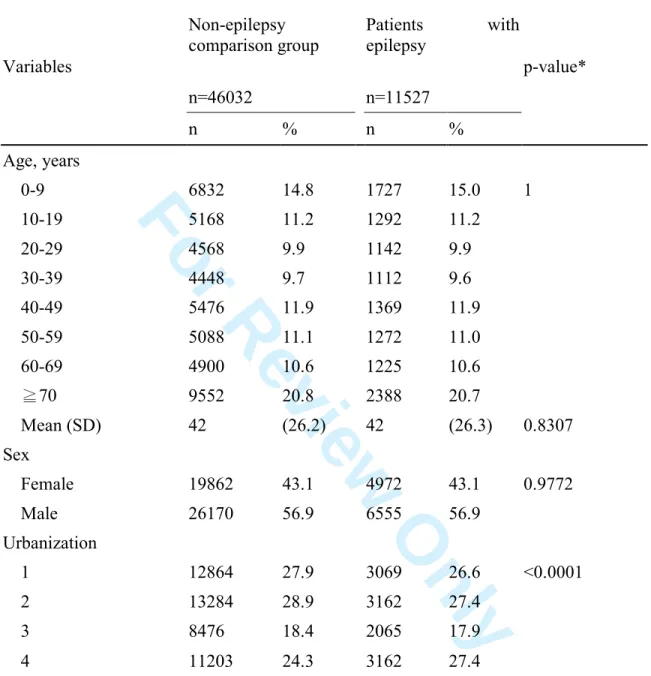
During the study period, we identified 11527 patients with epilepsy and 46032
subjects in the non-epilepsy comparison group (Table 3). Among patients with epilepsy,
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
20.7% were ≥ 70 years of age; 15.0% were < 10 years old; and the remaining patients
distributed in other age groups ranged 9.6%-11.9%. Forty three percent of patients were
women. Age distribution and the sex ratio of the comparison group were the same as
those in the epilepsy group after matching the two variables. Patients with epilepsy were
more likely to reside or work in less urbanized areas (level 4, i.e., the least urbanized,
27.4% vs. 24.3%), relative to the comparison group.
The median follow-up was 4.6 years in the epilepsy group and 5.5 years in the
comparison group. The incidence of schizophrenia was higher in the epilepsy group (3.53
vs. 0.46 per 1000 person-years) (Table 4). The Cox proportional hazard regression model
revealed that patients with epilepsy were 7.65 times more likely to develop schizophrenia
than patients in the comparison group (95% CI=6.04-9.69) after controlling for age, sex
and urbanization level. The risk of schizophrenia for patients with epilepsy was greater in
men than in women (HR [95% CI], 8.62 [6.28-11.83] for men and 6.49 [4.55-9.27] for
women). But the sex difference in the association was not statistically significant (p for
sex-epilepsy interaction = 0.22).
DISCUSSION
In this study, we discovered that patients with epilepsy are at an increased risk of
developing schizophrenia (adjusted hazard ratio [HR] = 7.65, 95% CI =6.04-9.69). This
result is consistent with the finding of a Danish population-based study that followed a
large cohort selected from longitudinal registers (Qin et al, 2005). Another case-control
study also suggests that patients with epilepsy is significantly associated with developing
schizophrenia-like illness (Stefansson, et al. 1998).
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
We observed that the incidence of schizophrenia was slightly higher in men than in
women among patients with epilepsy. The estimated HR for developing schizophrenia
in relation to epilepsy was also greater for men than for women. However, the test for
interaction between sex and epilepsy revealed that the relationship between epilepsy and
subsequent schizophrenia was not statistically different between men and women. The
results should be interpreted cautiously because failure to find statistical evidence may be
due to insufficient power to detect moderate interactions. Results of the Danish
longitudinal registers study (Qin, et al. 2005) also revealed no significant difference in
the schizophrenia incidences between men and women (test of interaction, p = 0.31), but
the sex-specific HRs was not provided in their report. Adachi et al. also have reported in
a case-control study that sex was not a predictor of interictal psychosis in patients with
epilepsy (Adachi, et al. 2000; Qin, et al. 2005). In the systematic reviews, McGrath
(McGrath 2006) found the incidence of schizophrenia was higher in men than in women.
This discrepancy may be accounted for by differences in methodology.
On the other hand, few studies have investigated whether schizophrenia is a risk
factor for epilepsy. Our study with the bidirectional analyses shows that patients with
schizophrenia are at an increased risk of developing epilepsy. A 28-year follow-up study
in northern Finland revealed that epilepsy was strongly associated with schizophrenia
(Makikyro, et al. 1998). A retrospective cohort study compared pediatric patients (age 6
to 17 years) with psychiatric diseases with patients without the diseases. The researchers
found that the risk of seizure disorders was 5-fold higher among children with psychiatric
diseases (McAfee, et al. 2007). In our study, we found that the risk of epilepsy was
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
almost 6-fold higher among children and adults with schizophrenia (HR = 5.88, 95% CI
=4.71-7.36).
In this study, mean age of patients newly diagnosed with schizophrenia and epilepsy
was 40 years and 42 years, respectively. However, it is notable that the onset age was not
normally distributed in both analyses 1 and 2. Almost half of patients newly diagnosed
with schizophrenia were aged 20-39 years. Of patients with epilepsy, more patients were
newly diagnosed at ≥ 70 years and < 9 years of age. Evidence has shown that the onset
age of schizophrenia is diverse and differs by sex in other studies. Rajji et al (Rajji, et al.
2009) found that the incidence of schizophrenia is the highest at 10-25 years old for men
and at 25-35 years old for women. The severity of the disease process is associated with
different ages at onset. Other peaks of onset ages appear in women aged 40 years and
older or after 60 years of age.
Abnormal neurodevelopment plays a role in both schizophrenia and epilepsy (Crow,
et al. 1989; Kanner, 2008c; McAfee, et al. 2007). Several studies have reported that
“alien tissue” such as harmartomas and focal dysplasia or medial temporal sclerosis is
more prevalent for patients of temporal lobe epilepsy with comorbidity of
schizophrenia-like psychosis (Falkai, et al. 2000; Roberts, et al. 1990; Taylor, 1975).
These investigators found that the majority of lesions are embryonic or perinatal in origin,
which supports the role of neurodevelopmental abnormalities in schizophrenia.
Abnormalities in neurodevelopment including those that affect neuronal migration, such
as lissencephaly and abnormal lissencephaly gene expression, reflect alterations that
predispose individuals to epileptogenesis, especially in medial temporal regions, and to
schizophrenia (Flint & Kriegstein, 1997).
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
Recent studies have revealed several candidate susceptibility genes for both
schizophrenia and epilepsy (Tan, et al. 2004). The LGI1 gene plays a role in migration
development of the central nervous system, which is related to temporal lobe epilepsy
and febrile seizures (Cascella, et al. 2009; Scheel, et al. 2002). It also plays a role in
regulating glutamatergic synaptic transmission, and is involved in the pathophysiology of
schizophrenia (Fukata, et al. 2006). Another recent study on the CNTNAP2 gene found
that it was associated with both epilepsy and schizophrenia (Friedman, et al. 2008).
CNTNAP2 plays a role in the organization of myelinated axons and may disrupt
neuroblast migration (Poliak, et al. 1999; Poliak, et al. 2003; Strauss, et al. 2006). Results
from both neuropathologic and genetic studies suggest that neurodevelopment and its
abnormalities might represent an etiological framework leading to the co-occurrence of
epilepsy and schizophrenia.
Our study is the first to simultaneously examine whether patients with schizophrenia
are at higher risk of developing epilepsy and whether patients with epilepsy are at higher
risk of developing schizophrenia. This study has demonstrated only the development of
schizophrenia or epilepsy is associated with each disorder; the causal relationship
remains unclear. The development of schizophrenia or epilepsy is likely associated with a
mutual etiological factor. Our further analyses showed that patients with epilepsy also
were more likely to have diabetes and TBI than those without epilepsy (data not shown).
Several limitations of our study must be addressed here. First, the accuracy of
medical coding in the claims data may affect the data validity. Schizophrenia and
epilepsy are considered as stigmata in Taiwan. The insurance system has mechanisms to
monitor the insurance claims. Thus, the diagnosis validity may compromise little our
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
findings. Second, this study may include schizophrenia or epilepsy patients who have not
been diagnosed in the comparison group. This sampling bias may slightly over estimate
the incidence in the comparison, not over 5% chance. Third, other medical conditions
may also associate with schizophrenia or other psychosis (Fujii & Ahmed 2001; Hsu, et
al. 2011). The premorbid medical or other characteristics that antedated the diagnoses,
such as traumatic brain injury, asphyxia, were not included in our study might be related
to develop disorders of schizophrenia or epilepsy. Forth, several recent studies have
reported bidirectional associations between psychiatric disorders and epilepsy, addressing
mainly the relationships between depression disorders and mood disorders (Kanner, 2007;
Kanner, 2008a; Kanner, 2008b; Kanner, 2009). Those studies provided evidence that
neurotransmitters, such as serotonin, norepinephrine and/or dopamine, play a role in
regulating the pathophysiologic relationship between psychiatric depression/mood
disorders and epilepsy (Kanner, 2008a; Kanner, 2008b). We were unable to observe the
etiological association between schizophrenia and epilepsy. This is because the
information on neurotransmitter measures is not available from the claims data for most
of the study subjects.
Conclusion
Despite these limitations, our data suggest a significant correlation between
schizophrenia and epilepsy. There is evidence of a complex bidirectional relationship
between schizophrenia and epilepsy; that is, patients with epilepsy are at greater risk of
developing schizophrenia and patients with schizophrenia are at higher risk of developing
epilepsy. Such a relationship may be explained by the existence of common pathogenic
mechanisms in both conditions, including changes in neuroanatomy, neural migration
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
dysfunction, genetic susceptibility, or environmental factors. Further studies on the
mechanism are warranted.
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
ACKNOWLEDGEMENTS
This study was supported in part by the Executive Yuan National Science Council
(grant number NSC 98-2621-M-039 -001), Taiwan Department of Health Clinical Trial
and Research Center of Excellence (grant number DOH100-TD-B-111-004) and Cancer
Research Center of Excellence (grant number DOH100-TD-C-111-005), and the China
Medical University Hospital (grant number 1MS1 and DMR-99-054).
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
DISCLOSURE OF CONFLICTS OF INTEREST
None of the authors has any conflict of interest to disclose.
We confirm that we have read the Journal’s position on issues involved in ethical
publication and affirm that this report is consistent with those guidelines.
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
REFERENCES
2009.06.05). Npu. (2010) Accessed at
http://www.nhi.gov.tw/webdata/AttachFiles/Attach_13787_1_NationalHealthInsu ranceinTaiwan2009.pdf
Adachi N, Matsuura M, Okubo Y, Oana Y, Takei N, Kato M, Hara T, Onuma T. (2000)
Predictive variables of interictal psychosis in epilepsy. Neurology 55:1310-1314.
Cascella NG, Schretlen DJ, Sawa A. (2009) Schizophrenia and epilepsy: is there a shared
susceptibility? Neurosci Res 63:227-235.
Crow TJ, Ball J, Bloom SR, Brown R, Bruton CJ, Colter N, Frith CD, Johnstone EC,
Owens DG, Roberts GW. (1989) Schizophrenia as an anomaly of development of
cerebral asymmetry. A postmortem study and a proposal concerning the genetic
basis of the disease. Arch Gen Psychiatry 46:1145-1150.
Falkai P, Schneider-Axmann T, Honer WG. (2000) Entorhinal cortex pre-alpha cell
clusters in schizophrenia: quantitative evidence of a developmental abnormality.
Biol Psychiatry 47:937-943.
Flint AC, Kriegstein AR. (1997) Mechanisms underlying neuronal migration disorders
and epilepsy. Curr Opin Neurol 10:92-97.
Forsgren L, Sidenvall R, Blomquist HK, Heijbel J, Nystrom L. (1990) An incident
case-referent study of febrile convulsions in children: genetical and social aspects.
Neuropediatrics 21:153-159.
Friedman JI, Vrijenhoek T, Markx S, Janssen IM, van der Vliet WA, Faas BH, Knoers
NV, Cahn W, Kahn RS, Edelmann L, Davis KL, Silverman JM, Brunner HG,
Geurts van Kessel A, Wijmenga C, Ophoff RA, Veltman JA. (2010) CNTNAP2
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
gene dosage variation is associated with schizophrenia and epilepsy. Mol
Psychiatry 15:1121.
Friedman JI, Vrijenhoek T, Markx S, Janssen IM, van der Vliet WA, Faas BH, Knoers
NV, Cahn W, Kahn RS, Edelmann L, Davis KL, Silverman JM, Brunner HG, van
Kessel AG, Wijmenga C, Ophoff RA, Veltman JA. (2008) CNTNAP2 gene
dosage variation is associated with schizophrenia and epilepsy. Mol Psychiatry
13:261-266.
Fujii DE, Ahmed I. (2001) Risk factors in psychosis secondary to traumatic brain injury.
J Neuropsychiatry Clin Neurosci 13:61-69.
Fukata Y, Adesnik H, Iwanaga T, Bredt DS, Nicoll RA, Fukata M. (2006)
Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic
transmission. Science 313:1792-1795.
Hesdorffer DC, Hauser WA, Annegers JF, Cascino G. (2000) Major depression is a risk
factor for seizures in older adults. Ann Neurol 47:246-249.
Hesdorffer DC, Hauser WA, Olafsson E, Ludvigsson P, Kjartansson O. (2006)
Depression and suicide attempt as risk factors for incident unprovoked seizures.
Ann Neurol 59:35-41.
Hesdorffer DC, Ludvigsson P, Olafsson E, Gudmundsson G, Kjartansson O, Hauser WA.
(2004) ADHD as a risk factor for incident unprovoked seizures and epilepsy in
children. Arch Gen Psychiatry 61:731-736.
Hsu JH, Chien IC, Lin CH, Chou YJ, Chou P. (2011) Incidence of diabetes in patients
with schizophrenia: a population-based study. Can J Psychiatry 56:19-26.
Kanner AM. (2007) Epilepsy and mood disorders. Epilepsia 48 Suppl 9:20-22.
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
Kanner AM. (2008a) Depression in epilepsy: a complex relation with unexpected
consequences. Curr Opin Neurol 21:190-194.
Kanner AM. (2008b) Mood disorder and epilepsy: a neurobiologic perspective of their
relationship. Dialogues Clin Neurosci 10:39-45.
Kanner AM. (2008c) Psychiatric comorbidity in children with epilepsy ... or is it:
epilepsy comorbidity in children with psychiatric disorders? Epilepsy Curr
8:10-12.
Kanner AM. (2009) Depression and epilepsy: a review of multiple facets of their close
relation. Neurol Clin 27:865-880.
Lindsay J, Ounsted C, Richards P. (1979) Long-term outcome in children with temporal
lobe seizures. III: Psychiatric aspects in childhood and adult life. Dev Med Child
Neurol 21:630-636.
Liu CY, Hung YT, Chuang YL, Chen YJ, Weng WS, Liu JS, Liang KY. (2006)
Incorporating development stratification of Taiwan townships into sampling design
of large scale health interview survey . J Health Manag 4:1-22.
Lu JF, Hsiao WC. (2003) Does universal health insurance make health care unaffordable?
Lessons from Taiwan. Health Aff 22:77-88.
Makikyro T, Karvonen JT, Hakko H, Nieminen P, Joukamaa M, Isohanni M, Jones P,
Jarvelin MR. (1998) Comorbidity of hospital-treated psychiatric and physical
disorders with special reference to schizophrenia: a 28 year follow-up of the 1966
northern Finland general population birth cohort. Public Health 112:221-228.
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
Matsuura M, Adachi N, Oana Y, Okubo Y, Kato M, Nakano T, Takei N. (2004) A
polydiagnostic and dimensional comparison of epileptic psychoses and
schizophrenia spectrum disorders. Schizophr Res 69:189-201.
McAfee AT, Chilcott KE, Johannes CB, Hornbuckle K, Hauser WA, Walker AM. (2007)
The incidence of first provoked and unprovoked seizure in pediatric patients with
and without psychiatric diagnoses. Epilepsia 48:1075-1082.
McGrath JJ. (2006) Variations in the incidence of schizophrenia: data versus dogma.
Schizophr Bull 32:195-197.
Mendez MF, Grau R, Doss RC, Taylor JL. (1993) Schizophrenia in epilepsy: seizure and
psychosis variables. Neurology 43:1073-1077.
Perez MM, Trimble MR. (1980) Epileptic psychosis--diagnostic comparison with process
schizophrenia. Br J Psychiatry 137:245-249.
Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P,
Peles E. (1999) Caspr2, a new member of the neurexin superfamily, is localized at
the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron
24:1037-1047.
Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X,
Chiu SY, Shrager P, Furley AJ, Peles E. (2003) Juxtaparanodal clustering of
Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J
Cell Biol 162:1149-1160.
Qin P, Xu H, Laursen TM, Vestergaard M, Mortensen PB. (2005) Risk for schizophrenia
and schizophrenia-like psychosis among patients with epilepsy: population based
cohort study. BMJ 331:23. 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
Rajji TK, Ismail Z, Mulsant BH. (2009) Age at onset and cognition in schizophrenia:
meta-analysis. Br J Psychiatry 195:286-293.
Roberts GW, Done DJ, Bruton C, Crow TJ. (1990) A "mock up" of schizophrenia:
temporal lobe epilepsy and schizophrenia-like psychosis. Biol Psychiatry
28:127-143.
Sachdev P. (1998) Schizophrenia-like psychosis and epilepsy: the status of the
association. Am J Psychiatry 155:325-336.
Scheel H, Tomiuk S, Hofmann K. (2002) A common protein interaction domain links
two recently identified epilepsy genes. Hum Mol Genet 11:1757-1762.
Slater E, Beard AW, Glithero E. (1963) The schizophrenialike psychoses of epilepsy. Br
J Psychiatry 109:95-150.
Stefansson SB, Olafsson E, Hauser WA. (1998) Psychiatric morbidity in epilepsy: a case
controlled study of adults receiving disability benefits. J Neurol Neurosurg
Psychiatry 64:238-241.
Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM,
Stephan DA, Morton DH. (2006) Recessive symptomatic focal epilepsy and
mutant contactin-associated protein-like 2. N Engl J Med 354:1370-1377.
Tan NC, Mulley JC, Berkovic SF. (2004) Genetic association studies in epilepsy: "the
truth is out there". Epilepsia 45:1429-1442.
Taylor DC. (1975) Factors influencing the occurrence of schizophrenia-like psychosis in
patients with temporal lobe epilepsy. Psychol Med 5:249-254.
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
FIGURE LENGENDS:
Figure 1. Flow chart showing selection of study subjects.
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
Table 1. Demographical characteristics of schizophrenia patients and the non-schizophrenia comparison group
Variables Non-schizophrenia comparison group Patients with schizophrenia p-value* n=20776 n=5195 n % n % Age, years < 10 56 0.3 14 0.3 1 10-19 2124 10.2 531 10.2 20-29 4888 23.5 1222 23.5 30-39 4676 22.5 1169 22.5 40-49 3680 17.7 920 17.7 50-59 2140 10.3 535 10.3 60-69 1436 6.9 359 6.9 ≧70 1776 8.5 445 8.6 Mean (SD) 40 (18.0) 40 (18.0) 0.9562 Sex Female 10060 48.4 2515 48.4 0.9904 Male 10716 51.6 2680 51.6 Urbanization 1 6305 30.3 1415 27.2 <0.0001 2 6101 29.4 1467 28.2 3 3807 18.3 968 18.6 4 4459 21.5 1306 25.1
*Chi-square test except for the p-value of mean age was from Wilcoxon two sample test
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
26
Table 2. Hazard ratios for incidence of epilepsy in relation to schizophrenia
Non-schizophrenia comparison
group Patients with Schizophrenia
Hazard ratio and 95% CI
(Patients with schizophrenia vs. comparison group)
Cases PY Incidence† Cases PY Incidence† Unadjusted adjusted#
All 134 112147 1.19 185 26461 6.99 5.83 (4.67-7.29) 5.88 (4.71-7.36)
Sex
Women 57 53848 1.06 87 12793 6.80 6.43 (4.60-8.98) 6.48 (4.63-9.06)
Men 77 58298 1.32 98 13668 7.17 5.39 (4.00-7.27) 5.42 (4.02-7.32)
#
Adjusted for age, sex, urbanization level PY: person-years at risk
† per1,000 person-years 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55
For Review Only
Table 3. Demographical characteristics of epilepsy patients and the non-epilepsy comparison group Variables Non-epilepsy comparison group Patients with epilepsy p-value* n=46032 n=11527 n % n % Age, years 0-9 6832 14.8 1727 15.0 1 10-19 5168 11.2 1292 11.2 20-29 4568 9.9 1142 9.9 30-39 4448 9.7 1112 9.6 40-49 5476 11.9 1369 11.9 50-59 5088 11.1 1272 11.0 60-69 4900 10.6 1225 10.6 ≧70 9552 20.8 2388 20.7 Mean (SD) 42 (26.2) 42 (26.3) 0.8307 Sex Female 19862 43.1 4972 43.1 0.9772 Male 26170 56.9 6555 56.9 Urbanization 1 12864 27.9 3069 26.6 <0.0001 2 13284 28.9 3162 27.4 3 8476 18.4 2065 17.9 4 11203 24.3 3162 27.4
*Chi-square test except for the p-value of mean age was from Wilcoxon two sample test
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
28
Table 4. Hazard ratios for incidence of schizophrenia in relation to epilepsy
Non-epilepsy comparison
group Patients with epilepsy
Hazard ratio and 95% CI
(Patients with epilepsy vs. comparison group)
Cases PY Incidence† Cases PY Incidence† Unadjusted adjusted#
All 111 240567 0.46 192 54444 3.53 7.62 (6.03-9.62) 7.65 (6.04-9.69)
Sex
Women 52 105226 0.49 75 24096 3.11 6.34 (4.45-9.03) 6.49 (4.55-9.27)
Men 59 135340 0.44 117 30348 3.86 8.75 (6.40-11.97) 8.62 (6.28-11.83)
#
Adjusted for age, sex, urbanization level PY: person-years at risk
† per1,000 person-years 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55