The effect of high and low frequency electroacupuncture in pain after
lower abdominal surgery
Jaung-Geng Lin
a,b, Ming-Wu Lo
b, Yeong-Ray Wen
c, Ching-Liang Hsieh
d,
Shen-Kou Tsai
e, Wei-Zen Sun
e,*
aAcupuncture Research Center, China Medical College, Taichung, Taiwan, ROC bInstitute of Chinese Medical Sciences, China Medical College, Taichung, Taiwan, ROC cDepartment of Anesthesiology, Shin-Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan, ROC dInternal Medicine of Chinese Medicine Department, China Medical College Hospital, Taichung, Taiwan, ROC e
Department of Anesthesiology, National Taiwan University Hospital College of Medicine, National Taiwan University, Taiwan, ROC Received 8 February 2002; accepted 26 June 2002
Abstract
In the present study, we examined the effects of preoperative electroacupuncture (EA) at classical bilateral acupuncture points (Zusanli, also known as ST-36) on postoperative pain and opioid-related side effects. One hundred healthy consenting women undergoing lower abdominal surgery were randomly assigned to four treatment regimens: Group I ðn ¼ 25Þ, control; Group II ðn ¼ 25Þ, sham-EA (needle insertion without electrical stimulation); Group III ðn ¼ 25Þ, low-EA (2 Hz of electrical stimulation); and Group IV ðn ¼ 25Þ, high-EA (100 Hz of electrical stimulation). EA groups received needle insertion with or without electrical stimulation 20 min prior to anesthesia. All patients received patient-controlled analgesia (PCA) of morphine postoperation. Postoperative pain was evaluated by recording (1) the time of the first required analgesic, (2) the number of PCA demands, (3) the total amount of morphine required by PCA, and (4) patients’ VAS pain score. We found that the time of first analgesic requested was 10, 18, 28, and 28 min in the control, sham-, low-, and high-EA groups, respectively. During the first 24 h, the total amount of morphine required was decreased by 21, 43 and 61% in the sham-, low- and high-EA groups, respectively. The incidence of nausea and dizziness during the first 24 h after surgery was significantly reduced in both the low-EA and high-EA groups compared with the control and sham-EA groups. We also found that sham-EA exerts a beneficial effect with respect to its pain relieving quality but not the side effect profiles. Our findings demonstrates that preoperative treatment with low-EA and high-EA can reduce postoperative analgesic requirements and associated side effects in patients undergoing lower abdominal surgery. q 2002 Interna-tional Association for the Study of Pain. Published by Elsevier Science B.V. All rights reserved.
Keywords: Electroacupuncture; Postoperative pain; Patient-controlled analgesia; Visual analogue scale
1. Introduction
Acupuncture, the practice of inserting needles into the skin and deeper tissues along ‘meridians’ to balance flow of bodily ‘energy’ or ‘Xi’, has been widely used in China since 2500 BC (Wu, 1996). Though its role in various medi-cal conditions remains largely controversial, recent evidence supports the worldwide use of acupuncture to relieve pain in clinical practice. Numerous studies have found that acupuncture activates multiple neurophysiologi-cal interactions, and thereby decreases nociceptive responses in animals receiving painful thermal, chemical, and electrical stimulations (reviewed by Mayer, 2000).
Manual or electrical stimulation through needles induces a particular pattern of afferent activity in peripheral nerves, mainly Ab, Ad and possibly C-fibers. This excitation leads to activation of the endogenous pain inhibition system through multiple neuronal pathways (Basbaum and Field, 1984; Kaufman et al., 1984; Andersson and Lundeberg, 1995). One of the main consequences of acupuncture is the release of endogenous opioids, including b-endorphins, enkephalins, dynorphins, and endomorphins-1 (Pomeranz and Chiu, 1976; Sjolund et al., 1977; He, 1987; Wang et al., 1990a,b; Han et al., 1999), as well as non-opioid substances such as serotonin, norepinephrine, and possibly GABA (Cheng and Pomeranz, 1981; Pomeranz, 1996; Sandkuehler et al., 1997). In addition, animal and human studies have shown that diffuse noxious inhibitory controls (DNIC) may also be involved in underlying acupuncture
0304-3959/02/$20.00 q 2002 International Association for the Study of Pain. Published by Elsevier Science B.V. All rights reserved. PII: S 0 3 0 4 - 3 9 5 9 ( 0 2 ) 0 0 2 6 1 - 0
www.elsevier.com/locate/pain
* Corresponding author. 7, Chung-Shan South Road, Taipei, Taiwan, ROC. Tel.: 1886-2-2312-3456; fax: 1886-2-2341-5736.
mechanisms (Bing et al., 1990). DNICs are part of the biolo-gical pain control system whereby a spatially remote noxious conditioning stimulus can reduce the response to a subsequent noxious stimulus elsewhere (Le Bars et al., 1979, 1992).
Although acupuncture is widely used in humans, its applicability in various painful disorders is challenged due to its weak and variable analgesic effect, as well as by its concomitant placebo or hypnotic effect (Moret et al., 1991; Amanzio and Benedetti, 1999), or by the patient’s psycho-logical expectation (Thomas and Lundeberg, 1996). Studies have demonstrated that acupuncture is especially, though not exclusively, effective in myofascial pain (Melzack et al., 1977; Lewit, 1979), renal colic (Lee et al., 1992), angina pectoris (Richter et al., 1991), osteoarthritis of the knees (Christensen et al., 1992), tension headache (Vincent, 1990), and fibromyalgia (Deluze et al., 1992).
To date, few studies have investigated acupuncture’s effect on postoperative pain, and they have shown conflict-ing results (Galloway et al., 1984; Martelete and Fiori, 1985; Christensen et al., 1989, 1993). A critical difference among these studies was the stimulation modality and the lack of sham control. None of these studies examined the effect of prestimulus acupuncture on postoperative pain and conco-mitant side effect profiles. Since acupoint stimulation has been widely used and proven to be effective in relieving profound nausea and vomiting caused by motion sickness and chemotherapy, electroacupuncture (EA) per se could potentially serve as an adjuvant for relieving opioid-related side effects during the postoperative period (Lee and Done, 1999). The present study was thus undertaken to evaluate whether preoperative application of different frequencies of EA stimulation can be effective in relieving postoperative pain, as well as postoperative opioid-related side effects.
2. Methods 2.1. Subjects
The study protocol was approved by the Hospital Research Committee. A total of 100 female patients were enrolled. After providing written informed consent, patients of ASA physical status I–II (American Society of Anesthe-siology nomenclature) who were scheduled for abdominal hysterectomy were randomly divided into four groups of 25 each by a computer-generated randomization sequence: Group I received neither needle insertion nor electric stimu-lation (control); Group II received needle insertion but with-out electrical stimulation (sham-EA); Group III received needle insertion with 2 Hz stimulation (low-EA); and Group IV received needle insertion with 100 Hz stimulation (high-EA). The use of the patient-controlled analgesia (PCA) device (Lifecare PCA Plus II Infusor, Abbott Labora-tories, IL, USA) and the administration of EA were explained to each patient at the time of their preoperative
visits. Patients with a history of opioid abuse, or significant cardiovascular, pulmonary, renal, hepatic or neurological disease were excluded.
2.2. Acupuncture
The acupuncture loci used were the bilateral Zusanli (also known as ST-36), which are located at one finger breadth below and anterior to the tibial tuberosity. We chose Zusanli because this point is traditionally considered to possess the most therapeutic effect on the lower abdomen. Two 30 gauge stainless steel acupuncture needles were inserted at the Zusanli point on both legs, with a distance between the needles of approximately 3 cm, i.e. one needle serving as the positive pole, and the other as the negative pole to allow electrical stimulation of the selected point. After the patient reported the sensation of ‘De-Xi’, a term used in acupunc-ture to describe a feeling of ‘heaviness’ in the area surround-ing the insertion locus, an electric current was delivered by a Functions Electrical Stimulator (Trio 300, I. T. O., Japan). Subjects and electrical equipment were placed such that subjects were unable to see any specifics regarding the type of current administered, and technicians maintained a normal persona to ensure that patients remained unaware of their grouping category. Electricity was generated as an output of constant current of 0.5 mA, 1 ms square pulse, at a maximal tolerable intensity (a strong, but not painful sensation as reported by the patient), and at 2 or 100 Hz depending on the group assignment. Sham-EA included needle insertion with the indicator light on but with no actual current. All patients were subject to the respective treatment modality for 20 min before the induction of anesthesia.
2.3. Anesthesia and postoperative care
After removal of the needles, anesthesia was induced with i.v. thiopental 5 mg/kg and succinylcholine 2 mg/kg for tracheal intubation. Isoflurane in nitrous oxide 60% with oxygen 40% and an intermittent dose of atracurium were used for anesthesia maintenance without the use of opioids. Following surgery, all patients were transported to the recovery room. The time interval of the patient’s first request for pain medication was recorded (either pethi-dine 1 mg/kg i.m. during the first hour, which was restricted to a single dose, or as recorded by PCA when no request was made during the first hour). At 1 h postoperation, the PCA system was connected to the patient. The PCA device was programmed to intravenously deliver 2 mg morphine as ‘on demand’ doses with a minimum lockout interval of 10 min during the following 23 h.
2.4. Efficacy measures
As soon as the patient complained of initial pain, the first PCA dose was provided. The average pain using the 100 mm visual analog scale (VAS) was recorded at
30 min, and at 1, 1.5, 2, 4, 8, 16 and 24 h by an observer who had no knowledge of the patient’s group assignment. The number of PCA demands, including those made during lockout intervals, was recorded by a printout attachment for 24 h. No other analgesics were administered during the PCA control period. Heart rate, blood pressure, and SpO2
were recorded by a pulse oximeter every 30 min for the first 2 h and at 4 h intervals thereafter. At the end of 24 h test period, patients were asked if they had experienced any opioid-related adverse effects, including nausea, vomiting, dizziness, or pruritus, and their responses were recorded. 2.5. Data analysis
This sample size permitted the detection of an inter-group mean difference using a type I error of 0.05 and a type II error of 0.2 (i.e. power ¼ 0.8). Patient’s age, weight, dura-tion of anesthesia and duradura-tion of surgery, VAS score, PCA demands and doses delivered were analyzed with one-way ANOVA followed by Bonferroni post hoc test. Incidence of side effects was analyzed using the chi-square test. A P value of less than 0.05 was considered statistically signifi-cant.
3. Results
The demographic data listed in Table 1 shows no signifi-cant difference among the four groups. All enrolled patients completed the study period with no withdrawals. The time
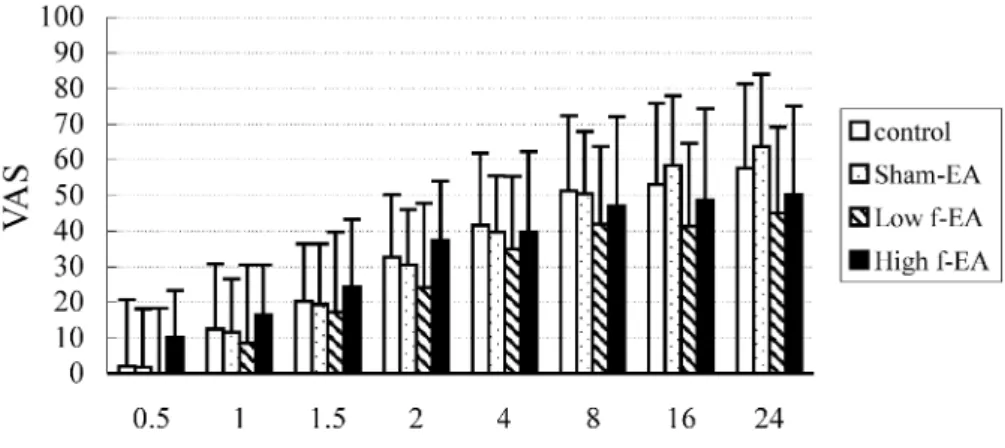
interval of the first request for analgesic was 10.6, 18.0, 27.9, and 28.1 min for the control, sham-EA, low-EA and high-EA, respectively. These intervals were all significantly longer than the control group, and the time intervals of both low- and high-EA groups were longer than the sham-EA group (Table 2). The number of PCA demands during the 24 h test period was significantly less in the high-EA (7.9 ^ 5.9) and low-EA (11.7 ^ 7.1) groups compared to that of the control (20.5 ^ 9.2) and the sham-EA (16.1 ^ 7.4) groups, as shown in Table 2. There were also highly significant differences in morphine delivered among the four groups, with the lowest dose used in the high-EA group (Table 2). Pain scores were not different among the four groups (Fig. 1); however, compared to the control group, the total amount of morphine required during the 24 h test period was reduced by 21, 43 and 61% in the sham-EA, low-EA and high-EA groups, respectively. The incidence of nausea and dizziness was significantly lower in both the low- and high-EA groups compared to the other two groups (Table 3). No patient demonstrated any respira-tory depression (respirarespira-tory rate ,10 min or SpO2, 90%).
4. Discussion
We found that sham-, low-, and high-EA treatments prior to lower abdominal surgery significantly reduced the post-operative PCA morphine requirement, and that low- and high-EA decreased opioid-induced nausea and dizziness throughout the first postoperative day. We also found that
Table 1
Demographic data for each of the four treatment groupsa
Group I (control) Group II (sham-EA) Group III (low-EA) Group IV (high-EA)
Age (year) 39 ^ 8 41 ^ 12 38 ^ 7 42 ^ 13
Weight (kg) 51 ^ 14 55 ^ 10 54 ^ 11 51 ^ 17
Duration of anesthesia (min) 113 ^ 38 110 ^ 19 121 ^ 14 115 ^ 21 Duration of operation (min) 101 ^ 13 105 ^ 20 110 ^ 21 102 ^ 15
a
Values are mean ^ S.D. ðn ¼ 25Þ.
Table 2
Postoperative analgesic requirements in each treatment groupa
Group I (control) Group II (sham-EA) Group III (low-EA) Group IV (high-EA)
Time for the first dose of pethidine after operation (min)
10.6 ^ 5.9 18.0 ^ 7.9* 27.9 ^ 12.3**,§ 28.1 ^ 13.8**,§ PCA demands in the first 24 h
1–8 h 9.0 ^ 3.6 7.0 ^ 3.6* 5.1 ^ 4.0**,1 3.3 ^ 3.2**,11,§ 8–16 h 8.2 ^ 5.3 5.8 ^ 3.8 4.0 ^ 2.6**,1 2.8 ^ 2.1**,11,§ 16–24 h 3.2 ^ 2.4 3.3 ^ 2.1 2.6 ^ 2.1 1.8 ^ 1.6**,11 Total in 24 h 20.5 ^ 9.2 16.1 ^ 7.4* 11.7 ^ 7.1**,1 7.9 ^ 5.9**,11,§ Morphine delivered (mg) 1–8 h 16.1 ^ 7.1 12.8 ^ 6.6* 9.2 ^ 7.1**,1 6.1 ^ 5.9**,11,§ 8–16 h 15.5 ^ 9.4 10.8 ^ 7.7* 7.6 ^ 5.4**,1 5.4 ^ 3.8**,11,§ 16–24 h 6.5 ^ 4.8 6.6 ^ 4.1 5.0 ^ 4.2 3.5 ^ 3.2**,11 Total in 24 h 38.1 ^ 16.0 30.2 ^ 14.4* 21.8 ^ 14.7**,11 15.0 ^ 10.7**,11,§
high-frequency EA stimulation produced better effects than low-frequency stimulation. Our results were consistent with several other animal and clinical studies that have reported that EA stimulation improved analgesia during the acute phase of posttraumatic pain. EA has been widely used and well documented in tooth extraction (Ekblom et al., 1991; Ernst and Pittler, 1998) and in oocyte aspiration (Stener-Victorin et al., 1999). EA-induced analgesia after major surgery, however, remains controversial. Christensen et al. (1993) demonstrated that patients receiving EA before and during hysterectomy exhibited no reduction in their post-operative analgesic requirement or pain ratings. The possi-ble reasons for this discrepancy are multiple. Christensen et al. selected an array of acupoints; the needles were inserted after induction of anesthesia and remained in place through-out the surgical procedure; and in addition to EA, patients received a relatively high dose of pethidine for induction and maintenance of anesthesia. The high level of pethidine used likely masked any benefit of EA that may have been present. This variation in methods versus those used in the present study may explain the vastly different results reported. Wang et al. (1997) found significantly greater pain relief after lower abdominal surgery among patients receiving transcutaneous electrical nerve stimulation (TENS) applied at Hogu (LI-4) during the postoperative period. Wang’s study showed that both high- and low-frequency TENS reduced i.v. PCA hydromorphone require-ment by 65 and 34%, respectively. Similar results were
obtained in our study whereby the total number of PCA demands and the opioid analgesic dose requirements were markedly decreased in both high- and low-frequency EA groups. These findings imply that both EA and TENS can produce adequate postoperative pain relief, even though their underlying mechanisms are likely dissimilar.
In the present study, EA-induced analgesic effects between 2 and 100 Hz were compared on the basis that low frequency (2 Hz) and high frequency (100 Hz) presum-ably induce a differential release of enkephalins and dynor-phins in both animals and humans (Chen and Han, 1992; Ulett et al., 1998). It has also been shown that the analgesic effect produced by low-frequency stimulation is naloxone-reversible, while high-frequency stimulation is not (Lee and Beitz, 1992; Guo et al., 1996). These results strongly suggest a distinct neuronal sensitization and characteristic spatial process in the central nervous system between these two frequencies. The primary difference between 2 and 100 Hz stimulations is the number of electric pulses provided during a given period of time. It is estimated that patients in the high-frequency group received a total of 20 times the number of electrical pulses than the low-frequency group during the 20 min test period. Previous study suggests that the inhibitory magnitude and duration of neuronal response to painful C-fiber activation is a function of the number of preceding conditioning stimuli (Ness and Gebhart, 1991). This previous speculation is consistent with the results observed in our study.
Fig. 1. Postoperative 24-h visual analogue scale (VAS, 0–100 mm) comparison. Open bars indicate control group; dotted bars, sham-EA; hatched bars, low-EA; shaded bars, high-EA.
Table 3
Postoperative side effects in the four treatment groupsa Number of opioid-related side effects
Group I (control) Group II (sham-EA) Group III (low-EA) Group IV (high-EA)
Nausea 11 (44) 10 (40) 4 (16)*,1 6 (24)*,1
Vomiting 0 (0) 1 (4) 1 (4) 0 (0)
Dizziness 14 (56) 16 (64) 7 (28)*,1 10 (40)*,1
Pruritis 2 (8) 0 (0) 1 (4) 1 (4)
a
The results of the present study also demonstrated that the opioid-sparing effect of EA is dependent on the frequency of the electrical stimulation. The morphine requirement after high-frequency EA was decreased by 31% compared with the low-EA group during the first 24 h postoperation. This opioid-sparing effect not only results in a decrease in the incidence of nausea and dizziness after surgery, but it may also increase the tolerability and availability of adequate analgesic effect among those who are susceptible to morphine overdose, e.g. debilitated or older patients. Furthermore, acupuncture per se has been shown to reduce the incidence of nausea through its direct antiemetic effect. Several studies have shown that acupuncture can decrease postoperative nausea and vomiting, in addition to reducing postoperative pain (Lee and Done, 1999).
In the present study, we did not deliberately attempt to influence subjects’ expectation regarding the effectiveness of EA. However, it is important to keep in mind that neuro-physiological and humoral events are related to psycholo-gical factors. Undoubtedly, sensory stimulation and particularly acupuncture has the potential to produce strong placebo effects. For example, while sham needle insertion showed no effect on opioid-related side effects, it did exert a moderate pain relieving effect. Indeed, acupuncture works by releasing endogenous opioids and so, it appears, does the placebo effect (Amanzio and Benedetti, 1999). To achieve optimal acupuncture therapy, physiological and psychologi-cal factors must interact in synergy, utilizing their respective endogenous mechanisms efficiently (Thomas and Lunde-berg, 1996). By utilizing two control groups in our study design, including PCA and sham-EA groups only, we were able to very clearly distinguish between the purely placebo elements involved versus clear evidence of physiological effects.
In the present study we also demonstrated that preopera-tive application of 20 min EA, whether high or low frequency, effectively reduced the 24 h postoperative morphine requirement. The concept of preemptive analgesia through the prevention of intense nociceptor activation and suppression of the subsequent hyperalgesic state has poten-tially dramatic implications for postoperative analgesia (Woolf and Chong, 1993; Sun et al., 1996). In animals, pretreatment with short-acting opioids or NMDA antago-nists has been shown to inhibit spinal dorsal horn sensitiza-tion and nocifensive behaviors (Yamamoto and Yaksh, 1992). However, results reported from human studies invol-ving preemptive analgesia in surgical situations are rela-tively less impressive than results shown in animal studies utilizing the same treatment schemes (Cousins et al., 2000). EA-produced analgesia generally lasts for up to 2 h (Chris-tensen et al., 1989; Ulett et al., 1998). Therefore, high- or low-frequency stimulation before surgical incision can be regarded as an alternative to conventional methods as a prestimulus analgesic modality. Whether EA is indeed useful as a preemptive analgesia requires further study that will require two additional treatment groups: one
receiving both pre- and postoperative EA, and another group receiving postoperative EA alone.
In conclusion, we found that preoperative EA is an adequate adjunct to PCA. Both high- and low-frequency electrical stimulation reduced the postoperative analgesic requirement. We found that high-frequency electrical stimu-lation provided the best results. In addition, the use of EA also resulted in a decrease in the incidence of opioid-related side effects after lower abdominal surgery. Together, our findings demonstrate that further studies are warranted regarding the efficacy of EA in both managing pain and in treating opioid-related symptoms.
Acknowledgements
This study was supported by grants from the National Science Council of the Republic of China (NSC90-2314-B002-302 and NSC 88-2314-B039-011).
References
Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci 1999;19:484–494.
Andersson S, Lundeberg T. Acupuncture-from empiricism to science: func-tional background to acupuncture effects in pain and disease. Med Hypotheses 1995;45:271–281.
Basbaum AI, Field HL. Endogenous pain control systems: brainstem spinal pathways and endogenous circuitry. Ann Rev Neurosci 1984;7:309– 338.
Bing Z, Villanueva L, Le Bars D. Acupuncture and diffuse noxious inhi-bitory controls: naloxone-reversible depression of activities of trigem-inal convergent neurons. Neuroscience 1990;37:809–818.
Chen XH, Han JS. Analgesia induced by electroacupuncture of different frequencies is mediated by different types of opioid receptors: another cross-tolerance study. Behav Brain Res 1992;47:143–149.
Cheng RS, Pomeranz B. Monoaminergic mechanism of electroacupuncture analgesia. Brain Res 1981;215:77–92.
Christensen PA, Noreng M, Andersen PE, Nielsen JW. Electroacupuncture and postoperative pain. Br J Anaesth 1989;62:258–262.
Christensen BV, Juhl IU, Vilbeck H, Bulow HH, Dreijer NC, Rasmunsen HF. Acupuncture treatment of severe knee osteoarthritis: a long-term study. Acta Anaesthesiol Scand 1992;36:519–525.
Christensen PA, Rotne M, Vedelsdal R, Jensen RH, Jacobsen K, Husted C. Electroacupuncture in anaesthesia for hysterectomy. Br J Anaesth 1993;71:835–838.
Cousins MJ, Power I, Smith G. 1996 Labat lecture: pain – a persistent problem. Reg Anesth Pain Med 2000;25:6–21.
Deluze CH, Bosia L, Zirbs A, Chantraine A, Vischer ThL. Electroacupunc-ture in fibromyalgia: results of a controlled trial. BMJ 1992;305:1249– 1252.
Ekblom A, Hansson P, Thomsson M, Thomas M. Increased postoperative pain and consumption of analgesics following acupuncture. Pain 1991;44:241–247.
Ernst E, Pittler MH. The effectiveness of acupuncture in treating acute dental pain: a systemic review. Br Dent J 1998;184:443–447. Galloway DJ, Boyle P, Burns HJ, Davidson PM, George WD. A clinical
assessment of electroanalgesia following abdominal operations. Surg Gynecol Obstet 1984;159:453–456.
Guo HF, Tian J, Wang X, Fang Y, Hou Y, Han J. Brain substrates activated by electroacupuncture of different frequencies (I): comparative study on
the expression of oncogene c-fos and genes coding for three opioid peptides. Mol Brain Res 1996;43:157–166.
Han Z, Jiang YH, Wan Y, Wanh Y, Chang JK, Han JS. Endomorphine-1 mediates 2 Hz but not 100 Hz electroacupuncture analgesia in the rat. Neurosci Lett 1999;274:75–78.
He LF. Involvement of endogenous opioid peptides in acupuncture analge-sia. Pain 1987;31:99–121.
Kaufman MP, Waldrop TG, Rybycki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Rec 1984;19:663–668.
Le Bars D, Dickensson AN, Besson JM. Diffuse noxious inhibitory controls (DNIC). II. Lack of effects on non-convergent neurons, supraspinal involvement and theoretical implications. Pain 1979;6:305–327. Le Bars D, Willer JC, De Broucker T. Morphine blocks descending pain
inhibitory controls in humans. Pain 1992;48:13–20.
Lee JH, Beitz AJ. Electroacupuncture modifies the expression of c-fos in the spinal cord induced by noxious stimulation. Brain Res 1992;577:80–91.
Lee A, Done ML. The use of nonpharmacologic techniques to prevent postoperative nausea and vomiting: a meta-analysis. Anesth Analg 1999;88:1362–1369.
Lee YH, Lee WC, Chen MT, Huang JK, Chung C, Chang LS. Acupuncture in the treatment of renal colic. J Urol 1992;147:16–18.
Lewit K. The needle effect in relief of myofascial pain. Pain 1979;6:83–90. Martelete M, Fiori AM. Comparative study of the analgesic effect of trans-cutaneous nerve stimulation (TNS); electroacupuncture (EA) and meperidine in the treatment of postoperative pain. Acupunct Electrother Res 1985;10:183–193.
Mayer DJ. Biological mechanisms of acupuncture. Prog Brain Res 2000;122:457–477.
Melzack R, Stillwell DM, Fox EJ. Trigger points and acupuncture points for pain: correlations and implications. Pain 1977;3:3–23.
Moret V, Forster A, Laverrie´re MC, Lambert H, Gaillard RC, Bourgeois P, Haynal A, Gemperle M, Buchser E. Mechanism of analgesia induced by hypnosis and acupuncture: is there a difference? Pain 1991;45:135–40. Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nocicep-tion in the rat. II Noxious visceral stimuli inhibit cutaneous nociceptive neurons and reflexs. J Neurophysiol 1991;66:29–39.
Pomeranz B. Scientific research into acupuncture for the relief of pain. J Altern Complement Med 1996;2:53–60.
Pomeranz B, Chiu D. Naloxone blockade of acupuncture analgesia: endor-phin implicated. Life Sci 1976;19:1757–1762.
Richter A, Herlitz J, Hjalmarson A. Effect of acupuncture in patients with angina pectoris. Eur Heart J 1991;12:175–178.
Sandkuehler J, Chen JG, Cheng G, Randic M. Low frequency stimulation of afferent Ad fibers induces long-term depression of primary afferent synapses with substantia gelatinosa neurons in the rat. J Neurosci 1997;17:6483–6491.
Sjolund B, Terenius L, Eriksson M. Increased cerebrospinal fluid levels of endorphins after electro-acupuncture. Acta Physiol Scand 1977;100:382–384.
Stener-Victorin E, Waldenstrom U, Nilsson L, Wikland M, Janson PO. A prospective randomized study of electroacupuncture versus alfentanil as anaesthesia during oocyte aspiration in in-vitro fertilization. Hum Reprod 1999;14:2480–2484.
Sun WZ, Shyu BC, Shieh JY. Nitrous oxide or halothane, or both, fail to suppress c-fos expression in the rat spinal cord dorsal horn neurons after subcutaneous formalin. Br J Anaesth 1996;76:99–105.
Thomas M, Lundeberg T. Does acupuncture work? Pain clinic. Updates 1996;4:1–4.
Ulett GA, Han S, Han JS. Electroacupuncture: Mechanisms and clinical application. Biol Psychiatry 1998;44:129–138.
Vincent CA. The treatment of tension headache by acupuncture: a controlled single case design with time series analysis. J Psychosom Res 1990;34:553–561.
Wang Q, Mao L, Han J. The arcuate nucleus of hypothalamus mediates low but not high frequency electroacupuncture analgesia in rats. Brain Res 1990a;513:60–66.
Wang Q, Mao L, Han J. The role of periaqueductal gray in mediation of analgesia produced by different frequencies of electroacupuncture stimulation in rats. Int J Neurosci 1990b;53:167–172.
Wang B, Tang J, White PF, Naruse R, Sloninsky A, Kariger R, Gold J, Wender RH. Effect of the intensity of transcutaneous acupoint electrical stimulation on the postoperative analgesic requirement. Anesth Analg 1997;85:406–413.
Woolf CJ, Chong MS. Preemptive analgesia-Treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 1993;77:362–379.
Wu JN. A short history of acupuncture. J Altern Complement Med 1996;2:19–21.
Yamamoto T, Yaksh TL. Comparison of the antinociceptive effects of pre-and posttreatment with intrathecal morphine pre-and MK801, an NMDA antagonist, on the formalin test in the rat. Anesthesiology 1992;77:757– 763.