Morphology and Growth Kinetics of Ag
3
Sn During
Soldering Reaction Between Liquid Sn and an Ag Substrate
T.L. Su, L.C. Tsao, S.Y. Chang, and T.H. Chuang (Submitted 25 February 2002; in revised form 28 March 2002)
For the soldering of recycled Ag sputtering targets, the interfacial reaction between liquid Sn and an Ag substrate at temperatures ranging from 250 -425 °C has been investigated. Experimental results show that a scallop-shaped layer of Ag3Sn intermetallic compounds formed during the soldering reaction. Kinetics analysis indicated that the growth of such interfacial Ag3Sn intermetallic compounds is diffusion-controlled with activation energy of 70.3kJ/mol. During the reaction, the Ag substrate dissolves into the molten Sn solder and causes the appearance of needle-shaped Ag3Sn precipitates in the Sn matrix.
Keywords Ag3Sn intermetallic compound, interfacial reaction,
kinetic analysis, target bonding
1. Introduction
For the soldering of recycled sputtering targets with their back plates, pure tin has been widely used,[1] while Ag has been regarded as a popular sputtering material. During the soldering reaction, intermetallic compounds form at the inter-face, which lead to the bonding effect between the Ag target material and the Sn solder. However, an overgrowth of inter-metallic compounds causes brittle cracking at the interfaces. Therefore, phase identification and growth analysis of the re-acted intermetallics during the Ag/Sn soldering reaction pro-vide vital links in such an application. The interfacial reactions of Sn thin-film, Sn-Pb, and Sn-Ag bulk solders with Ag sub-strates have been investigated by a number of researchers.[2-8] The studies all showed that the intermetallic compound formed during these interfacial reactions were of the Ag3Sn phase. However, measurements of the growth kinetics of such an intermetallic compound at the Ag/Sn interface are scarce and are what this study is concerned with. For this purpose, the intermetallic compounds appearing during the soldering reac-tions between liquid Sn and Ag substrates in a wide tempera-ture range from 250-425 °C were identified. The morphology these compounds was observed, and their thicknesses were measured as a function of reaction time, allowing the activation energy for the growth of these intermetallic compounds to be obtained.
2. Experimental
Pure Sn solders (3N) were rolled into a foil of 0.2 mm thickness. Ag substrates with a dimension of 8 ×12 mm were cut from a 1 mm thick Ag plate (3N5), were ground with SiC
paper, and were polished with 1m and 0.3m Al2O3powders.
For the study of interfacial reactions, the Sn solder foil was placed on the Ag substrate and was heated in an infrared fur-nace under a vacuum of 10−3 torr. Through a water-cooling
system installed within the furnace, the specimens were cooled to room temperature in 2 min. Soldering reactions were con-ducted at temperatures between 250 and 425 °C for various times.
For the observations of the morphology of intermetallic compounds formed at the Ag/Sn interface after soldering re-actions, scanning electron microscopy (SEM) was used. The specimens were cross-sectioned, ground with SiC paper, and polished with 1m and 0.3m Al2O3powders. The chemical
composition of the intermetallic compounds was determined by electron probe microanalysis (EPMA). For growth kinetics analysis, the thickness of intermetallic compounds that are formed for various temperatures and time periods were deter-mined by dividing the total area of intermetallic cells in a transverse section by the total width. For further clarification of the formation mechanism of intermetallic compounds during the soldering reaction, the original Ag/Sn interface was marked by the sputter deposition of a Ta thin film with a thickness of about 1000 Å on a partial region of the Ag substrate. Ta does not react with Ag and liquid Sn, and the deposition of such a thin film acted as a diffusion barrier identifying the original Ag/Sn interface.
3. Results and Discussion
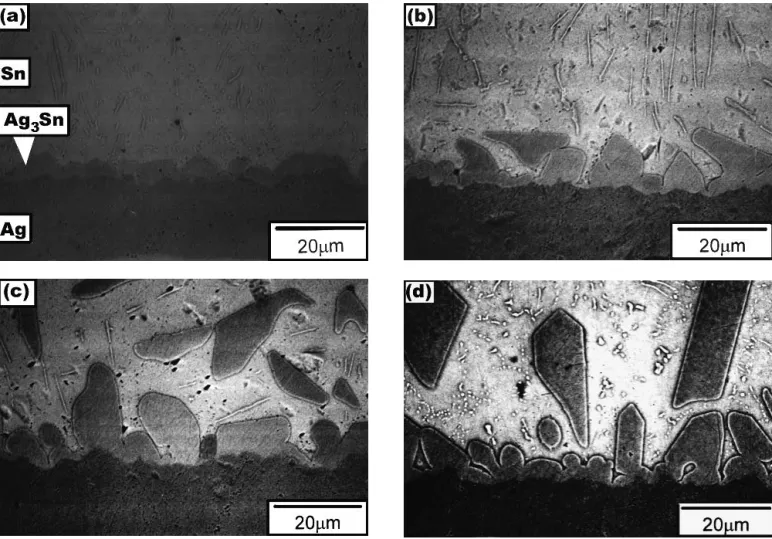
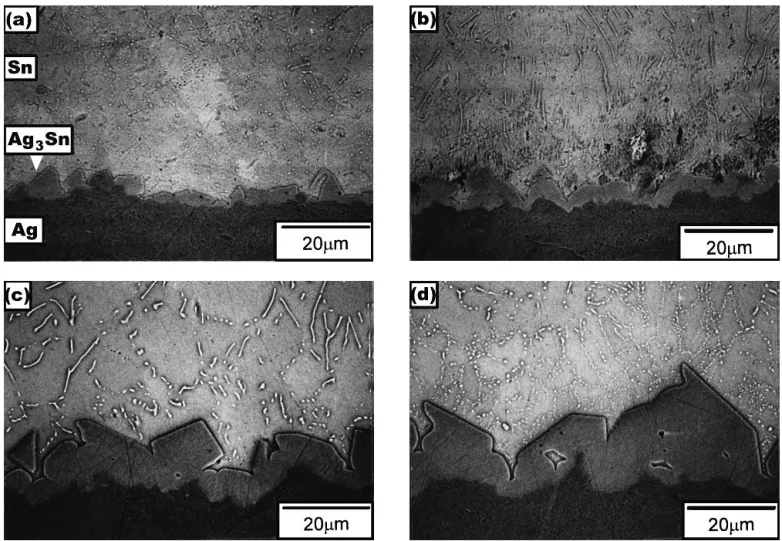
After soldering reactions between liquid Sn and Ag sub-strates take place, a scallop-shaped layer of intermetallic com-pounds appears at the Ag/Sn interface, as shown in Fig. 1 and 2, which constitutes EPMA evidence that the intermetallic compounds have the composition Ag3Sn. Accompanying this formation of interfacial intermetallic Ag3Sn, a large number of
needle-shaped precipitates were observed in the Sn matrix. The chemical composition of these precipitates, as analyzed by EPMA, was also Ag3Sn. The formation of such a needle-shaped Ag3Sn phase is attributed to the dissolution of Ag
into the liquid Sn matrix during the Ag/Sn interfacial reaction, which precipitates after solidification of the Sn solder.
T.L. Su, L.C. Tsao, S.Y. Chang,and T.H. Chuang, Department of Materials Science and Engineering, National Taiwan University, Tai-pei 106, Taiwan. Contact e-mail: tunghan@ccms.ntu.edu.tw.
JMEPEG (2002) 11:365-368 ©ASM International
Figures 1 and 2 show that with increasing reaction tempera-tures and time, the long precipitate needles divided into par-ticles under the driving force of surface reduction. The scallop-shaped intermetallic compounds at the Ag/Sn interface grew with an increase of reaction temperatures and time periods. Figure 1 shows that at higher temperatures the scallop-shaped Ag3Sn intermetallic compounds have separated from the Ag/Sn
interface and moved into the Sn matrix. Even in reactions occurring at lower temperatures such as 250 °C (Fig. 2), even for a long period of 120 min, such a phenomenon of interme-tallic compound separating from the Ag/Sn interface was not found.
Through the sputter deposition of the Ta thin film on the partial region of the Ag substrate, the original Ag/Sn interface can be marked after the Ag/Sn soldering reaction. Figure 3 shows that the Ag/Sn interface accompanying the Ag3Sn mi-grates some distance toward the Ag substrate. The result is attributed to the relatively high solubility of Ag in molten Sn. The severe dissolution of Ag into the liquid Sn solders also causes the precipitation of a needle-shaped Ag3Sn phase in the Sn matrix after the solidification of the Sn solder. Adding an adequate amount of Ag into the Sn solder can prevent a prob-lem such as consumption of the Ag substrate.
For kinetics analysis, the square of the thickness (x2) of the
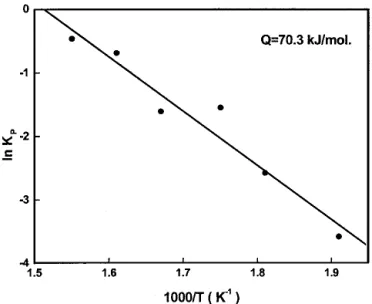
Ag3Sn at the Ag/Sn interface vs the reaction time (t) is plotted in Fig. 4 for various soldering temperatures. It can be seen that the plots are linear. The result indicates that the growth of Ag3Sn during the interfacial reactions between liquid Sn and
the Ag substrates is diffusion-controlled. The growth rate con-stants (Kp⳱ x2/t) for various reaction temperatures are calcu-lated and plotted in an Arrhenius relation, as shown in Fig. 5. From the slope of the Arrhenius plot, the activation energy Q for the growth of Ag3Sn intermetallic compounds during the
Ag/Sn soldering reactions is estimated to be 70.3 kJ/mol. The activation energy for lattice diffusion of Ag in bulk Sn single crystals is 51.5-77.0 kJ/mol, depending on the crystal orienta-tion,[9] and that of Sn in bulk Ag is 165-171 kJ/mol.[10]This implies that the growth of Ag3Sn intermetallic compounds during the Ag/Sn soldering reactions was predominantly controlled by the diffusion of Ag into the Sn solders, which is consistent with the morphology observation shown in Fig. 3.
4. Conclusions
A discontinuous layer of scallop-shaped Ag3Sn intermetal-lic compounds at the Ag/Sn interface results from the soldering
Fig. 1 Typical morphology of intermetallic compounds formed at the Ag/Sn interface and in the Sn matrix after soldering reactions between liquid Sn and Ag substrates at various temperatures for 15 min: (a) 300 °C; (b) 325 °C; (c) 350 °C; and (d) 375 °C
reaction between liquid Sn and an Ag substrate. Accompanying this reaction, Ag from the substrate is found to dissolve in the molten Sn, which results in the formation of a large number of needle-shaped Ag3Sn precipitates in the Sn matrix after
solidi-fication. The interfacial Ag3Sn intermetallic compounds grow
with increasing reaction time and temperature. The growth ki-netics are diffusion-controlled with an activation energy of
Fig. 2 Typical morphology of intermetallic compounds formed at the Ag/Sn interface and in the Sn matrix after soldering reactions between liquid Sn and Ag substrates at 250 °C for various time periods: (a) 30 min; (b) 60 min; (c) 90 min; and (d) 120 min
Fig. 3 Migration of the Ag/Sn interface during the soldering reaction between liquid Sn and the Ag substrate at 325 °C for 15 min. (The
original Ag/Sn interface is marked with a Ta thin film.) Fig. 4 The x
2of Ag
3Sn intermetallic compounds as a function of t for
soldering reactions between liquid Sn and an Ag substrate at various temperatures (°C)
70.3 kJ/mol, which is near that of the lattice diffusion of Ag in bulk Sn (51.5-77.0 kJ/mol). The result implies that the growth of Ag3Sn during the Ag/Sn soldering reaction is dominated by
the diffusion of Ag into Sn solders.
References
1. F. Hillen, D. Pickart-Castillo, and J. Rass: “Solder Alloy and Soldering Processes for Flux-Free Soldering of Difficult-to-Wet Materials,” Welding Cutting, 2000, 52(8), pp. E162-65.
2. V. Simic and Z. Marinkovic: “Room Temperature Interactions in Ag-Metals Thin Film Couples,” Thin Solid Films, 1979, 61(2), pp. 149-60. 3. Z. Marinkovic and V. Simic: “Kinetics of Reaction at Room-Temperature in Thin Silver Metal Couples,” Thin Solid Films, 1991, 195(1-2), pp. 127-35.
4. V. Simic and Z. Marinkovic: “Stability of Compounds in Thin-Metal Couples in the Course of Long Aging at Room-Temperature,” Thin Solid Films, 1992, 209(2), pp. 181-97.
5. X.H. Wang and H. Conrad, “Effect of Sn Content of Pb-Sn Solder Alloys on Wetting Dynamics,” Scrip. Metal Mater., 1994, 31(4), pp. 375-80.
6. C.J. Thwaites and M. Woodall: “Silver-Palladium Metallisation Inter-actions with Reflowed Solder Pastes,” Brazing Soldering, 1987, 12, pp. 57-60.
7. Y. Moriya, Y. Yamade, and R. Shinya: “Interface Reaction Between Ag-Pd Conductor and Pd-Sn Solder,” IEEE Trans. CPMT B, 1998, 21(4), pp. 394-97.
8. G.Y. Li and Y.C. Chan: “Interactions Between Silver-Palladium Met-allization Tin-Lead-Silver Solder,” Phys. Stat. Sol., 1998, 166(2), pp. R13-R14.
9. B.F. Dyson: J. Appl. Phys., 1996, 37, p. 2375, cited: Diffusion in Solid Metals and Alloys, H. Mehrer, ed., Landorf Bornstein Handbook, Springer Verlag, 1990, 26, p. 157.
10. V.K. Kaygorodov, S.M. Klotsman, A.N. Jimofeyev, and I.Sh. Trakhtenberg: Phys. Met. Metallog., 1969, 28, pp. 128, 146.
Fig. 5 Arrhenius plot of the growth rate constant Kp of Ag3Sn
formed by the Ag/Sn interfacial reaction