Transcription of Epstein–Barr Virus-Encoded Nuclear Antigen 1 Promoter Qp Is Repressed by
Transforming Growth Factor-
 via Smad4 Binding Element in Human BL Cells
Chih-Lung Liang,* Chi-Neu Tsai,† Pei-Jung Chung,‡ Jo-Lin Chen,§ Cheng-Ming Sun,* Ruey-Hwa Chen,¶ Ji-Hong Hong,储 and Yu-Sun Chang*,†,1
*Institute of Microbiology and Immunology, National Yang-Ming University, Shih-Pai, Taipei; †Graduate Institute of Basic Medical Sciences,
Chang-Gung University School of Medicine, Kwei-Shan, Taoyuan; ‡Graduate Institute of Life Sciences, National Defense Medical Center, Taipei;
§Graduate Institute of Life Sciences, National Tsing-Hua University, Hsin-Chu;¶Department of Molecular Medicine, National Taiwan University
School of Medicine, Taipei; and储Department of Radiation Oncology, Chang-Gung Memorial Hospital,
Kwei-shan, Taoyuan, Taiwan, Republic of China
Received June 22, 2000; returned to author for revision July 24, 2000; accepted August 15, 2000
In Epstein–Barr virus (EBV)-infected BL cells, the oncogenic EBV-encoded nuclear antigen 1 (EBNA 1) gene is directed from the latent promoter Qp. Yeast one-hybrid screen analysis using the⫺50 to ⫺37 sequence of Qp as the bait was carried out to identify transcriptional factors that may control Qp activity. Results showed that Smad4 binds the⫺50 to ⫺37 sequence of Qp, indicating that this promoter is potentially regulated by TGF-. The association of Smad4 with Qp was further confirmed by supershift of EMSA complexes using Smad4-specific antibody. The transfection of a Qp reporter construct in two EBV(⫹) BL cell lines, Rael and WW2, showed that Qp activity is repressed in response to the TGF- treatment. This repression involves the interaction of a Smad3/Smad4 complex and the transcriptional repressor TGIF, as determined by cotransfection assay and coimmunoprecipitation analysis. Results suggest that TGF- may transcriptionally repress Qp through the Smad4-binding site in human BL cells. © 2000 Academic Press
Key Words: EBV; BL cells; Qp; TGF-; Smad4; TGIF.
INTRODUCTION
Epstein–Barr virus (EBV) is a herpesvirus associated with many malignant diseases, including Africa Bur-kitt’s lymphoma (BL), nasopharyngeal carcinoma (NPC), Hodgkin’s disease, and T-cell lymphoma (Rick-inson and Kieff, 1996). In these infected tumor cells, the expression of EBV latent genes is limited. For example, EBNA 1 is the only antigen expressed in BL cells (Rowe et al., 1986). Transgenic mice specifically expressing EBNA 1 in B cells show B-cell neoplasia (Wilson et al., 1996) and overexpression of EBNA 1 enhances the malignant progression of human NPC cells, indicating an important role of EBNA 1 in EBV-associated malignancies (Sheu et al., 1996). EBNA 1 is a DNA-binding protein that binds to the ori-P region of the EBV genome and allows the viral genome to be present as an episome in infected cells (Yates et al., 1985). Transcription of the EBNA 1 gene in BL and NPC cells is initiated from a latent promoter, Qp (Tsai et al., 1995). Several studies show that Qp expression can be
regulated by interferon regulatory factors (IRFs) through interaction with the basal transcription ma-chinery (Nonkwelo et al., 1995, 1997) and Qp is posi-tively regulated by JAK/STAT activation through two potential STAT-binding sites (Chen et al., 1999), indi-cating that Qp is potentially regulated by cytokines. Xu et al. (1999, 2000) also reported that the levels of TGF- in serum samples from patients with EBV-asso-ciated BL and NPC are elevated compared to those from healthy individuals. TGF- is a cytokine that plays important regulatory roles in cell growth, morphogen-esis, cell differentiation, and apoptosis (Massague, 1998).
Following TGF- stimulation, the constitutively ac-tive type II receptor kinase (TRII) phosphorylates and activates the kinase TRI and its downstream signal-ing mediators, or Smads. Activated Smad2/Smad3 forms a heteromeric complex with Smad4 and is then translocated into the nucleus to affect the transcrip-tional activity of target genes (Zhang et al., 1997). Smad complexes participate in transactivation by as-sociation with a coactivator, the p300/CREB-binding protein, through the interaction between Smad3 and the C-terminal fragment of p300 in a temporal and phosphorylation-dependent manner (Feng et al., 1998). On the other hand, upon activation, the Smad complex can associate with a repressor (or corepressor) to
1To whom correspondence and reprint requests should be
ad-dressed at Chang-Gung University School of Medicine, Graduate Insti-tute of Basic Medical Sciences, 259 Wen-Hwa 1st Road, Kwei-Shan, Taoyuan, Taiwan, Republic of China. Fax: 886-3-328-5683. E mail: ysc@mail.cgu.edu.tw.
Virology 277, 184–192 (2000)
doi:10.1006/viro.2000.0582, available online at http://www.idealibrary.com on
0042-6822/00 $35.00
Copyright © 2000 by Academic Press All rights of reproduction in any form reserved.
down-regulate the transcription. To date, several core-pressors including TGT-interacting factor (TGIF) (Wot-ton et al., 1999) have been identified. In the course of determining Qp regulation in BL cells, we found Qp was repressed through the Smad4-binding element (SBE) located at the region between ⫺49 and ⫺45 of Qp in response to TGF- treatment. The Qp repression was caused by the TGF--induced association of the Smad3/Smad4 complex with TGIF, a transcription corepressor.
RESULTS
TGF- effector protein Smad4 is identified as a Qp-interacting protein
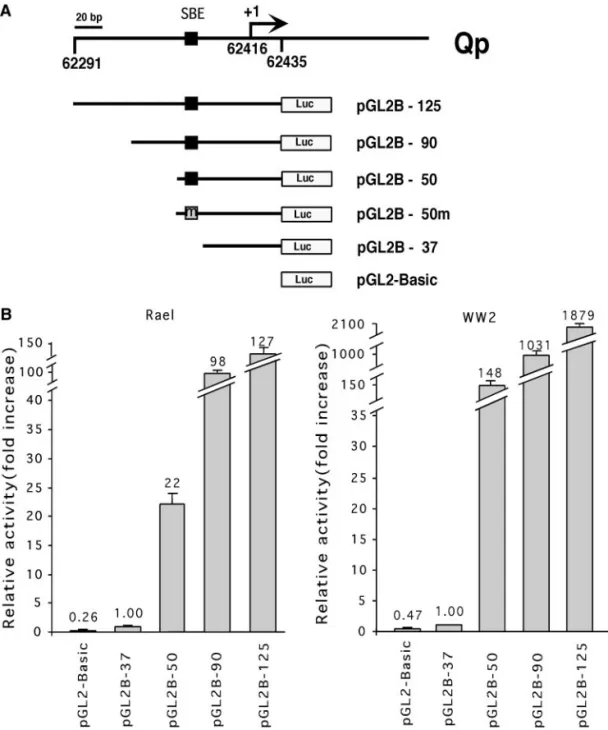
To determine the transcription factors that may regu-late EBNA 1 promoter Qp, we first identified the shortest promoter sequence of Qp that possesses a promoter activity. Qp constructs (Fig. 1A) containing various lengths of a previously identified 125-bp region (Tsai et al., 1995) were introduced into two BL cell lines, Rael and
FIG. 1. Schematic representation of the Qp-containing reporter constructs and their activities in type 1 BL cells. (A) Qp-containing promoter construct and its mutants. pGL2B-50m contained mutations (5⬘-GTCTGGTC-3⬘ to 5⬘-ATGTAGTC-3⬘) in the SBE of Qp. pGL2B-37 was generated as a deletion mutant, which did not contain SBE. pGL2-Basic is the vector control. The transcription initiation site of Qp is marked as “⫹1”, which corresponds to 62415 on the EBV genome of B95-8 strain. (B) Qp-containing reporter gene constructs were transfected into Rael and WW2 cells. The promoter activity was determined by the luciferase activity. pGL2-Basic was used as the negative control plasmid. Relative activity (fold activation) was measured by dividing the luciferase activity of each construct with that of pGL2B-37. Data were the average of six independent experiments. The standard deviations (SD) are shown by vertical bars.
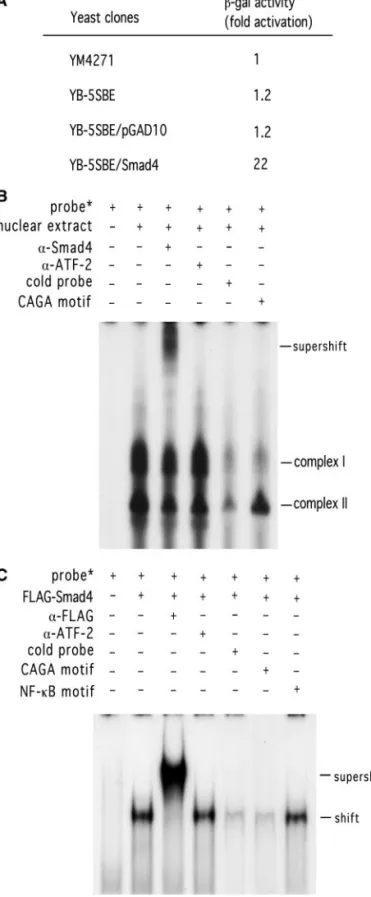
WW2. As shown in Fig. 1B, pGL2B-50 possessed a pro-moter activity, which was at least 20 times higher than the basal activity of pGL2B-37 in Rael cells. In addition, pGL2B-90 and pGL2B-125 showed at least 100-fold higher activity. A similar, but more obvious, effect was observed in WW2 cells, in which pGL2B-50 showed ⬃150-fold higher activity than pGL2B-37, and pGL2B-90 and pGL2B-125, more than 1000-fold higher activity. Thus, five copies of the sequence between⫺50 and ⫺37 were used as the bait in yeast one-hybrid screen. From 47 transformants grown on SD/Ura⫺/Leu⫺ plates, three clones showed positive reactions. DNA sequence anal-ysis revealed that one of three clones contained an insert whose sequence was identical to nt ⫹134 to ⫹1427 of Smad4, an effector in TGF- signal transduc-tion. This cDNA clone was designated as pGAD/Smad4. The binding specificity of pGAD/Smad4 was measured by-galactosidase activity assay in the yeast one-hybrid system. Yeast cells (YB-5SBE/Smad4) harboring both pLacZ-5SBE and pGAD/Smad4 showed an activity that was 22-fold higher than that of the parental yeast cells (YM4271), yeast cells (YB-5SBE) harboring pLacZ-5SBE alone, or yeast cells (YB-5SBE/pGAD10) harboring both pLacZ-5SBE and pGAD10 vector (Fig. 2A). Smad4 is a DNA-binding protein, which recognizes the SBE site (Zawel et al., 1998). Sequence analysis of the⫺50 to ⫺37 region of Qp reveals a potential SBE site between⫺49 and ⫺45. Therefore, identification of Smad4 binding to Qp suggests the function of TGF- in regulation of EBNA 1 gene expression.
To test whether endogenous Smad4 recognized the potential SBE site of Qp, the sequence between⫺54 and ⫺40 of Qp was used as the probe in the electrophoretic mobility shift assay (EMSA). As shown in Fig. 2B, using nuclear extracts from TGF--treated Rael cells, two com-plexes were formed. Both comcom-plexes were affected by unlabeled probe, whereas only Complex I was com-pletely competed off by the 200⫻ CAGA box, a
Smad4-FIG. 2. Association of Smad4 with Qp determined by yeast one-hybrid screen and as analyzed by electrophoretic mobility shift assay (EMSA). (A) The sequence between ⫺50 and ⫺37 (5⬘-TGTCTG-GTCGCTAG-3⬘) relative to the transcription initiation site of Qp was used as the bait in a yeast one-hybrid screen. The interaction of Smad4 and Qp was examined by-galactosidase activity in the yeast one-hybrid system. YM4271 is the parental yeast strain. YB-5SBE indicates the yeast cells harboring the LacZ reporter plasmid pLacZ-5SBE, which contains five copies of the⫺50 to ⫺37 region of Qp. YB-5SBE/pGAD10 indicates the yeast cells harboring both plasmid pLacZ-5SBE and the vector control pGAD10. YB-5SBE/Smad4 indicates the yeast cells
har-boring both the reporter plasmid pLacZ-5SBE and the Smad4 cDNA clone pGAD10/Smad4. The fold activation was determined as de-scribed under Materials and Methods. (B) Binding of SBE of Qp with the endogenous and ecotopically expressed Smad4. TGF--treated Rael cell extracts were reacted with 5⬘-end-labeled oligonucleotide (⫺54 to ⫺40 of Qp). For the supershift experiment, the anti-Smad4 antibody or anti-ATF-2 antibody (as a negative control) was used. For competition assay, the unlabeled probe or CAGA sequence (which is a Smad4 consensus-binding sequence) was used as the competitor. (C) For binding of SBE of Qp with ecotopically expressed Smad4, 293 cells were infected with adenovirus carrying a Flag-tagged Smad4 gene and then treated with TGF-. Nuclear extracts isolated from treated cells were incubated with 5⬘-end-labeled oligonucleotide (⫺54 to ⫺40 of Qp). For the supershift experiment, the anti-Flag antibody or anti-ATF-2 antibody (as a negative control) was used, respectively. For competition assay, the unlabeled CAGA sequence or NF-B motif was used as the competitor. The products were analyzed on 4% polyacrylamide gel. These results are representative of at least three independent experi-ments.
binding site. Furthermore, an anti-Smad4 antibody super-shifted and reduced the intensity of Complex I. On the other hand, anti-ATF2 antibody (as a negative control) had no effect on either complex. A similar pattern was detected, but less obviously, when nuclear extracts were isolated from Rael cells without TGF- treatment (data not shown). To further confirm the interaction of Smad4 and Qp, the Qp probe was incubated with the nuclear extract isolated from 293 cells, which was infected with a recombinant adenovirus carrying the Flag-tagged Smad4 gene. As shown in Fig. 2C, only one complex was detected, which was competed off by the unlabeled probe and the CAGA box, but not by the NF-B-binding motif. Furthermore, this complex was supershifted by anti-Flag antibody, but not by anti-ATF-2 antibody. Taken
together, these results indicated that Smad4, induced endogenously or introduced exogenously, interacts with the SBE site of Qp.
TGF- represses EBNA 1 gene expression and its promoter activity in type 1 BL cells
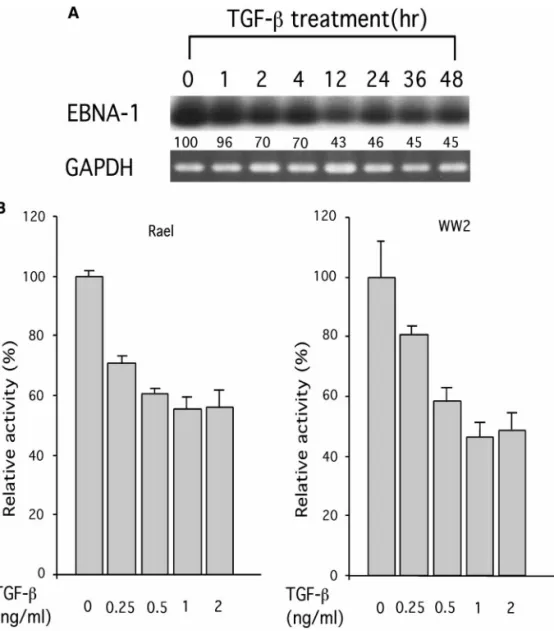
To investigate the effect of TGF- on Qp, BL cells were treated with TGF- and RNA was extracted from cells at 1, 2, 4, 12, 24, 36, and 48 h, respectively, after the treat-ment. The presence of EBNA 1 transcripts was deter-mined by RT-PCR and EBNA 1 gene-specific primers. As shown in Fig. 3A, TGF- reduced the expression of EBNA 1 transcripts in Rael cells by ⬃30% at 1 h after the treatment, and by⬃50% 12 to 48 h after the treatment.
FIG. 3. TGF- represses EBNA 1 gene expression in type 1 BL cells. (A) Type 1 BL cells Rael were treated with TGF- at 1 ng/ml and total RNA was isolated from the treated cells at 1, 2, 4, 12, 24, 36, and 48 h, respectively, after the treatment. RT-PCR was carried out with RNA from treated cells and EBNA 1 gene-specific primers. The RT-PCR products were then subjected to Southern blot analysis for detection of EBNA 1 gene expression. The percentage decrease of EBNA 1 transcripts is shown at the bottom. Expression of GAPDH gene is used as the internal control. (B) Qp-containing reporter construct pGL2B-50 was electroporated into Rael and WW2 cells, respectively. Cells were treated without or with TGF- at 0.25, 0.5, 1, and 2 ng/ml, respectively. Relative activity was determined by dividing the luciferase activity from each transfection assay after treatment with the indicated concentrations of TGF- with that of pGL2B-50 without the TGF- treatment (as 100%). Data were the average of at least six independent experiments. Vertical bars show the standard deviations (SD).
To test whether TGF- affects Qp activity, pGL2B-50 was introduced into Rael and WW2 cells, which were then treated with TGF-. As shown in Fig. 3B, the Qp activity was repressed by⬃20% upon TGF- treatment at 0.25 ng/ml, by ⬃40% at 0.5 ng/ml, and by ⬃50% at 1 ng/ml or higher concentrations, as compared to the ac-tivity in the absence of TGF- in both Rael and WW2 cells. Similar results were detected when pGL2B-125 and pGL2B-90 were examined (data not shown). In this set of experiments, an almost eightfold activation by TGF- treatment at 1 ng/ml was observed for p3TP-Lux (Keeton et al., 1991), a well-studied TGF--inducible re-porter construct, in both cell lines (data not shown). Taken together, these results indicated that Qp is spe-cifically repressed by TGF- in BL cells.
TGIF is involved in TGF- repression of Qp activity in type 1 BL cells
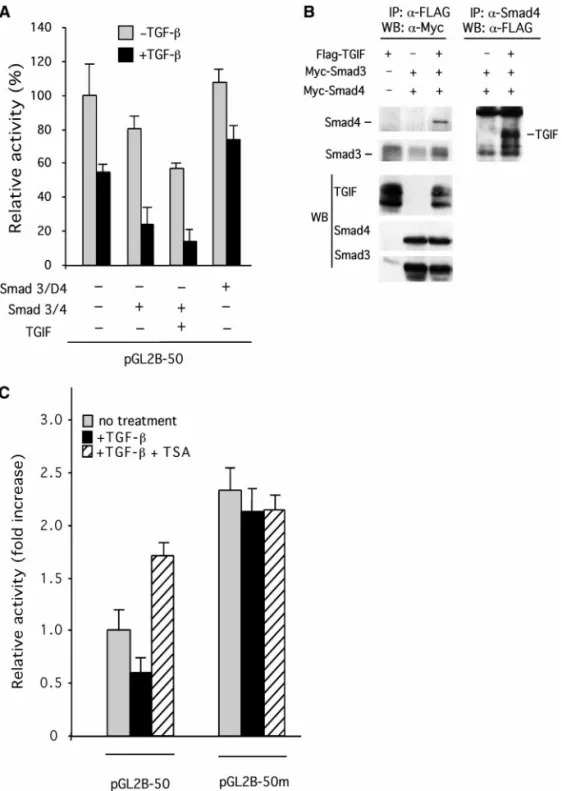
A homeoprotein TGIF has been identified as a Smad2/ Smad3-binding protein and functions as a corepressor for TGF--inducible transcription (Wotton et al., 1999). To determine whether TGIF was involved in TGF- repres-sion of Qp activity, Flag-tagged TGIF was cotransfected with pGL2B-50 into Rael cells and transfected cells were then treated with TGF- (1 ng/ml). As shown in Fig. 4A, cotransfection of cells with Smad3 and Smad4 showed an almost 20% reduction of Qp activity, which was further reduced to⬃75% after TGF- treatment. Cotransfection of Smad3/Smad4 and TGIF-expressing vector resulted in more than ⬃40% reduction of Qp activity and ⬃85% reduction after treatment with TGF-. This TGF--in-duced repression was inhibited by cotransfection of a Smad4 dominant-negative mutant. These results sug-gest that TGIF and Smad3/Smad4 cooperatively repress the Qp activity in response to TGF- treatment.
Association of Smad3/Smad4 with TGIF was then con-firmed by the coimmunoprecipitation method. Flag-tagged TGIF-expressing vector was cotransfected with Myc-tagged Smad3- and Smad4-expressing vectors into 293 cells, followed by TGF- treatment. As shown in Fig. 4B, the complex immunoprecipitated by Flag anti-body (for TGIF) contained Smad3 and Smad4, as de-tected by the subsequent Western blot analysis using anti-Myc antibody. Similarly, the anti-Smad4 antibody was able to immunoprecipitate the Flag-tagged TGIF. The ecotopic expression of TGIF, Smad3, and Smad4 was confirmed by Western blot analysis of the whole-cell lysates using anti-Flag and anti-Myc antibody, respec-tively (Fig. 4B). Results confirmed the physical associa-tion of TGIF and Smad proteins in this study.
Histone deacetylase (HDAC) is involved in TGF- -repressed Qp activity in type 1 BL cells
A recent report showed that the TGF--induced inhi-bition of the TGF-/activin response element
(ARE)-con-taining promoter (A3-Lux) activity results from the recruit-ment of a histone deacetylase (HDAC) by TGIF (Wotton et al., 1999). To determine whether HDAC participates in TGF- repression of Qp activity, Rael cells were trans-fected with pGL2B-50 and pGL2B-50m, and then treated with either 1 ng/ml of TGF- or 1 ng/ml of TGF- plus 100 ng/ml of trichostatin A (TSA), a specific inhibitor for HDAC. Plasmid pGL2B-50 contains ⫺50 to ⫹17 se-quence of Qp and plasmid pGL2B-50m contains muta-tions at the SBE site of pGL2B-50. As shown in Fig. 4C, pGL2B-50m showed elevated activity and showed loss of responsiveness to TGF- repression (gray bars) as com-pared to that of pGL2B-50. Treatment with TSA alone (data not shown) or combined treatment with TGF- and TSA (striped bars) also resulted in the release of Qp repression. Importantly, the promoter activity of pGL2B-50m was elevated more than threefold over that of pGL2B-50 in the presence of TGF-, and this activity was not affected by the addition of TSA. These results sug-gest that HDAC is involved in TGF- repression of Qp activity and this repression is mediated through the SBE site within Qp. Similar results were obtained using WW2 cells (data not shown).
DISCUSSION
EBV is known to infect and immortalize human B cells into continuously growing LCLs in vitro and is associated with many human malignant diseases. Earlier studies demonstrated that EBV and its gene products can induce TGF- production and secretion in human cells (Cayrol and Flemington, 1995). In sera of patients with EBV-associated BL and NPC, the concentration of TGF- is higher than that of healthy individuals (Xu et al., 1999, 2000), thus suggesting a role of this cytokine in the pathogenesis of EBV-associated diseases. In this study, we identified a transcription factor Smad4, which binds the SBE site within Qp. We also found that Smad proteins cooperated with TGIF to repress the Qp via the SBE site in type 1 BL cells in response to TGF- stimulation.
Qp is a latent promoter, selectively used in type 1 BL cells. Upon TGF- stimulation, this promoter was re-pressed through the SBE site. Mutation of the SBE site, therefore, released the TGF--induced repression (Fig. 4C). The SBE site is located within the previously iden-tified positive element QRE-1. QRE-1 is the potential binding site of the cellular transcription factor LBP-1, which shows significant promoter activity in conjunction with the interferon regulatory factor (IRF) binding site QRE-2 located between⫺12 and ⫹2 of Qp (Nonkwelo et al., 1995, 1997). In this study, we found that pGL2B-50 showed an almost 20-fold higher promoter activity than that of pGL2B-37. Promoter construct pGL2B-50 contains both the QRE-1 and the QRE-2 sites, while promoter construct pGL2B-37 contains only the QRE-2 site. As discussed above, both QRE-1 and QRE-2 sites are
quired for a significant promoter activity. This may ex-plain the low promoter activity of pGL2B-37 observed in this study (Fig. 1B).
The QRE-2- or IRF-binding site within Qp is also
over-lapped with two STAT sites (⫺15 to ⫺6 and ⫹1 to ⫹9 of Qp), which are important for positive regulation of Qp by the JAK/STAT pathway (Chen et al., 1999). However, this activation can be repressed by Zta, which up-regulates
FIG. 4. Transcriptional repression of Qp by cooperation of TGF--inducible Smads and TGIF. (A) Transcriptional repression of Qp by Smad3/Smad4 and TGIF. Rael cells were cotransfected with pGL2B-50 and 1 g each of the expressing plasmids of Smad3 and Smad4 with or without TGIF-expressing vector, and then treated with or without 1 ng/ml of TGF-. The plasmid pcDNA3 was used as the vector control. (B) Interaction of TGIF with Smad3/Smad4 by immunoprecipitation analysis. Flag-tagged TGIF and Myc-tagged Smad3- and Smad4-expressing vectors were cotransfected into 293 cells. Cells were first immunoprecipitated with anti-Flag antibody and analyzed by Western blot analysis with anti-Myc antibody, or first precipitated with anti-Smad4 antibody and then analyzed by Western blot analysis with anti-Flag antibody. The bands corresponding to Smad3, Smad4, and TGIF are indicated by horizontal bars. Lower panel: total cell lysates were directly subjected to Western blot analysis using anti-Flag for Flag-TGIF identification and anti-Myc antibody for both Myc-Smad3 and Myc-Smad4 identification. (C) Effect of TSA and TGF- on Qp activity. Qp construct pGL2B-50 and its mutant pGL2B-50m were individually electroporated into Rael cells and transfected cells were treated with 100 ng/ml of TSA, 1 ng/ml of TGF-, or both. Relative activity (fold increase) was measured by dividing the luciferase activity of each treatment with that of pGL2B-50 without any treatment. Results were the average of six independent transfections. Vertical bars show the standard deviations (SD).
p53 gene expression, and in turn leads to p53-mediated interference of JAK/STAT signaling (Chen et al., 1999). It is also known that Zta expression is activated by TGF-. Thus, Qp can be negatively regulated through the Zta-mediated interference of the JAK/STAT signaling path-way. Therefore, TGF- might activate Zta, which, in turn, represses Qp through the STAT sites. However, the pro-moter activity of pGL2B-50 was still repressed by TGF- in two EBV-negative BL cell lines, Ramos and Akata(⫺), to a level similar to that observed in EBV(⫹) BL cells (data not shown). This eliminates the possibility that Qp repression was the result of induction of the Zta protein by TGF-. It is also true that p53 is often mutated in BL cells. Therefore, Qp repression examined in this study was more likely a direct effect in response to TGF-. Physically, the potential IRF- and STAT-binding sites are near the transcription initiation site of Qp, whereas the SBE identified in this study is located⬃30 nt upstream from these sites. It is conceivable that TGF--induced Smad4 binding interferes with the LBP-1/IRF interaction at the transcriptional level and vice versa. This may suggest that the regulatory actions, either by STAT and IRF or by LBP-1 and Smad4, are mutually exclusive.
Transcriptional control in eukaryotic cells is involved in multiple layers of cooperativity (Cayrol and Flemington, 1995). TGF--induced transcription regulation is not lim-ited to interactions only between Smad proteins. Asso-ciation of Smad proteins with different transcriptional factors may contribute to the opposite effect, either tran-scriptional activation or trantran-scriptional repression. Tran-scriptional repression is often a result of recruitment of histone deacetylase (HDAC) to transcriptional corepres-sors such as TGIF (Wotton et al., 1999; this study) by forming a specific transcription complex. In this study, Qp repression was caused by recruitment of HDAC to the Smad/TGIF complex. Therefore, this supports a model in which TGIF is recruited to TGF--targeted promoters such as Qp, and functions as an adapter to link Smad to HDAC 1, leading to transcriptional repression. However, we cannot rule out that other repressors or unidentified factors may be also involved in this Qp repression. EBNA 1 is an EBV-encoded oncogene, which plays an impor-tant role in maintaining the viral episomes in infected cells. Qp is the key promoter in regulating EBNA 1 expression in EBV-associated tumors. Down-regulation of Qp by agents such as Smad4 and transcriptional corepressors might be a possible therapeutic approach to eradicate the EBV genome from those tumors.
Recent reports by Xu et al. (1999, 2000) indicated that the levels of TGF- in serum samples from patients with EBV-associated BL and NPC are elevated compared to those from healthy individuals. Levels of this cytokine could correlate with the severity of NPC (Xu et al., 1999), although it is not clear whether this is also true for BL patients. Furthermore, other cytokines may regulate the EBV lytic cycle in addition to TGF-. It has been reported
before that TGF- induces latent EBV to enter into the lytic cycle (di Renzo et al., 1994). Currently, we are testing whether the BZLF1 gene, a latent-to-lytic switching gene, is also regulated by TGF- at the transcriptional level. Study of the regulation of EBV genes by TGF- will be important to further understand the role of EBV in the development of tumors.
MATERIALS AND METHODS Cell lines
Rael and WW2 are EBV-positive type 1 Burkitt’s lym-phoma (BL) cell lines. These cells were maintained at 37°C in RPMI 1640 medium containing 15% (v/v) fetal bovine serum (FBS), 100 g/ml streptomycin, 100 U/ml penicillin, and 2 mM L-glutamine. The 293 cells were maintained in DMEM supplemented with 10% (v/v) FBS, 100g/ml streptomycin, and 100 g/ml penicillin. RNA extraction, RT-PCR, and Southern blot hybridization
Total RNA was isolated from Rael cells treated with 1 ng/ml of TGF- by using REzol C&T protocol (PROtech Technology, Taiwan, ROC). A 5-g sample of total RNA was heated at 70°C for 10 min and rapidly cooled on ice. Reverse transcription was carried out in a reaction con-taining 1M of gene-specific 3⬘ primer, 3⬘K 5⬘-CCATT-TCCAGGTCCTGTA-3⬘ for EBNA 1, 0.125 mM dNTP, 8 units of avian myeloblastosis virus reverse transcriptase (AMV-RT; Promega, Madison, WI), and 20 units of RNase inhibitor (Promega) at 42°C for 1 h. PCR assays were carried out by adding 10 pmol of 5⬘ primer 5⬘ Qp 5⬘-GCGTTTCTTGAGCTT-3⬘ for EBNA 1 transcripts and 2.5 units of Taq DNA polymerase (Promega) to one-third of the reverse transcription reaction mixture (Tsai et al., 1995). RT-PCR products were separated by electrophore-sis in 2% agarose gel and then transferred to a nitrocel-lulose membrane. The membrane was fixed in an XL-1000 UV CROSSLINKER (Spectronics, Westbury, NY) be-fore hybridization with [␣-32P]dCTP-labeled RT-PCR products. After the hybridization reaction, the mem-branes were washed in washing buffer containing 0.2⫻ SSC and 0.1% SDS for 30 min before exposure to X-ray films.
Yeast one-hybrid system
Five copies of⫺50 to ⫺37 (5⬘-TGTCTGGTCGCTAG-3⬘) of EBV Qp was inserted into the SmaI site of pLacZi and pHisi-1 (Clontech, Palo Alto, CA) to generate pLacZi-5SBE and pHisi-pLacZi-5SBE. Plasmid pLacZi-pLacZi-5SBE was linear-ized by NcoI and plasmid pHisi-5SBE was digested with XhoI. The linearized plasmids were transformed into yeast YM4271, to establish two stable clones, YB-5SBE and YH-5SBE. YH-5SBE yeast strain was transformed with the metastasized NPC biopsy cDNA library (2.6⫻
106individual colonies) generated with pGAD10 (custom-made by Clontech) and then selected on SD/His⫺/Leu⫺ plates with 45 mM 3-amino-1,2,4-triazole (3-AT; Sigma, St. Louis, MO). A total of 47 clones was obtained from the 2.6⫻ 106 colonies of the cDNA library. YB-5SBE yeast cells were transformed with the purified plasmid DNA and selected on SD/Ura⫺/Leu⫺ plates. The clones that showed positive -galactosidase (-gal) activity were isolated and the plasmid DNAs were purified before being subjecting to DNA sequence analysis. The-gal activity was assayed with n-nitrophenyl--D -galactopyr-anoside (ONPG) as the substrate by using the -galac-tosidase enzyme assay system (Promega).
Plasmid construction
Qp-containing reporter plasmids were constructed as follows. Various regions of the Qp (spanning nts 62291 to 62416 of the EBV genome) were individually generated by PCR and then cloned into the pGL2-Basic vector (Promega). Briefly, PCR products for pGL2B-125, -90, -50, -50m, and -37 were generated
by Q3/Fb, Q5(5⬘-GAAATTGGGTGACCACTG-3⬘)/Fb,
Q4(5⬘-TGTCTGGTCGCTAGATGG-3⬘)/Fb, Q4m(5⬘-TATG-TAGTCGCTAGATGG-3⬘)/Fb, and Q2/Fb, respectively, and were inserted into HincII-digested pUC18 (Gibco BRL, Life Technologies, Paisley, UK). The recombinant plasmids digested with HindIII and BamHI were eluted and subcloned into HindIII- and BglII-treated pGL2-Basic (Promega). These reporter plasmids were des-ignated as pGL2B-125, -90, -50, -50m, and -37, respec-tively (Fig.1) (Tsai et al., 1995).
The expression vectors of pcDNA3-Flag-Smad3, Smad4, and dominant-negative Smad4 mutant (SmadD4) were described previously (Zhang et al., 1997).
The full-length TGIF cDNA was generated by RT-PCR using primers TGIF-F (5⬘-AATGAAAGGCAAAGGT-3⬘) and TGIF-R (5⬘-TTAAGCCGTAAGTTTTGCC-3⬘). This RT-PCR product was digested with BglII and EcoRI, and then cloned to the BglII- and EcoRI-digested pFlag-CMV2 (Kodak, Rochester, NY). The plasmid was designated as pFlag-TGIF.
The sequences of all the constructs were confirmed by Sanger’s dideoxy chain-termination method (Sanger et al., 1977), using the T7 sequence version 2.0 DNA se-quencing kit (Amersham Life Science, Buckinghamshire, UK) according to the manufacturer’s protocol.
DNA transfection and TGF- treatment
Reporter plasmids (2.5g) and the indicated amounts of expression plasmids were introduced into Rael and WW2 cells by electroporation at a capacitance of 960F and a voltage of 0.22 kV using the Gene Pulser (Bio-Rad, Richmond, CA). After transfection, cells were harvested and lysed in 50l of lysis buffer (Promega). The concen-tration of the cell lysates was measured by using Bio-Rad
protein assay reagent. A 50-g sample of protein ex-tracts was used to assay the luciferase activity in an illuminometer (autolumat model LB953; Berthold, Ger-many).
For TGF- treatment, cells were first transfected with indicated plasmid DNA and cultured in RPMI 1640 sup-plemented with 0.2% FBS for 12 h, prior to treatment with 1 ng/ml of recombinant human TGF- 1 (R&D Systems, Minneapolis, MN) for 24 h. All of the plasmid DNAs were purified by the cesium chloride/ethidium bromide gradi-ent cgradi-entrifugation method.
Western blot analysis and immunoprecipitation For the Western blot analysis, cells were washed three times with phosphate-buffered saline (PBS) before being harvested and lysed in the buffer containing 10 mM Tris–HCl, pH 7.1, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM EDTA, and 1% Triton X-100. Cells were disrupted by sonication, after which the cell debris was removed by centrifugation at 10,000 g. The supernatants of the whole-cell lysates were then separated by NaDodSO4–polyacrylamide gel electrophoresis and then electrotransferred to nitrocellulose membranes and im-munoblotted with the indicated antibodies. Anti-Flag an-tibody (Kodak), Myc anan-tibody, and Smad4 anti-body (Santa Cruz Biotechnology, Santa Cruz, CA) were obtained commercially. The blot was then subjected to the ECL system (Amersham, Arlington Heights, IL) for the detection of TGIF or Smad3/Smad4 proteins.
Coimmunoprecipitation of Smads and TGIF was per-formed as previously described by Wotton et al. (1999). The immunoprecipitates were analyzed by SDS–PAGE, followed by the Western blot analysis as described above.
Electrophoretic mobility shift assay
The double-stranded oligonucleotide containing two copies of the⫺54 to ⫺40 sequence of Qp promoter was used as the probe in the electrophoretic mobility shift assay (EMSA). In brief, the oligonucleotide was labeled with T4 polynucleotide kinase and [␥-32P]ATP. Nuclear extracts prepared from either Rael cells or 293 cells infected by a recombinant adenovirus carrying Flag-tagged Smad4 (kindly provided by Dr. Kohei Miyazono) were incubated with 15,000 cpm of [␥-32P]-labeled oligo-nucleotide for 10 min at room temperature in a buffer containing 0.13 g/l poly dI:dC, 4% glycerol, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 50 mM NaCl, and 10 mM Tris–HCl, pH 7.5. For competition assay, a 200-fold excess of the individual cold double-stranded oligonu-cleotides was added into the reaction mixture before addition of the [␥-32P]-labeled probe. After a 30-min in-cubation at room temperature, the samples were loaded onto 4% nondenaturing acrylamide gel and electrophore-sis was carried out in 0.5⫻ TBE at 100 V for 2.5–3 h. For
the supershift assay, the reaction mixture was incubated at 4°C with 4 g of anti-Smad4 antibody (Santa Cruz Biotechnology), anti-ATF-2 antibody (Santa Cruz Biotech-nology), and anti-Flag antibody (Kodak), respectively. The gel was subsequently dried and exposed to Kodak BioMax film.
ACKNOWLEDGMENTS
We thank Drs. R. Derynck and M. Kawabata for Smad constructs, Dr. Kohei Miyazono for Smad4-containing recombinant adenovirus, and Dr. A. Rickinson for type 1 BL cells. We also thank Dr. J. S. Huang for critical reading and helpful discussion of this work. This study was supported by Grant CMRP721 from Chang-Gung University and Chang-Gung Me-morial Hospital, and by Grants NSC 88-2314-B-182-022 and NSC 89-2320-B-182-001 to Y.S.C. and NSC 88-2312-B-002-050 to R.H.C. from the National Science Council, Taiwan, Republic of China.
REFERENCES
Cayrol, C., and Flemington, E. K. (1995). Identification of cellular target genes of the Epstein–Barr virus transactivator Zta: Activation of transforming growth factor beta igh3 (TGF-beta igh3) and TGF-beta 1.
J. Virol. 69, 4206–4212.
Chen, H., Lee, J. M., Wang, Y., Huang, D. P., Ambinder, R. F., and Hayward, S. D. (1999). The Epstein–Barr virus latency BamHI-Q promoter is positively regulated by STATs and Zta interference with JAK/STAT activation leads to loss of BamHI-Q promoter activity. Proc.
Natl. Acad. Sci. USA 96, 9339–9344.
di Renzo, L., Altiok, A., Klein, G., and Klein, E. (1994). Endogenous TGF-beta contributes to the induction of the EBV lytic cycle in two Burkitt’s lymphoma cell lines. Int. J. Cancer 57, 914–919.
Feng, X. H., Zhang, Y., Wu, R. Y., and Derynck, R. (1998). The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for smad3 in TGF-beta-induced transcriptional activa-tion. Genes Dev. 12, 2153–2163.
Keeton, M. R., Curriden, S. A., van Zonneveld, A. J., and Loskutoff, D. J. (1991). Identification of regulatory sequences in the type 1 plasmin-ogen activator inhibitor gene responsive to transforming growth factor beta. J. Biol. Chem. 266, 23048–23052.
Massague, J. (1998). TGF-beta signal transduction. Annu. Rev. Biochem. 67, 753–791.
Nonkwelo, C., Henson, E. B., and Sample, J. (1995). Characterization of the Epstein–Barr virus Fp promoter. Virology 206, 183–195.
Nonkwelo, C., Ingrid, K. R., and Sample, J. (1997). The Epstein–Barr virus EBNA-1 promoter Qp requires an initiator-like element. J. Virol. 71, 354–361.
Rickinson, A. B., and Kieff. E. (1996). Epstein–Barr virus. In “Field’s Virology” (B. N. Field, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Straus, Eds.), Vol. 2, 3rd ed., pp. 2397–2446. Lippincott-Raven, Philadephia.
Rowe, D. T., Rowe, M., Evan, G. I., Wallace, L. E., Farrell, P. J., and Rickinson, A. B. (1986). Restricted expression of EBV latent genes and T-lymphocyte-detected membrane antigen in Burkitt’s lymphoma cells. EMBO J. 5, 2599–2607.
Sanger, F., Nicklen, S., and Coulson, A. R. (1977). DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74, 5463– 5467.
Sheu, L. F., Chen, A., Meng, C. L., Ho, K. C., Lee, W. H., Leu, F. J., and Chao, C. F. (1996). Enhanced malignant progression of nasopharyn-geal carcinoma cells mediated by the expression of Epstein–Barr nuclear antigen 1 in vivo. J. Pathol. 180, 243–248.
Tsai, C. N., Liu, S. T., and Chang, Y. S. (1995). Identification of a novel promoter located within the BamHI Q region of the Epstein–Barr virus genome for the EBNA 1 gene. DNA Cell Biol. 14, 767–776. Wilson, J. B., Bell, J. L., and Levine, A. J. (1996). Expression of Epstein–
Barr virus nuclear antigen-1 induces B cell neoplasia in transgenic mice. EMBO J. 15, 3117–3126.
Wotton, D., Lo, R. S., Lee, S., and Massague, J. (1999). A Smad tran-scriptional corepressor. Cell 97, 29–39.
Xu, J., Ahmad, A., Jones, J. F., Dolcetti, R., Vaccher, E., Prasad, U., and Menezes, J. (2000). Elevated serum transforming growth factor 1 levels in Epstein–Barr virus-associated diseases, and their correla-tion with virus-specific immunoglobulin A (IgA), and IgM. J. Virol. 74, 2443–2446.
Xu, J., Menezes, J., Prasad, U., and Ahmad, A. (1999). Elevated serum levels of transforming growth factor beta1 in Epstein–Barr virus-associated nasopharyngeal carcinoma patients. Int. J. Cancer 84, 396–399.
Yates, J. L., Warren, N., and Sugden, B. (1985). Stable replication of plasmids derived from Epstein–Barr virus in various mammalian cells. Nature 313, 812–815.
Zawel, L., Dai, J. L., Buckhaults, P., Zhou, S., Kinzler, K. W., Vogelstein, B., and Kern, S. E. (1998). Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cells 1, 611–617.
Zhang, Y., Musci, T., and Derynck, R. (1997). The tumor suppressor Smad4/DPC 4 as a central mediator of Smad function. Curr. Biol. 7, 270–276.