Impact of EZH2 Polymorphisms on Urothelial Cell Carcinoma Susceptibility and Clinicopathologic Features
Yung-Luen Yu1,2,3,#, Kuo-Jung Su2,#, Shun-Fa Yang4,5,*
1Graduate Institute of Cancer Biology, and Center for Molecular Medicine, China Medical University, Taichung 404, Taiwan
2The Ph.D. Program for Cancer Biology and Drug Discovery, China Medical University, Taichung 404, Taiwan
3Department of Biotechnology, Asia University, Taichung 413, Taiwan 4Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
5Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan
#These authors contributed equally to the work.
*Address correspondence to: Shun-Fa Yang, Ph.D., Institute of Medicine, Chung Shan Medical University, Taichung 402, Taiwan; Tel: +886-4-24739595 ext. 34253; Fax: +886-4-24723229; E-mail: ysf@csmu.edu.tw.
Competing Interests: The authors declare that no competing interests exist.
Running title: EZH2 Polymorphisms in UCC [Authors: The short title should be included during online submission but not in the manuscript.]
Funding:
This work was supported by the following grants: NSC101-2321-B-039-004, and NHRI-EX102-10245BI. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
Abstract
Background: The gene EZH2, the polycomb group protein enhancer of zeste 2, encodes a transcriptional repressor that also serves as a histone methyltransferase that is associated with progression to more advanced disease in a variety of malignancies. EZH2 expression level in urothelial cell carcinoma (UCC) is highly correlated with tumor aggressiveness, but it has not been determined if specific EZH2 genetic variants are associated with UCC risk. This study investigated the potential associations of EZH2 single-nucleotide polymorphisms with UCC susceptibility and its clinicopathologic characteristics.
Methodology/Principal Findings: A total of 233 UCC patients and 552 cancer-free controls, all of whom were from Taiwan, were analyzed for four EZH2 single-nucleotide polymorphisms (rs6950683, rs2302427, rs3757441, and rs41277434) using real-time PCR genotyping. After adjusting for other co-variants, we found that individuals carrying at least one C allele at EZH2 rs6950683 or at least one G allele at rs2302427 had a lower risk of developing UCC than did wild-type carriers. The CCCA or TGTA haplotype among the four EZH2 sites was also associated with a reduced risk of UCC. Furthermore, UCC patients who carried at least one G allele at rs2302427 had a lower invasive tumor stage than did patients carrying the wild-type allele.
Conclusions: The rs6950683 and rs2302427 polymorphic genotypes of EZH2 might contribute to the prediction of UCC susceptibility. This is the first study to provide insight into risk factors associated with EZH2 variants in carcinogenesis of UCC in Taiwan.
Keywords: Enhancer of zeste 2, urothelial cell carcinoma, single-nucleotide polymorphism 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51
Introduction
The urothelium covers the epithelial lining of the urinary tract from the renal calyces to the bladder. The vast majority of urothelial cell carcinomas (UCCs) occur in the bladder, whereas only approximately 5–10% of UCCs occur in the upper urinary tract . UCC is a highly aggressive malignancy that causes substantial morbidity and mortality . The major clinical distinction is between the non-muscle invasive Ta and T1 tumors and the muscle invasive T2–T4 tumors . The molecular biology of UCC has been extensively studied in recent years, and many genetic alterations and modified expression patterns of certain oncogenes and tumor suppressor genes have been linked to its tumorigenesis and progression .
Epigenetic changes by DNA methylation at CG dinucleotide sites (CpGs) are frequent events in tumor development . Most differential DNA methylation has been attributed to genes that are essential for developmental processes, often polycomb repressive complex 2 (PRC2)–regulated genes . EZH2 is a subunit of PRC2 and is involved in chromatin compaction and gene repression . Recently, EZH2 was linked to the aggressiveness of human cancers, including breast cancer and prostate cancer . Overexpression of EZH2 correlates with advanced stages of human cancer progression and poor prognosis . In addition, EZH2 promotes the epithelial– mesenchymal transition, a process that is associated with cancer progression and metastasis .
Single-nucleotide polymorphisms (SNPs) are easy-to-detect genetic variants because they can be analyzed from blood samples . Epidemiological studies suggest that SNPs are important in mediating an individual’s susceptibility to many types of cancer . Although EZH2 contributes to the formation of many types of cancer, the association between EZH2 variants and UCC risk and prognosis has been poorly investigated. We therefore performed a case-control study of four SNPs located in the promoter, exonic, and intronic regions of EZH2 to assess the associations between these SNPs and UCC susceptibility and clinicopathologic characteristics.
Materials and Methods
Study subjects and specimen collection
This hospital-based case-control study recruited 233 UCC patients between 2010 and 2012 at the Taichung Veterans General Hospital in Taichung, Taiwan. The diagnosis of UCC was made according to the criteria specified in the national 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85
guidelines for UCC. An additional 552 race- and ethnic group–matched non-cancer patients who entered the hospital for health check-ups were enrolled as the control group.
UCC patients were clinically staged at the time of diagnosis according to the tumor/node/metastasis staging system of the American Joint Committee on Cancer (2002) [16]. The patients’ clinicopathological characteristics, including clinical staging, lymph node metastasis, and histopathologic grading levels, were verified by chart review. Whole-blood specimens collected from the controls and UCC patients were placed in tubes containing EDTA, immediately centrifuged, and stored at −80°C. Before commencing the study, approval was obtained from the Institutional Review Board of Taichung Veterans General Hospital, and informed written consent was obtained from each individual.
Selection of EZH2 Polymorphisms
A total of four SNPs in EZH2 (NM_004456) were selected from the International HapMap Project data for this study. We included the non-synonymous SNP rs2302427 (D185H in exon 6) in the coding sequence of the gene. The other SNPs (rs6950683, rs3757441, and rs41277434) were selected in this study because they have been found in cancer patients .
Genomic DNA extraction
Genomic DNA was extracted using QIAamp DNA blood mini kit reagents (Qiagen, Valencia, CA). DNA was dissolved in buffer containing 10 mM Tris (pH 7.8) and 1 mM EDTA and then quantified by measurement of the optical density at 260 nm. Final DNA preparations were stored at −20°C and used as templates for PCR .
Real-time PCR
Allelic discrimination of the EZH2 polymorphisms rs6950683, rs2302427, rs3757441, and rs41277434 was assessed using an ABI StepOneTM Real-Time PCR System (Applied Biosystems), SDS v3.0 software (Applied Biosystems), and the TaqMan assay. The final volume for each reaction was 5 μL, containing 2.5 μL TaqMan Genotyping Master Mix, 0.125 μL TaqMan probes mix, and 10 ng genomic DNA. The reaction conditions included an initial denaturation step at 95°C for 10 min 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119
followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min . Statistical analysis
Hardy–Weinberg equilibrium was assessed using a χ2 goodness-of-fit test for biallelic markers. A Mann-Whitney U-test and a Fisher’s exact test were used to compare differences of age and demographic characteristics distributions between controls and UCC patients. The odds ratios (ORs) with 95% confidence intervals (CIs) were estimated by logistic regression models. The adjusted odds ratios (AORs) with 95% CIs of the association between genotype frequencies and UCC risk as well as clinical pathological characteristics were estimated by multiple logistic regression models after controlling for other covariates. The haplotype-based analysis was carried out using the Phase program. All p values < 0.05 were considered significant. The data were analyzed using SAS statistical software (Version 9.1, 2005; SAS Institute Inc., Cary, NC).
Results
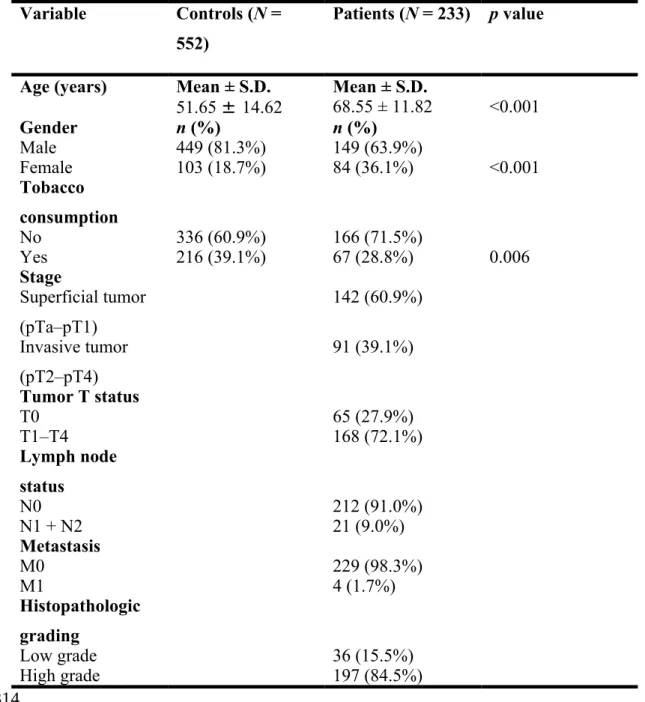
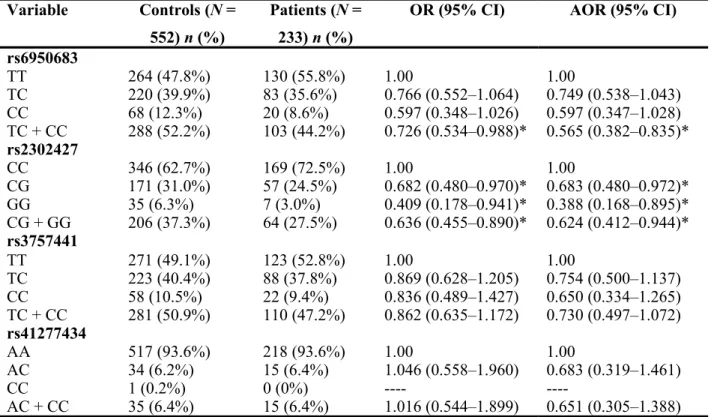
This study analyzes the demographic characteristics of sample specimens and found that there were significantly different distributions of age (control: 51.65 ± 14.62; UCC: 68.55 ± 11.82; p < 0.001) and gender (p < 0.001) between healthy controls and UCC patients (Table 1). To reduce possible interference of confounding variables, AORs with 95% CIs were estimated by multiple logistic regression models after controlling for age and gender in each comparison. Table 2 shows the genotype distributions and the association between UCC and EZH2 polymorphisms. The alleles with the highest distribution frequency at EZH2 rs6950683, rs2302427, rs3757441, and rs41277434 in both UCC patients and controls were homozygous T/T, homozygous C/C, homozygous T/T, and homozygous A/A, respectively. Individuals carrying TC + CC at rs6950683 showed a 0.565-fold (95% CI: 0.382–0.835) lower risk of UCC, and those carrying CG, GG, or CG + GG at rs2302427 showed a 0.683-fold (95% CI: 0.480–0.972), 0.388-0.683-fold (95% CI: 0.168–0.895) and a 0.624-0.683-fold (95% CI: 0.412–0.944) lower risk of UCC, respectively, compared with individuals carrying the wild-type allele. Individuals with polymorphisms at rs3757441 and rs41277434 showed no reduction in UCC risk compared with wild-type individuals.
The distribution of clinical status/EZH2 genotypes in UCC patients was estimated to clarify the role of EZH2 polymorphisms in the clinicopathologic state of 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153
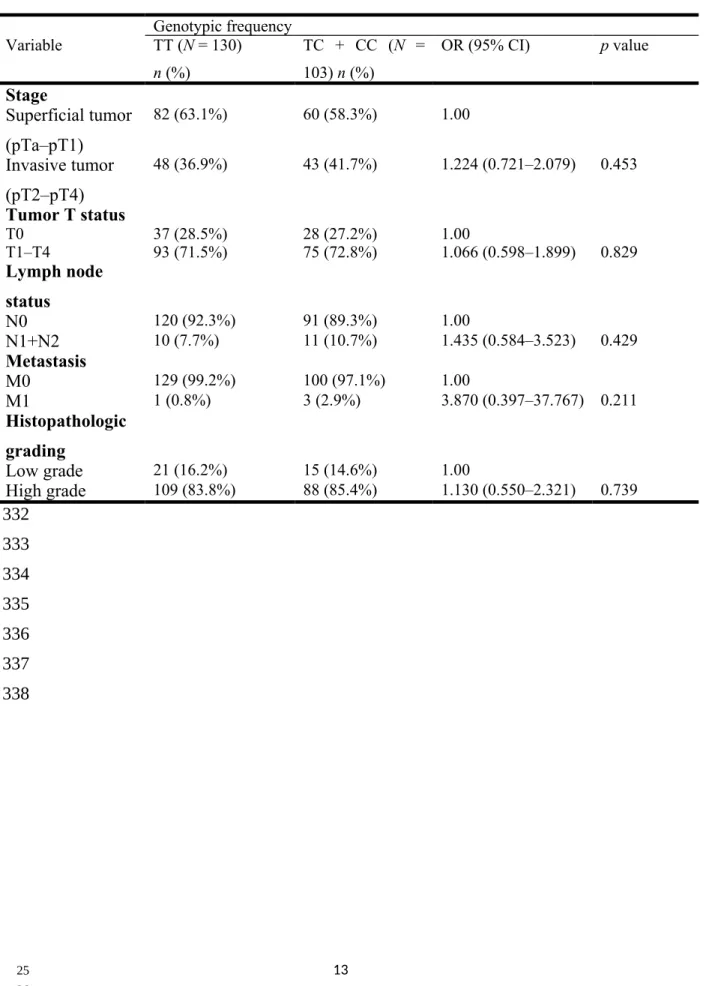
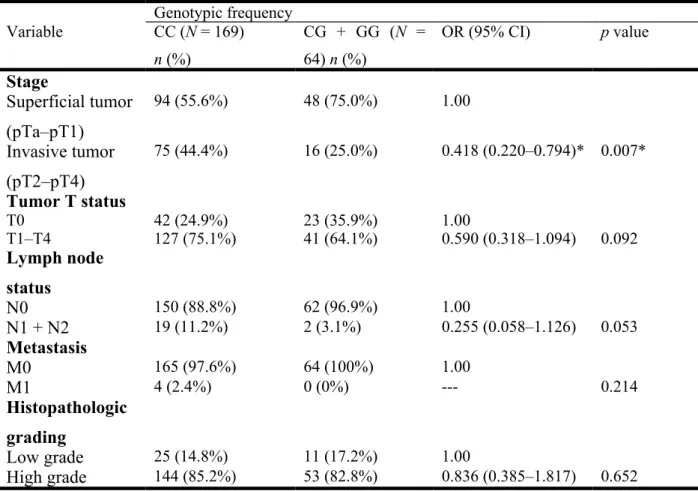
UCC patients. Clinical status assessments included tumor/node/metastasis staging, lymph node status, metastasis, and histopathologic grading. Compared with control subjects having the wild-type genotype, patients with at least one polymorphic C allele at EZH2 rs6950683 showed no significant differences between EZH2 genotypic frequencies and clinicopathological variables (Table 3); however, patients with at least one polymorphic G allele at EZH2 rs2302427 showed a 0.418-fold (95% CI: 0.220–0.794) decrease in invasive tumor stage (Table 4). No significant differences were observed between other EZH2 genotypic frequencies and any clinicopathological variables (data not shown).
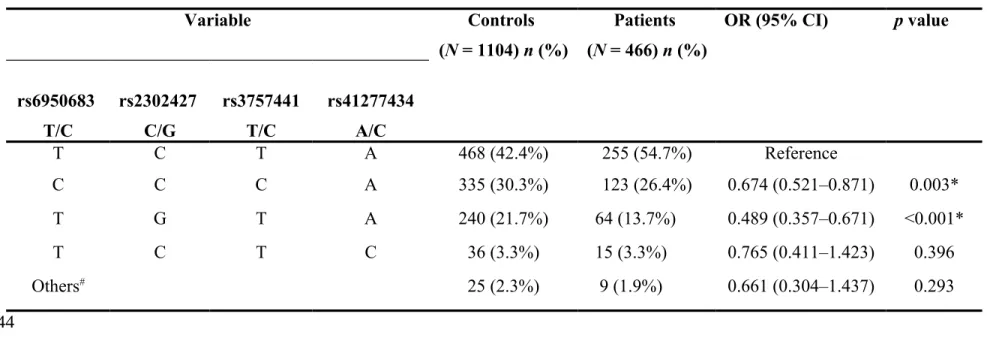
The haplotype distributions of EZH2 rs6950683, rs2302427, rs3757441, and rs41277434 were further evaluated, and eight haplotypes were derived from these four SNPs in our recruited individuals. The most common haplotype in the control group was TCTA (42.4%), and it was, therefore, chosen as the reference. Compared with this reference, two minor haplotypes, CCCA and TGTA, significantly reduced the risk of UCC by 0.674-fold (95% CI: 0.521–0.871) and 0.489-fold (95% CI: 0.357–0.671), respectively (Table 5).
Discussion
Epigenetic gene regulation has evolved as a key mechanism contributing to cell-cycle control and cell-fate determination . The polycomb group proteins function in an epigenetic regulatory system associated with gene silencing . EZH2 plays an important role in cell-cycle regulation, and its gene has emerged as a novel oncogene and putative target for therapy . Previous studies have suggested that EZH2 expression is high in UCC tumors with advanced stage and higher grades . Therefore, EZH2 polymorphisms may be associated with UCC development. However, the predictive value of EZH2 for susceptibility to UCC has not previously been investigated.
SNPs in genes encoding cancer susceptibility factors have been documented to influence gene expression, protein function, and disease susceptibility in certain individuals . We performed a genetic association analysis of EZH2 variants with UCC. EZH2 contains 20 exons, 19 introns, and 41 identified SNPs and encodes two isoforms of different transcript sizes . In our present hospital-based case-control study, four EZH2 SNPs were genotyped in 233 patients with UCC and 552 healthy controls. We observed that SNPs rs6950683 and rs2302427 were strongly associated 154 155 156 157 158 159 160 161 162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177 178 179 180 181 182 183 184 185 186 187
with reduced UCC risk (Table 2).
SNP rs6950683 is located in a tissue-specific CpG island within the EZH2 promoter region upstream of the EZH2 coding sequence. Thus, it may impact gene expression by affecting promoter function. SNP rs2302427 is located in exon 6 and causes a non-synonymous amino-acid change from aspartic acid to histidine, which might affect the protein function. Further functional studies are needed to confirm the specific mechanisms by which these EZH2 polymorphisms influence UCC development.
In recent years, haplotype-based association studies have identified genetic variants that predict cancer predisposition . Although many SNPs have no direct effect on gene products, they can still be used as genetic markers to locate adjacent functional variants that contribute to disease. When each SNP involved the haplotypes has a true contribution to disease susceptibility, haplotype analysis provides greater statistical power. Therefore, haplotype analysis is sometimes advantageous over analysis of individual SNPs for detecting an association between alleles and a disease phenotype . Our haplotype analysis of the four EZH2 SNPs rs6950683, rs2302427, rs3757441, and rs41277434 revealed that the CCCA and TGTA haplotypes were associated with a lower risk of UCC (Table 5). However, it is possible that these EZH2 SNPs are linked with other functional polymorphisms and are, therefore, not directly responsible for the decreased susceptibility to UCC.
To the best of our knowledge, this is the first study to associate EZH2 polymorphisms with risk of UCC. Results showed two SNPs of EZH2 decreased UCC susceptibility, and UCC patients with the polymorphism rs2302427 had a lower risk of invasive tumor stage. These results suggest that EZH2 variants might be novel susceptibility markers linked to UCC. However, the number of case and control subjects in this study was relatively small, and thus additional studies with larger sample sizes are needed to validate the relevance of EZH2 polymorphisms to UCC. Further investigations should focus on EZH2 sequence variants and their biological function in UCC patients.
[Authors: Consider adding an Acknowledgments section here. See the Author Instructions for further guidance.]
References 188 189 190 191 192 193 194 195 196 197 198 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216 217 218 219 220 221
1. Munoz JJ, Ellison LM (2000) Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol 164: 1523-1525.
2. Stenzl A, Cowan NC, De Santis M, Kuczyk MA, Merseburger AS, et al. (2011) Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol 59: 1009-1018.
3. Lauss M, Aine M, Sjodahl G, Veerla S, Patschan O, et al. (2012) DNA methylation analyses of urothelial carcinoma reveal distinct epigenetic subtypes and an association between gene copy number and methylation status. Epigenetics 7: 858-867.
4. Hinz S, Kempkensteffen C, Christoph F, Hoffmann M, Krause H, et al. (2008) Expression of the polycomb group protein EZH2 and its relation to outcome in patients with urothelial carcinoma of the bladder. J Cancer Res Clin Oncol 134: 331-336.
5. Esteller M (2007) Epigenetic gene silencing in cancer: the DNA hypermethylome. Hum Mol Genet 16 Spec No 1: R50-59.
6. Hansen KD, Timp W, Bravo HC, Sabunciyan S, Langmead B, et al. (2011) Increased methylation variation in epigenetic domains across cancer types. Nat Genet 43: 768-775.
7. Schlesinger Y, Straussman R, Keshet I, Farkash S, Hecht M, et al. (2007) Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat Genet 39: 232-236.
8. Yu YL, Chou RH, Shyu WC, Hsieh SC, Wu CS, et al. (2013) Smurf2-mediated degradation of EZH2 enhances neuron differentiation and improves functional recovery after ischaemic stroke. EMBO Mol Med 5: 531-547.
9. Heyn H, Esteller M (2013) EZH2: an epigenetic gatekeeper promoting lymphomagenesis. Cancer Cell 23: 563-565.
10. Kleer CG, Cao Q, Varambally S, Shen R, Ota I, et al. (2003) EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A 100: 11606-11611.
11. Ren G, Baritaki S, Marathe H, Feng J, Park S, et al. (2012) Polycomb protein EZH2 regulates tumor invasion via the transcriptional repression of the metastasis suppressor RKIP in breast and prostate cancer. Cancer Res 72: 3091-3104. 12. Sauvageau M, Sauvageau G (2010) Polycomb group proteins: multi-faceted
regulators of somatic stem cells and cancer. Cell Stem Cell 7: 299-313.
13. Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani RS, et al. (2008) Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 27: 7274-7284.
14. Di Paolo A, Danesi R, Del Tacca M (2004) Pharmacogenetics of neoplastic 222 223 224 225 226 227 228 229 230 231 232 233 234 235 236 237 238 239 240 241 242 243 244 245 246 247 248 249 250 251 252 253 254 255 256 257 258 259
diseases: new trends. Pharmacol Res 49: 331-342.
15. Shastry BS (2002) SNP alleles in human disease and evolution. J Hum Genet 47: 561-566.
16. Yu YL, Su KJ, Hsieh YH, Lee HL, Chen TY, et al. (2013) Effects of EZH2 polymorphisms on susceptibility to and pathological development of hepatocellular carcinoma. PLoS One 8: e74870.
17. Crea F, Fornaro L, Paolicchi E, Masi G, Frumento P, et al. (2012) An EZH2 polymorphism is associated with clinical outcome in metastatic colorectal cancer patients. Ann Oncol 23: 1207-1213.
18. Yoon KA, Gil HJ, Han J, Park J, Lee JS (2010) Genetic polymorphisms in the polycomb group gene EZH2 and the risk of lung cancer. J Thorac Oncol 5: 10-16.
19. Yiang GT, Harn HJ, Yu YL, Hu SC, Hung YT, et al. (2009) Immunotherapy: rAAV2 expressing interleukin-15 inhibits HeLa cell tumor growth in mice. J Biomed Sci 16: 47.
20. Yu YL, Wei CW, Chen YL, Chen MH, Yiang GT (2010) Immunotherapy of breast cancer by single delivery with rAAV2-mediated interleukin-15 expression. Int J Oncol 36: 365-370.
21. Wu CS, Yen CJ, Chou RH, Li ST, Huang WC, et al. (2012) Cancer-associated carbohydrate antigens as potential biomarkers for hepatocellular carcinoma. PLoS One 7: e39466.
22. Yu YL, Su KJ, Chen CJ, Wei CW, Lin CJ, et al. (2012) Synergistic anti-tumor activity of isochaihulactone and paclitaxel on human lung cancer cells. J Cell Physiol 227: 213-222.
23. Cantone I, Fisher AG (2013) Epigenetic programming and reprogramming during development. Nat Struct Mol Biol 20: 282-289.
24. Yu YL, Chou RH, Wu CH, Wang YN, Chang WJ, et al. (2012) Nuclear EGFR suppresses ribonuclease activity of polynucleotide phosphorylase through DNAPK-mediated phosphorylation at serine 776. J Biol Chem 287: 31015-31026.
25. Simon JA, Lange CA (2008) Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res 647: 21-29.
26. Raman JD, Mongan NP, Tickoo SK, Boorjian SA, Scherr DS, et al. (2005) Increased expression of the polycomb group gene, EZH2, in transitional cell carcinoma of the bladder. Clin Cancer Res 11: 8570-8576.
27. Arisan S, Buyuktuncer ED, Palavan-Unsal N, Caskurlu T, Cakir OO, et al. (2005) Increased expression of EZH2, a polycomb group protein, in bladder carcinoma. Urol Int 75: 252-257.
260 261 262 263 264 265 266 267 268 269 270 271 272 273 274 275 276 277 278 279 280 281 282 283 284 285 286 287 288 289 290 291 292 293 294 295 296 297
28. Chung TT, Pan MS, Kuo CL, Wong RH, Lin CW, et al. (2011) Impact of RECK gene polymorphisms and environmental factors on oral cancer susceptibility and clinicopathologic characteristics in Taiwan. Carcinogenesis 32: 1063-1068. 29. International HapMap C (2003) The International HapMap Project. Nature 426:
789-796.
30. Cardoso C, Mignon C, Hetet G, Grandchamps B, Fontes M, et al. (2000) The human EZH2 gene: genomic organisation and revised mapping in 7q35 within the critical region for malignant myeloid disorders. Eur J Hum Genet 8: 174-180.
31. Chung YT, Hsieh LL, Chen IH, Liao CT, Liou SH, et al. (2009) Sulfotransferase 1A1 haplotypes associated with oral squamous cell carcinoma susceptibility in male Taiwanese. Carcinogenesis 30: 286-294.
298 299 300 301 302 303 304 305 306 307 308 309 310 311
Table 1. Demographic characteristics of controls and patients with UCC.
Variable Controls (N =
552)
Patients (N = 233) p value
Age (years) Mean ± S.D. Mean ± S.D.
51.65 ± 14.62 68.55 ± 11.82 <0.001 Gender n (%) n (%) Male 449 (81.3%) 149 (63.9%) Female 103 (18.7%) 84 (36.1%) <0.001 Tobacco consumption No 336 (60.9%) 166 (71.5%) Yes 216 (39.1%) 67 (28.8%) 0.006 Stage Superficial tumor (pTa–pT1) 142 (60.9%) Invasive tumor (pT2–pT4) 91 (39.1%) Tumor T status T0 65 (27.9%) T1–T4 168 (72.1%) Lymph node status N0 212 (91.0%) N1 + N2 21 (9.0%) Metastasis M0 229 (98.3%) M1 4 (1.7%) Histopathologic grading Low grade 36 (15.5%) High grade 197 (84.5%)
Mann-Whitney U test or Fisher’s exact test was used to determine the significance of differences between healthy controls and patients with UCC.
312 313 314 315 316 317 318 319
Table 2. Distribution frequency of EZH2 genotypes in controls and patients with UCC. Variable Controls (N = 552) n (%) Patients (N = 233) n (%)
OR (95% CI) AOR (95% CI) rs6950683 TT 264 (47.8%) 130 (55.8%) 1.00 1.00 TC 220 (39.9%) 83 (35.6%) 0.766 (0.552–1.064) 0.749 (0.538–1.043) CC 68 (12.3%) 20 (8.6%) 0.597 (0.348–1.026) 0.597 (0.347–1.028) TC + CC 288 (52.2%) 103 (44.2%) 0.726 (0.534–0.988)* 0.565 (0.382–0.835)* rs2302427 CC 346 (62.7%) 169 (72.5%) 1.00 1.00 CG 171 (31.0%) 57 (24.5%) 0.682 (0.480–0.970)* 0.683 (0.480–0.972)* GG 35 (6.3%) 7 (3.0%) 0.409 (0.178–0.941)* 0.388 (0.168–0.895)* CG + GG 206 (37.3%) 64 (27.5%) 0.636 (0.455–0.890)* 0.624 (0.412–0.944)* rs3757441 TT 271 (49.1%) 123 (52.8%) 1.00 1.00 TC 223 (40.4%) 88 (37.8%) 0.869 (0.628–1.205) 0.754 (0.500–1.137) CC 58 (10.5%) 22 (9.4%) 0.836 (0.489–1.427) 0.650 (0.334–1.265) TC + CC 281 (50.9%) 110 (47.2%) 0.862 (0.635–1.172) 0.730 (0.497–1.072) rs41277434 AA 517 (93.6%) 218 (93.6%) 1.00 1.00 AC 34 (6.2%) 15 (6.4%) 1.046 (0.558–1.960) 0.683 (0.319–1.461) CC 1 (0.2%) 0 (0%) ---- ----AC + CC 35 (6.4%) 15 (6.4%) 1.016 (0.544–1.899) 0.651 (0.305–1.388)
The odds ratios (ORs) and their 95% confidence intervals (CIs) were estimated by logistic regression models. The adjusted odds ratios (AORs) with their 95% CIs were estimated by multiple logistic regression models after controlling for age and gender. *p value < 0.05. 320 321 322 323 324 325 326 327 328
Table 3. Distribution frequency of the clinical status and of the EZH2 rs6950683 genotype in patients with UCC.
Genotypic frequency Variable TT (N = 130) n (%) TC + CC (N = 103) n (%) OR (95% CI) p value Stage Superficial tumor (pTa–pT1) 82 (63.1%) 60 (58.3%) 1.00 Invasive tumor (pT2–pT4) 48 (36.9%) 43 (41.7%) 1.224 (0.721–2.079) 0.453 Tumor T status T0 37 (28.5%) 28 (27.2%) 1.00 T1–T4 93 (71.5%) 75 (72.8%) 1.066 (0.598–1.899) 0.829 Lymph node status N0 120 (92.3%) 91 (89.3%) 1.00 N1+N2 10 (7.7%) 11 (10.7%) 1.435 (0.584–3.523) 0.429 Metastasis M0 129 (99.2%) 100 (97.1%) 1.00 M1 1 (0.8%) 3 (2.9%) 3.870 (0.397–37.767) 0.211 Histopathologic grading Low grade 21 (16.2%) 15 (14.6%) 1.00 High grade 109 (83.8%) 88 (85.4%) 1.130 (0.550–2.321) 0.739 329 330 331 332 333 334 335 336 337 338
Table 4. Distribution frequency of the clinical status and of the EZH2 rs2302427 genotype in patients with UCC.
Genotypic frequency Variable CC (N = 169) n (%) CG + GG (N = 64) n (%) OR (95% CI) p value Stage Superficial tumor (pTa–pT1) 94 (55.6%) 48 (75.0%) 1.00 Invasive tumor (pT2–pT4) 75 (44.4%) 16 (25.0%) 0.418 (0.220–0.794)* 0.007* Tumor T status T0 42 (24.9%) 23 (35.9%) 1.00 T1–T4 127 (75.1%) 41 (64.1%) 0.590 (0.318–1.094) 0.092 Lymph node status N0 150 (88.8%) 62 (96.9%) 1.00 N1 + N2 19 (11.2%) 2 (3.1%) 0.255 (0.058–1.126) 0.053 Metastasis M0 165 (97.6%) 64 (100%) 1.00 M1 4 (2.4%) 0 (0%) --- 0.214 Histopathologic grading Low grade 25 (14.8%) 11 (17.2%) 1.00 High grade 144 (85.2%) 53 (82.8%) 0.836 (0.385–1.817) 0.652 339 340 341 342
Table 5. Distribution frequency of EZH2 haplotype in controls and UCC patients. Variable Controls (N = 1104) n (%) Patients (N = 466) n (%) OR (95% CI) p value rs6950683 T/C rs2302427 C/G rs3757441 T/C rs41277434 A/C T C T A 468 (42.4%) 255 (54.7%) Reference C C C A 335 (30.3%) 123 (26.4%) 0.674 (0.521–0.871) 0.003* T G T A 240 (21.7%) 64 (13.7%) 0.489 (0.357–0.671) <0.001* T C T C 36 (3.3%) 15 (3.3%) 0.765 (0.411–1.423) 0.396 Others# 25 (2.3%) 9 (1.9%) 0.661 (0.304–1.437) 0.293
#Others: CCTA (20), TGCA (7), TCCA (6), CGTA (1) 343
344 345 346 347