IMMUNODIAGNOSIS OF ANGIOSTRONGYLIASIS WITH
MON~CLONAL ANTIBODIES RECOGNIZING A CIRCULATING
ANTIGEN OF MOL.WT 91,000 FROM A~Gr~STR~NGY~U~
CANTONENSIS
H. H. SHIH
and S. N.
CHENDepartment of Zoology, National Taiwan University, Taipei, Taiwan, Republic of China (Received 29 March 1990; accepted 23 October 1990)
AbstTaCt-SHIH H. H. and CHEN S. N. 1991. Immunodiagnosis of angiostrongyliasis with monoclonal antibodies recognizing a circulating antigen of mol.wt 91,000 from Angiostrongyhu cuntonensis. ZnternationaiJournaifor Parasitology 21: 171-l 77. In the analysis of excretory-secretory (ES) antigens from infective third-stage larvae (L3) of A~giostro~gy~~ cu~fo~ens~, one major ~mponent of mol.wt 91,000 was not precipitated by pooled sera of patients with eosinophilic meningoen~phalitis. Monoclonal antibodies (MC Ab) secreted from two hybridoma cell lines, established against somatic antigens of L3, recognized this molecule but with different epitope specificities indicated by an additivity index (A.I.) of 83%. The 2 MC Ab (TD2 and 3A5) belonged to IgG2a and IgM classes, respectively. Combinations of TD2 and 3A5 were used in a sensitive enzyme-linked fluorescent assay (ELFA) for the immunodiagnosis of human angio- strongyliasis. The double-antibody sandwich ELFA method was applied firstly to sera from experimentally infected rats using either TD2 or 3A5 to coat the assay plates. Two fluorescence unit (F.U.) peaks appeared in sera from infected rats collected 18 and 44 days after infection. Specimens from 35 patients were tested, all cerebrospinal fluids (CSF) and most sera (88%) showed positive reactions and the average F.U. of CSF was greater than that of serum.
INDEX KEY WORDS: Angiosrrongylus cantonensis; monoclonal antibody; enzyme-linked fluorescence assay; immunodiagnosis.
INTRODUCTION
Angiostrongylus cantonensiscauses eosinophilicmeningo- encephalitis in humans and is found commonly in Southeast Asia and the Pacific Islands (Alicata & Jindrak, 1970; Cross, 1978). Hundreds of human cases were recorded over the last decade in Taiwan. Definite diagnosis of angiostrongyliasis relies on the discovery of either larval or juvenile worms in the CSF of patients. Such diagnoses are difficult to make because worms are seldom found in the limited volume of CSF. recovered. Immunodiagnosis can be useful in such circumstances. The indirect enzyme-linked immuno- sorbent assay @LISA) is preferred for detecting the presence and titre of antibody (Ab) in serum and CSF against either crude extracts of juveniles and adults or metabolites of adult worms (Kamiya, 1975; Tharavanij,
1979; Chen, 1986).
The detection of antigen (Ag) rather than Ab may have greater clinical use as has been exemplified in monkeys expe~mentally infected with A. ~~tonen~is larvae (Chen, Suzuki & Lin, 1973). The detection of antigens which circulate (cir-Ag) has also achieved emphasis elsewhere; these molecules usually have weak antigenicity and unbalanced Ag/Ab ratios (Smith, Karr, Lykins & Ristic, 1972; Phillips &
Draper, 1975). There were at least two kinds of cir-Ag (Yamashita, Saito, Sate, Takai, Watanabe t Otsuru, 1979) in the blood of rates experimentally infected with A. cantonensis. However, the detection of cir-Ag was not easy and cross-reactivity occurred like that reported in Bancroftian filariasis (Prasad, Reddy & Harinath, 1983). Hybridoma technology provides one way of increasing the specificity and reproducibility of the diagnostic test.
In this study, we analysed the components of L3 ES of A. cantonensis and evaluated their antigenicity. Two hybridoma cell lines were established and each MC Ab secreted recognized a single circulating ES molecule from the L3 but with different epitope specificities. We developed sensitive ELFA immunodiagnosis using combined monoclonal antibodies.
MATERIALS AND METHODS
Parasite antigens. A strain of Angiostrongylus cantonensis from Taiwan has been maintained for more than 10 years in our laboratory in adult Wistar rats and water snails, Biomphafaria gfubrarus. Third-stage larvae (L3) were har- vested and collected (Chen, Tang & Lee, 198 1) and more than 1000 were homogenized by sonication and extracted over- night at 4°C in a refrigerator. The extract was centrifuged (30 min, 11,000 g) and the supernatant, ‘somatic antigens’, was 171
172 H. H. SHIH and S. N. CHEN
assayed for protein (Bradford, 1976). These antigens were SDS-PAGE. Samples were reduced (Parkhouse & Clark, used as: (1) Ag to immunize BALB/c mice to produce 1983) and electrophoresed on 10% gel slabs using Bio-Rad hybridoma cell lines; (2) in indirect ELISA to screen the equipment. The gel slabs were fixed, dried and autoradio- hybridomas; and (3) to establish total L3 protein profiles for graphed. Apparent mol.wt of A. cantonensis components immunoblot analysis. Somatic preparations of Toxocara cati were determined against standard marker proteins (Pharmacia, and C~onorc~j~ sinerr& were extracted by the same pro- Sweden). Thereafter a mini-gel system with a 50 mm running cedures as described above and stored at - 20°C until use. gel and 20 mm stacking gel was used. Proteins electro- These last two species were collected from cats infected phoresed on the mini-gel slabs were then transferred to experimentally by another group in our laboratory. nitrocellulose (NC) paper to analyse their antigenicity.
Radiolabelled metabolic products. Intact and alive L3 were cultured at 37’C for 1-2 days in l-2 ml of tissue culture medium minus methionine (Leibovitz’s L 1.5, Gibco Ltd), containing penicillin (100 i.u. mll’), streptomycin (0.1 mg ml ‘) and-317 mBq ml- ’ of “S-methionine (specific activity > 43.03 TBa mmol-‘. New England Nuclear). At the end of the incubation, radiolabelled proteins were recovered from culture medium by precipitation with ice-cold ethanol (80%, v/v, final concentration) (Parkhouse & Clark, 1983). The total radioactivity present in the ES was estimated by assays on precipitates of aliquots with 10% (w/v) TCA in a liquid scintillation counter (LKB 1217 RACK BETA).
Protein blotting. The transfer technique of Gershoni & Palade (1983) was modified slightly. The mini-gel slabs were equilibrated in Tris-glycine transfer buffer, overlaid with NC paper (Immobilon transfer membranes, Millipore, U.S.A.), sandwiched between filter papers in a gel holder, and the proteins transferred for 1 h at 0.5-1.0 A in a transfer tank (Hoefer scientific instruments, U.S.A.). The NC paper was washed overnight with 6 M-urea dissolved in PBS containing 0.05% Tween-20 (PBST) to recover electrophoresed proteins which had been transferred. The NC paper was rinsed in PBST and blocked in PBS containing 3% skimmed milk for 1 h at room temperature, rinsed twice in PBS, cut into strips, and exposed individually to each MC Ab in ascites at the 1:500 dilution level in PBS for 3 h at 37°C. The treated strips were rinsed three times in PBST and exposed to horseradish peroxidase-labelled goat anti-mouse Ig antiserum (kpl) purified by affinity chromatography and diluted 1:1000_in PBS for 2 h at 37X The reacted NC strips were developed in PBS containing 0.1 ,ug ml-’ 3,3’-diammobenzidine (DAB, Sigma, U.S.A.) and 0.01% H,Op
~ybridomas. Cell ~ fusion was effected between spienic lymphocytes from BALB/c mice immunized three times with total L3 somatic antigens and myeloma cells from the FO cell line. The hybridomas were developed according to the procedures of Kiihler & Milstein (1975) using polyethylene glycol (Merck, mol.wt 1500). Antibody activity of culture supernatant was assessed by indirect ELISA using A. cuntonens~s L3 extract. Ascites containing MC Ab produced in p~stane-p~med mice were collected and stored at - 2o’C until use.
Isotyping and puriJication of monocional antibodies. An isotyping kit (Southern Biotechnology Associates Inc., U.S.A.) was used to distinguish the classes and subclasses of the MC Ab secreted. Ascites containing MC Ab of the IgG2a subclass were purified with a Protein A-Sepharose CL&B immunosor~nt column (Bio-Rad Laboratories, U.S.A.) (Goding, 1978). Eluted Ig were collected, neutralized to pH 7.0, desalted with a prepacked PD-10 column (Sephadex G- 25, bed volume 9 ml, Pharmacia Fine Chemicals AB, Sweden), and then concentrated by lyophilization. The MC Ab of IgM class was purified by gel filtration chromato- graphy with a Sephacryl S 300 (Pharmacia) column. Fractions of the first absorbance peak eluted at 280 nm were collected, desalted and lyophili~d as stated above.
Ra~o-imm~oprecip~tat~on. Soluble radioactive material was incubated with pooled sera (5-15 ~1) either from rats infected with A. cantonensis or from patients with eosino- philic meningoencephalitis, respectively. Antigen-antibody complexes were precipitated with an excess of either goat anti-rat Ig or goat anti-human Ig antisera (Kirkegaard & Perry Laboratories, kpl, U.S.A.), and washed as described by Parkhouse & Clark (1983). Control precipitates were pre- pared from pooled normal uninfected rat and human sera. ‘S-Labelled precipitates were divided into two parts: one was counted for total radioactivity, and the other was resolubil- ized in PBS and analvsed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). _ _ _
Sera. Rat sera were obtained at intervals from Wistar rats infected orally with 80 L3 of A. cantonensis (see Yoshimura & Soulsby, 1976) and stored at - 2o’C until use. Human sera and CSF, obtained from patients with the clinical symptoms of eosinophilic meningoencephalitis, were collected mainly at the National Taiwan University Hospital. Control sera were collected from confirmed patients of schistosomiasis, toxo- cariasis, clonorchiasis and taeniasis.
ELZSA a~itivity tesf. To distinguish whether or not MC Ab recognized different antigen epitopes (Friguet, Djavadi- Ohaniance, Pages, Bussard & Goldberg, 1983), a basic ELISA test (Voller, Bidwell & Bartlett, 1979) was performed using horseradish peroxidase as the enzyme tracer. The saturation curves of the Ag by each MC Ab were determined by the indirect ELISA, then the epitope specificity of the MC Ab was analysed by adding two as&tic fluids to each well in amounts determined to be sufficient for each to saturate the coated Ag. The amount of bound Ab was assayed and the additivity index (AI.) was calculated following the equation proposed by Friguet et al. (1983).
ELF’A. The ELISA procedure was adapted to ELFA (Yolken & Stopa, 1979) by substituting a fluorogenic sub- strate for the color-producing substrate. Optimal dilutions of reagents were determined by checkerboard titration. A double-Ab sandwich method was used to detect cir-Ag in sera from immune rats, patients and in CSF. An aliquot of 0.010 pug ascitic protein of TD2 was coated per well on the MicroFLUOR ‘B’ plate (Dynatech Laboratories Inc., U.S.A.). The plates were incubated overnight at 4”C, washed four times with PBST using a 96-well plate washer (Dynateck), and 50~~1 samples of either a clinical or an experimental specimen were added to the wells and incubated for 3 hat 37’C and overnight at 4°C. The plates were washed and 0.015 pg of ascitic protein of 3A5 was added to each well and incubated for 1 h at 37’C. The plates were washed, and affinity-purified phosphatase-labelled goat anti-mouse Ig heavy- chain p antiserum (human serum absorbed, kpl) diluted IOOO-fold was added. The plates were incubated for 1 h at 37”C, washed and a 100~~1 volume of ~methyl~~lli- fervl phosphate (MUP, Sigma) diluted to 10m4 M in 0.03 M- dieih&olamine buffer (pH 9.i) containing 10m5 M-MgCt, 6H,O was added to each well and incubated for 30 min at 37°C. The amount of fluorescence in each well was measured directly in a MicroFLUOR reader (Dynatech) and expressed as F.U. A background reading was taken from the well
Immunodiagnosis of A. cantonensis by ELFA 173
containing the substrate alone and the value subtracted from those of the specimens, either normal rat or human sera were run with each test as negative controls.
RESULTS
Antigenicity of excretory-secretory antigens from third-stage larvae
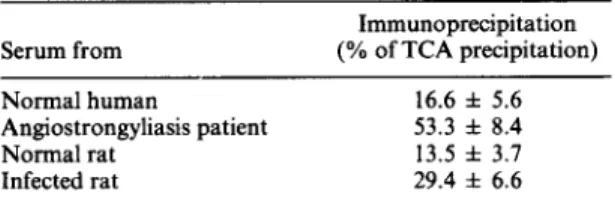
The components detected in the secretions of Angio- strongylus cantonensis L3 are highly antigenic to in- fected hosts. The immunoprecipitates of sera from either infected rats or patients showed significantly higher values than did sera from the normal controls (Table 1). Further analysis by resolubilization of immunoprecipitates of ES Ag with SDS-PAGE (Fig.
1) illustrated that only molecules of mol.wts 79,000, 65,000 and 43,000 were precipitated by pooled sera from infected rats. One major band of mol.wt 91,000 did not appear in the autoradiograph of electro- phoresed immunoprecipitates (Fig. 1, lane 2).
TABLE I-ANTIGENICITY OF A. cantonensis L3 ES
Serum from Normal human Angiostrongyliasis patient Normal rat Infected rat Immunoprecipitation (% of TCA precipitation) 16.6 f 5.6 53.3 f 8.4 13.5 f 3.7 29.4 f 6.6
Hybridoma production and characterization
Six hybridoma cell lines secreting Ab against either somatic or ES Ag from L3 were established. Two MC Ab recognized a molecule of molwt 91,000 as shown by immunoblot (Fig. 2) and radio-immunoprecip- itation (Fig. 3). The antibodies are of IgG2a subclass (TD2) and of IgM class (3A5). The AI. for TD2 and
1 2 3 4 e *‘r-r f ’ 1 2 3 330 - A B 67 - 60, 36 -
FIG. 1. Autoradiograph of labelled L3 excretory-secretory products (ES) co-precipitated by pooled normal human sera (I) and pooled sera from patients (2) with eosinophilic meningoencephalitis. Protein profile of L3 ES paralleled (3). Position of molwt standards shown on the left. Arrow at
right indicates position of molecules mol.wt 91,000.
3A5 was 83.0% (Table 2) which revealed that the two MC Ab showed additive binding and that the MC Ab from TD2 and 3A5 cell lines have different epitope specificities within the molwt 9 1,000 molecule of L3 ES. Detection of circulating antigens of moI.wt 91,000 in
sera from infected rat
Circulating Ag of mol.wt 91,000 was detected by a
1 2 3 4
FIG. 2. Immunoblots of worm somatic extracts and excretory-secretory products (ES). Protein blots of somatic extract of Angiostrongylus canfonensis L3 (I), of Toxocara cati adult (3), of Clonorchir sinensis adult (4) and L3 ES of A. contonensis (2) were probed with monoclonal antibodies (MC Ab) TD2 (A) and
174 H. H. SHIH and S. N. CHEN TABLE 2-THE ELISA ADDITIVITY TEST wrrn MONOCLONAL
ANTIBODIES AGAINST A. cantonensis L3 Monoclonal Optical density Theoretical Additivity
antibodies at 492 nm sum index (%)
TD2 (1:80) 0.712 _ _
3A5 (1:160) 0.873 _
TD2 + 3A5 1.450 1.585 83.0
TD2 + TD2 0.771 1.424 8.3
3A5 + 3A5 0.887 1.746 1.6
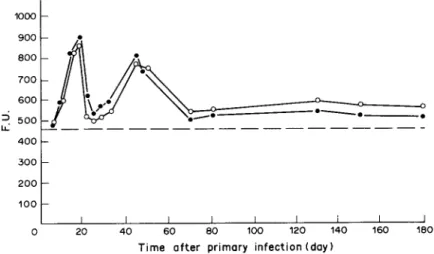
double-Ab sandwich ELFA in sera from infected rats collected from 8 to 180 days after infection (Fig. 4). Similar periodicity appeared in two circumstances: when the plate was coated with TD2 and the test sera were screened by 3A5 and phosphatase-labelled anti- mouse p chain conjugate; and alternately when the plate was coated with 3A5 and screened by TD2 and phosphatase-labelled anti-mouse y chain conjugate. Two F.U. peaks of reactivity were recorded for sera taken from rats 18 and 44 days after infection (Fig. 4). Immunodiagnosis of human angiostrongyliasis
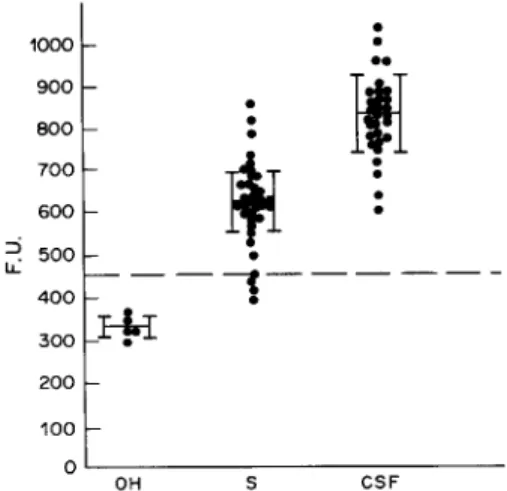
Qualitative and quantitative assays for the mol.wt 91,000 molecules in sera and CSF of 35 patients with eosinophilic meningoencephalitis were done by double- Ab sandwich ELFA against TD2. The. negative/ positive cutoff was calculated as the mean F.U. from six normal human sera run with the test plus 2 S.D. values. All CSF and most sera (88%, 31/35) were positive (Fig. 5). The average F.U. of CSF (837 f 95)
1 2 3 4
42”-- _
FIG. 3. Autoradiograph of labelled L3 somatic components (1 and 3) and L3 excretory-secretory products (ES) (2 and 4) co-precipitated by monoclonal antibodies (MC Ab) TD2 (1 and 2) and 3A5 (3 and 4). Molecules of mol.wt 91,000 are
indicated by an arrow.
was greater than that for sera (627*71). Cross reactivity was tested on five pools of infected sera collected from confirmed patients of schistosomiasis, toxocariasis, clonorchiasis and taeniasis. Cross-re- actions were significantly lower than the cutoff value calculated from reactions between normal human serum in the test system.
1000 t 200 100
1
I I I I I I I I J 0 20 40 60 80 100 120 140 160 160Time after primary infection (day)
FIG. 4. Double antibody sandwich ELFA detecting circulating antigen of mol.wt 91,000 in infected rat sera. Rats infected with 80 Angiostrongylus cantonensis L3. MicroFLUOR ‘B’ plate coated first with 0.010 ng ascitic protein from monoclonal antibody (MC Ab) TD2/ well, and screened by MC Ab 3A5 and phosphatase-labelled anti-mouse /.I chain antiserum (0). Plate coated first with 0.015 pg ascitic protein from 3A5, and then screened by MC Ab TD2 and phosphatase-labelled anti-mouse chain y antiserum (0). A value exceeding 467 fluorescence unit (F.U.) (---), equal to the average counts from normal rat sera plus 3 s.D.,
Immunodiagnosis of A. cantonensis by ELFA 175 1000
c
. .
:
900.
600L
4r
:
iti
700 . . 600 : 2 500 400 __+---__ .3,: *
t
200 100 LO/
OH SFIG. 5. Double antibody sandwich ELFA detecting circu- lating antigens of mol.wt 91,000 in sera (S) and cerebrospinal fluids (CSF) of patients with eosinophilic meningoenceph- alitis. Inter-helminth cross reactivity examined on five pools of sera from other helminth-infected (OH) patients with schistosomiasis, toxocariasis, clonorchiasis and taeniasis. A value exceeding 370 fluorescence unit (F.U.) (---), equal to the average counts from normal human sera plus 3 s.D., was
considered to be positive.
DISCUSSION
The level of circulating Ab detected either in the serum or CSF of patients with A. cantonensis may not be clearly diagnostic of infection because of the delay in the appearance, persistence in the circulation, and the high level of cross-reactivity of the Ab (des Moutis, Ouaissi, Grzych, Yarzabal, Haque & Capron, 1983). We therefore sought to detect cir-Ag. Indeed, such Ag is present in the circulation before specific Ab appears and unlike Ab does not persist for long in the circulation after the parasite is lost. Circulating Ag from A. cantonensis was first recovered in the sera of experimentally infected rats by Yamashita et al. (1979). They demonstrated Ag in sera of 33.3% of cyclophosphamide-treated and 20.0% of untreated rats infected with 100 A. cantonensis larvae and proposed that the detection of cir-Ag was a highly sensitive method for diagnosis. Our results support this proposition.
The L3 ES of A. cantonensis is composed of many complex molecules (Fig. 1, lane 3) as traced by radioisotope-35S. More than half of the total radio- activity appeared in immunoprecipitates from reactions with sera from patients with eosinophilic meningoencephalitis (Table 1). The electrophoresis patterns of resolubilized immunoprecipitates indicate that many molecules, including some major ones, were not precipitated (Fig. 1, lane 2). That is, the molecules show weak antigenicity in both humans and rats.
Two MC Ab which recognize weak antigenic mole- cules of mol.wt 91,000 were found by chance when screening hybridoma secretions. Figure 3 shows that
both TD2 and 3A5 can catch the mol.wt of 91,000 molecules among radiolabelled ES and somatic anti- gens. These two MC Ab belonged to different Ig classes and showed different epitope specificities. Observa- tions on combinations of reactions between the MC Ab and the A. cantonensis antigens established ELFA as a sensitive immunoassay for angiostrongyliasis. The system was tested firstly against sera from experi- mentally infected rats either with TD2 or 3A5 as the primary reaction antibody to detect molecules with a molwt of 91,000 in the circulation of the infected rats. Two peaks of Ag activity at 18 and 44 days after infection (Fig. 4) indicated that the cir-Ag of mol.wt 91,000 is common between stages of A. cantonensis (data not shown). The antigen is secreted into the rat circulation largely when the worms migrated from rat brain to the pulmonary arteries. In human cases, F.U. obtained from sera from most patients and all CSF are significantly higher than those from normal sera; the average value for the CSF was greater than that for sera. The difference between sera and CSF may result because L3 of A. cantonensis do not usually develop into the adult stage in humans and are always retained in the central nervous system (CNS), so that the amount of ES materials is greater in CSF than in the serum. Alternatively it may arise because the protein content of the CSF is only about 0.5% of that of serum (Hochwald, 1970), although y-globulin within the CNS which was synthesized locally has been reported in patients with a variety of neurological diseases (Lippincott, Korrnan, Lax & Corcoran, 1965). The cir- Ag of molwt 91,000 which traversed the brain-blood barrier and appeared in human circulation may combine with circulating Ab to be eliminated as an immune complex. Molecules which remain in the CSF may persist longer at increasing concentrations as the worms continue to secrete and excrete them into the brain.
Application of MC Ab in immunodiagnosis is recommended by Felice & Siracusano (1987). MC Ab offer several advantages including: decreased false- positive reactions; decreased cross reactivity (because each MC Ab usually recognizes only one antigen determinant unique to the parasite of interest); and increased reproducibility and standardization of the test. However sensitivity in diagnosis may be lost because of the single epitope specificity of Mc Ab. This problem was overcome here by using two MC Ab which react with different epitopes on the same major cir-Ag. Sensitivity was increased remarkably by sub- stituting a fluorogenic for the colorigenic substrate usually used in ELBA. ELFA required about lOO-fold less enzyme than did ELISA (Yolken & Stopa, 1979). Some problems within the present immunodiag- nostic system remain to be resolved. The sources of the cir-Ag have not yet been confirmed, but we are sure that they are not shed from the L3 cuticle because the surface of the L3 did not react in an indirect fluorescent Ab test with TD2 (data not shown). Secondly, al- though both of these two MC Ab could not react with
176 H. H. SHIH and S. N. CHEN somatic extracts of either Toxocura canis or Clonorchis
sinensis (Fig. 2), the sample size of cross reactivity is still too small and only species from the classes Trematoda and Cestoda were studied, and only one serum pool from another nematode infection was used. Finally, ES molecules were more abundant in human CSF than in serum and CSF is therefore a better test material than serum for immunodiagnosis, but spinal puncture is a cumbersome technique which requires educated skill. The assay could be done however whenever patients with neurological symp- toms are tapped for CSF for other tests. The ELFA on CSF nevertheless is not as convenient to do as other assays for cir-Ag on blood and urine developed so far, e.g. for Trypanosoma lewisi by D’Alesandro (1972); Plasmodium species by Smith et al. (1972); Onchocera volvulus by des Moutis et al. (1983); Wuchereria bancrofti by Franks (1946) and Prasad et al. (1983); Schistosoma mansoni by Gold, Rosen & Weller (1969), Deelder, Klappe, van den Aardweg & van Meerbeke (1976) and Bout, Santoro, Carlier, Bina & Capron (1977); and for S. japonicum by Hirata & Akusawa (1975) and Hirata (1976).
REFERENCES
AUCATA J. E. & .J~NDRAK K. 1970. Angiostrongyliasis in the Pacific and Southeast Asia. In: American Lectures in Tropical Medicine (Edited by THOMAS C. C.). Springfield, Illinois.
BOUT D., SANTORO F., CARLIER Y., BINA J. C. & CAPRON A. 1977. Circulating immune complexes in schistosomiasis. Immunology 33: 17-22.
BRADFORD M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochem- istry 72: 248-254.
CHEN S. N., SUZUKI T. & LIN K. H. 1973. Studies on immunodiagnosis of angiostrongyliasis. 1. Detection of antigen and antibody in serum and cerebrospinal fluid. Journal of Formosan Medical Association 12: 161-166. CHEN S. N., TANG T. & LEE S. J. 198 1. The in vitro cultivation
of the first and third stage larvae and adult worm of Angiostrongylus cantonensis. Proceedings of the National
Science Council, Republic of China, Part B 5: 375-384.
CHEN S. N. 1986. Enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to Angiostrongylus cantonensis. Transactions of the Royal Society of Tropical Medicine and Hygiene 80: 398405.
CROSS J. H. 1978. Clinical manifestation and laboratory diagnosis of eosinophilic meningitis syndrome associated with angiostrongyliasis. Southeast Asian Journal of
Tropical Medicine and Public Health 9: 161-170.
D’ALESANDRO P. A. 1972. Trypanosoma lewisi: production of exoantigens during infection in the rat. Experimental Parasitology 32: 149-164.
DEELDER A. M., KLAPPE H. T. M., AARDWEG G. J. M. J. VAN DEN & MEERBEKE E. H. E. M. VAN 1976. Schistosoma mansoni: demonstration of two circulating antigens in infected hamsters. Experimental Parasitology 40: 189-197. FELICE G. K. & SIRACUSANO A. 1987. Monoclonal antibodies for immunodiagnosis of human hydatidosis. Parasitology Today 3: 25-26.
FRANKS M. B. 1946. Specific soluble antigen in the blood of
filarial patients. Journal of Parasitology 32: 4WO6. FRIGUE~ B., DJAVADI-OHANIANCE L., PAGES J., BUSSARD A. &
GOLDBERG M. 1983. A convenient enzyme-linked immuno- sorbent assay for testing whether monoclonal antibodies recognize the same antigenic site. Application to hybrid- omas specific for the a-subunit of Escherichia coli trypto- phan synthase. Journal of Immunological Methods 60: 35 l- 358.
GERSHONI J. M. & PALADE G. E. 1983. Protein blotting: principles and applications. Analytical Biochemistry 131:
l-15.
GODING J. W. 1978. Use of staphylococcal protein A as an immunological reagent. Journal of Immunological Methods 20: 241-254.
GOLD R., ROSEN F. S. & WELLER T. H. 1969. A specific circulating antigen in hamsters infected with Schistosoma mansoni. Detection of antigen in serum and urine, and correlation between antigenic concentration and worm burden. American Journal of Tropical Medicine and Hygiene 18: 545-551.
HIRA~A M. SC AKUSAWA M. 1975. Circulating antigen in animals infected with Schistosoma japonicum. 1. Detection and characteristics of circulating antigen in infected rabbits. Japanese Journal of Parasitology 24: 250-254.
HIRAPA M. 1976. Circulating antigen in animals infected with Schistosoma japonicum. 2. Appearance of circulating anti- gen in infected mice. Japanese Journal of Parasitology 25: 396-401.
H~CHWALD G. M. 1970. Influx of serum proteins and their concentration in spinal fluid along the neuraxis. Journal of the Neurological Sciences 10: 269-278.
KAMIYA M. 1975. Immunodiagnosis of Angiostrongylus cantonensis infection. In: Diagnostic Methodsfor Important Helminthiasis & Amoebiasis in Southeast Asia & the Far East (Edited by HARINASUTA C. & REYNOLDS D. C.), pp.
140-163. A Publication of the Central Coordination Board, SEAMEO-TROPMED Project, Bangkok, Thailand. KOHLER G. & MILSTEIN C. 1975. Continuous cultures of fused
cells secreting antibodv of nredefined specificity. Nature (London) 256: 495497.
LIPPINCOT~ S. W.. KORMAN S., LAX L. C. & CORCORAN C.
1965. Transfer rates of y-globulin between cerebrospinal fluid and blood plasma (results obtained on a series of multiple sclerosis patients). Journal of Nuclear Medicine 6: 632-644.
MOUTHS I. DES, OUAISSI A., GRZYCH J. M., YARZABAL L., HAQUE A. & CAPRON A. 1983. Onchocerca volvulus: detec- tion of circulating antigen of monoclonal antibodies in human onchocerciasis. American Journal of Tropical
Medicine and Hygiene 32: 533-542.
PARKHOLJSE R. M. E. & CLARK N. W. T. 1983. Stage specific secreted and somatic antigens of Trichinella spiralis. Molecular and Biochemical Parasitology 9: 3 19-327. PHILLIPS T. M. & DRAPER C. C. 1975. Circulating immune
comulexes in schistosomiasis due to Schistosoma mansoni. Brit&h Medical Journal 2: 476-477.
PRASAD G. B. K. S.. REDDY M. V. R. & HARINATH B. C. 1983. Detection of fifarial antigen in immune complexes in Bancroftian filariasis by ELISA. Indian Journal of Medical Research 78: 78&783.
SMITH A. R., KARR L. J., LYKINS J. D. & RIS-~IC M. 1972. Serum-soluble antigens of malaria: a review. Experimental Parasitology 31: 120-125.
THARAVANIJ S. 1979. Immunology of angiostrongyliasis. In: Studies on Angiostrongyliasis in Eastern Asia & Australia (Edited by CROSS J. H.), pp. 151-164. Special Publication of
Immunodiagnosis of A. canlonensis by ELFA 177 the U.S. Naval Medical Research Unit, No. 2, Taipei,
Taiwan.
VOLLER A., BIDWELL D. E. & BARTLETT A. 1979. Setting up ELISA. In: The Enzyme Linked Immunosorbent Assay (ELISA). A Guide with Abstracts of Microplate Applica- tions (Edited by VOLLER A., BIDWELL D. E. & BARTLETT A.), pp. 35-40. Dynatech Europe, Borough House, Rue du Pre, Guernsey, G.B.
YAMASHITA T., SAITO Y., SATO Y., TAKAI A., WATANABE H. & OTSURU M. 1979. Circulating antigens and immune com-
plexes in the serum of rats infected with Angiosrrongylus cantonensis. Japanese Journal of Parasitology 281 393401. YOLKEN R. H. & STOPA P. J. 1979. Enzyme-linked fluor-
escence assay: ultrasensitive solid-phase assay for detection of human rotavirus. Journal of Clinical Microbiology September: 3 17-32 1.
YOSHIMURA K. & SOULSBY E. J. L. 1976. Angiostrongylus cantonensis: lymphoid cell responsiveness and antibody production in rats. American Journal of Tropical Medicine and Hygiene 25: 99-107.