YC-1 [3-(5
⬘-Hydroxymethyl-2⬘-furyl)-1-benzyl Indazole] Inhibits
Endothelial Cell Functions Induced by Angiogenic Factors
in Vitro and Angiogenesis in Vivo Models
Shiow-Lin Pan, Jih-Hwa Guh, Chieh-Yu Peng, Shih-Wei Wang, Ya-Ling Chang,
Fong-Chi Cheng, Jau-Hsiang Chang, Sheng-Chu Kuo, Fang-Yu Lee, and Che-Ming Teng
Pharmacological Institute (S.-L.P., C.-Y.P., S.-W.W., Y.-L.C., C.-M.T.) and School of Pharmacy (J.-H.G.), College of Medicine, National Taiwan University, Taipei, Taiwan; MDS Pharma Service, Taipei, Taiwan (F.-C.C.); Graduate Institute of Pharmaceutical Chemistry, China Medical College, Taichung, Taiwan (S.-C.K.); and Yung-Shin Pharmaceutical Industry Co., Ltd., Taichung, Taiwan (J.-H.C., F.-Y.L.)
Received February 17, 2005; accepted March 18, 2005
ABSTRACT
Angiogenesis is a process that involves endothelial cell prolif-eration, migration, invasion, and tube formation, and inhibition of these processes has implications for angiogenesis-mediated disorders. The purpose of this study was to evaluate the anti-angiogenic efficacy of YC-1 [3-(5 ⬘-hydroxymethyl-2⬘-furyl)-1-benzyl indazole] in well characterized in vitro and in vivo sys-tems. YC-1 inhibited the ability of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) in a dose-dependent manner to induce proliferation, migration, and tube formation in human umbilical vascular endothelial cells; these outcomes were evaluated using [3H]thymidine
incorpo-ration, transwell chamber, and Matrigel-coated slide assays, respectively. YC-1 inhibited VEGF- and bFGF-induced p42/p44
mitogen-activated protein kinase and Akt phosphorylation as well as protein kinase C␣ translocation using Western blot analysis. The effect of YC-1 on angiogenesis in vivo was eval-uated using the mouse Matrigel implant model. YC-1 adminis-tered orally in doses of 1 to 100 mg/kg/day inhibited VEGF- and bFGF-induced neovascularization in a dose-dependent manner over 7 days. These results indicate that YC-1 has antiangio-genic activity at very low doses. Moreover, in transplantable murine tumor models, YC-1 administered orally displayed a high degree of antitumor activity (treatment-to-control life span ratio ⬎ 175%) without cytotoxicity. YC-1 may be useful for treating angiogenesis-dependent human diseases such as cancer.
Angiogenesis is the formation of new blood vessels from preexisting endothelial vasculature (Folkman et al., 1971). Physiologically, angiogenesis plays a crucial role in embry-onic development, placental implantation, and wound heal-ing. In contrast, the same process also supports pathological conditions such as solid tumor growth, diabetic retinopathy, psoriasis, and rheumatoid arthritis. Complex and diverse cellular actions such as extracellular matrix degradation, proliferation and migration of endothelial cells, and morpho-logical differentiation of endothelial cells to form tubes have all been implicated in angiogenesis (Bussolino et al., 1997).
Although all of these processes are regulated under normal conditions, abnormal vascularization is clearly implicated in malignant tumor growth and metastasis. The extreme growth of tumors to volumes larger than a few cubic milli-meters requires continuous recruitment of new blood vessels (Folkman, 1990). These newly synthesized blood vessels also provide a route for cancer cells to enter the systemic circula-tion and spread to distant organs (Fidler and Ellis, 1994). A growing number of angiogenic factors have been recognized as contributors to regulation of tumor angiogenesis. When a balance between angiogenic and angiostatic factors is dis-rupted, uncontrolled angiogenic factors such as growth fac-tors, cytokines, lipid metabolites, and cryptic fragments of hemostatic proteins can be released from tumor cells. Among these angiogenesis-related factors are vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) (Klein et al., 1997; Ferrara, 2001). These factors are This work was supported by the National Science Council of the Republic of
China (Grant NSC 93-2811-B002-026).
S.-L.P. and J.-H.G. contributed equally to this work.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.105.085126.
ABBREVIATIONS: VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor; YC-1, 3-(5⬘-hydroxymethyl-2⬘-furyl)-1-benzyl indazole; sGC, soluble guanylyl cyclase; HUVEC, human umbilical vascular endothelial cell; FBS, fetal bovine serum; PBS, phosphate-buffered saline; PKC, protein kinase C; ERK1/2, extracellular signal-regulated protein kinase 1/2; MST, median survival time; ODQ, 1H-[1,2,4]oxadia-zole[4,3-a]quinoxalin-1-one;L-NAME, NG
-nitro-L-arginine methyl ester; HIF-1␣, hypoxia-inducible factor-1␣.
THEJOURNAL OFPHARMACOLOGY ANDEXPERIMENTALTHERAPEUTICS Vol. 314, No. 1
Copyright © 2005 by The American Society for Pharmacology and Experimental Therapeutics 85126/3035439
JPET 314:35–42, 2005 Printed in U.S.A.
produced by many pathologic (tumor) and normal cells, and they stimulate endothelial cell proliferation, sprouting, mi-gration, and morphogenesis. They act via receptor tyrosine kinases and influence normal and abnormal angiogenesis (Friesel and Maciag, 1999; Cross and Claesson-Welsh, 2001). YC-1 is a novel NO-independent type of soluble guanylyl cyclase (sGC) activator that mimics many of the known func-tions of NO and NO donors. YC-1 was discovered in Teng’s laboratory (Ko et al., 1994). YC-1 and NO synergistically activate sGC in vitro, and YC-1 potentiates the effects of exogenous and endogenous carbon monoxide on sGC (Friebe et al., 1996). YC-1 at a high concentration level (e.g., ⬎30 M) induces these cellular and physiological functions via cGMP-dependent or independent pathways (Wang et al., 2002; Chien et al., 2003; Pan et al., 2004). However, we have not found published studies reporting an inhibitory effect of YC-1 on endothelial cells at a very low concentration. In this study, we determined that low levels of YC-1 suppressed endothelial cell proliferation, migration, and tube formation in vitro and inhibited VEGF- and bFGF-induced angiogenic signaling pathways. YC-1 also markedly inhibited neovascu-larization induced by VEGF and bFGF in vivo in the Matrigel plug implantation model. Finally, YC-1 prolonged the life-span of tumor-treated animals in an antitumor activity experiment without producing any apparent cytotoxicity. Taken together, these results suggest that YC-1 may inhibit tumor activity via the antiangiogenic properties demon-strated at a low level of exposure.
Materials and Methods
Cell Culture. Human umbilical vein endothelial cells (HUVECs) were obtained from human umbilical cord veins with collagenase, isolated according to protocols from Jaffe et al. (1973), and cultured in 75-cm2plastic flasks in M199 medium containing 20% fetal bovine serum (FBS) and 15 g/ml endothelial cell growth supplements. Confirmation of cell identity as endothelial cells was provided by detection of CD31 (PECAM-1) by immunostaining. The human lung cancer cell line A549 was cultured in Dulbecco’s modified Eagle’s medium containing 10% inactivated FBS. Cells were incubated at 37°C in a humidified atmosphere of 5% CO2 in air. Medium was changed every 2 days, and cells were passaged after treatment with a solution of 0.05% trypsin/0.02% EDTA. Experiments were con-ducted on HUVECs that had gone through two to five passages.
[3H]Thymidine Incorporation Assay. Confluent HUVECs were trypsinized, suspended in M199 medium supplemented with 20% FBS, and seeded at 1.0⫻ 104cells per well into 96-well plates. After 24 h, the cells were washed twice with phosphate-buffered saline (PBS) and starved with 2% FBS-M199 medium for 24 h. The cells were then incubated with or without indicated reagents and growth factors (e.g., VEGF and bFGF at 10 ng/ml) for 24 h and harvested. Before the harvest, cells were incubated with [3 H]thymi-dine (2 Ci/ml) for 4 h. Cells were harvested with Filter-Mate (PerkinElmer Life and Analytical Sciences, Boston, MA), and incor-porated radioactivity was determined.
Migration Assay. Chemotactic migration of HUVECs was mea-sured with a transwell migration apparatus as described previously by Pan et al. (2003). Cell migration was assayed in 24-well, 6.5-mm-internal diameter chamber cluster plates (8-m pore size; Costar, Cambridge, MA). Briefly, HUVECs (1⫻ 105 cells/well) were sus-pended in 200 l of serum-free M199 medium with 0.1% bovine serum albumin and loaded into the upper chamber of a transwell cluster plate coated with 0.1 mg/ml gelatin. VEGF or bFGF was diluted to 10 ng/ml in 0.6 ml of M199/0.1% bovine serum albumin and added to the lower wells of the chamber. YC-1 (3–30M) was
added at the indicated concentration 1 h before the assay. The chambers were incubated for 24 h at 37°C in an atmosphere of 95% air and 5% CO2. At the end of incubation, cells were fixed and stained with hematoxylin. Nonmigrated cells on top of the filters were wiped off, filters were mounted, and migrated cells attached to the bottom of the filter were counted in six randomly chosen (⫻400) high-power fields. Cell migration was calculated as the difference between the number of migrated cells in the YC-1-treated samples and the num-ber of migrated cells in the control samples.
Tube Formation Assay. HUVECs (2⫻ 105cells) were seeded on a layer of polymerized Matrigel with or without YC-1 (3–30M) and VEGF or bFGF (10 ng/ml) in a chamber slide (Nalge Nunc Interna-tional, Naperville, IL). Matrigel cultures were incubated at 37°C. After 24 h, cell morphology was evaluated using a phase-contrast microscope, and cells were photographed.
PKC Fractionation in HUVECs. Cells were homogenized in 0.2 ml of buffer A containing 20 mM HEPES (pH 7.5), 0.33 M sucrose, 2 mM EGTA, 2 mM EDTA, 5 mM dithiothreitol, 20 mM sodium fluo-ride, 0.1 mM sodium orthovanadate, 20g/ml leupeptin, 10 g/ml aprotinin, and 0.2 mM phenylmethyl sulfonyl fluoride. The cytosolic fraction (supernatant) was separated by centrifugation at 100,000g for 60 min. The pellet was resuspended in homogenization buffer A without sucrose but with 0.1% Triton X-100, followed by centrifuga-tion and colleccentrifuga-tion of the particulate fraccentrifuga-tion (supernatant). The samples were processed for SDS-polyacrylamide gel electrophoresis and immunoelectrophoresis.
Western Blotting. After exposure of cells to indicated agents over specified time courses, cells were washed twice with ice-cold PBS, and reaction was terminated by addition of 100l of ice-cold lysis buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EGTA, 0.5 mM phenylmethyl sulfonyl fluoride, 10g/ml aprotinin, 10 g/ml leupep-tin, and 1% Triton X-100). Protein (60g/lane) was separated on 7.5 to 12% SDS-polyacrylamide gel electrophoresis. The nitrocellulose membrane was immunoreacted with the primary antibody to ERK1/2, phosphorylated-ERK1/2 (BD Biosciences, San Jose, CA), Akt, phosphorylated-Akt,␣-tubulin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and PKC␣ (Serotec, Oxford, UK) for overnight incubation at 4°C. After four washings with PBS/0.1% Tween 20, the secondary antibody (diluted 1:2000) was applied to the membranes for 1 h at room temperature. Antibody-reactive bands were detected with an enhanced chemiluminescence kit (ECL; Amersham Bio-sciences UK, Ltd., Little Chalfont, Buckinghamshire, UK).
In Vivo Matrigel Plug Assay. Nude mice (6 weeks of age) were given subcutaneous injections of 500l of Matrigel (BD Biosciences) at 4°C with growth factor (150 ng/ml VEGF or bFGF). After injection, the Matrigel rapidly formed a plug. YC-1 (1–100 mg/kg/day) was given orally starting on day 1. After 7 days, the skin of the mouse was easily pulled back to expose the Matrigel plug, which remained intact. After quantitative differences were noted and photographed, hemoglobin was measured as an indication of blood vessel formation, using the Drabkin method (Drabkin reagent kit 525; Sigma-Aldrich, St. Louis, MO). The concentration of hemoglobin was calculated from a known amount of hemoglobin assayed in parallel.
In Vivo Antitumor Activity Assay. A549 (1⫻ 107) cells in a volume of 100l were transplanted through the chest wall into the left pleural space of nude mice using a 26-gauge needle on day 0; a dose of 10 mg/kg/day YC-1 was given orally starting on day 1. Antitumor activity was assessed as the ratio of median survival time (MST) in the treatment group (T) to MST in the control group (C), and the results are shown as T/C: life span T/C (%) ⫽ (MST of drug-treated group/MST of control group)⫻ 100. Long-term survi-vors were recorded until each animal died.
Cytotoxicity Assay. The cytotoxicity assay was carried out using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay method. MTT (Sigma-Aldrich) was dissolved in PBS at a concentration of 5 mg/ml and sterilized by filtration (Millipore Corporation, Billerica, MA). From this stock solution, 10l/100 l medium was added to each well, and plates were gently shaken and
incubated at 37°C for 2 h. After the incubation period, cells were lysed with dimethyl sulfoxide and quantified at OD550with an en-zyme-linked immunosorbent assay reader.
Data Analysis and Statistics. Data are presented as mean⫾ S.E.M. or as percentage of control. Statistical comparisons between groups were performed using the Student’s t test. P⬍ 0.05 was considered statistically significant.
Results
Effect of YC-1 on VEGF- and bFGF-Induced Cell Pro-liferation of HUVECs. The ability of YC-1 to affect the mitogen activity of VEGF and bFGF was assessed in a [3H]thymidine incorporation assay. YC-1 (0.01–1M)
inhib-ited, in a dose-dependent manner, cell growth induced by 10 ng/ml VEGF and bFGF (IC50values of 1.4⫻ 10⫺7and 9.0⫻
10⫺8M, respectively) (Fig. 1A). YC-1 was also tested for its capacity to affect HUVEC proliferation stimulated by 20% FBS to assess the specificity of this effect. Under the same experimental conditions, YC-1 inhibited the mitogenic activ-ity stimulated by VEGF and bFGF but not by FBS in the same concentration range (data not shown).
YC-1 may inhibit cell proliferation through effects on a cyclic GMP-dependent pathway or potentate a nitric oxide-mediated pathway in endothelial cells. Accordingly, we in-vestigated the effect of ODQ (a sGC inhibitor, 3 and 10M), 100 M dibutyl cGMP (a cyclic GMP analog), and 1 mM
L-NAME (a nitric oxide synthase inhibitor) on YC-1
antipro-liferative activity. ODQ andL-NAME did not reverse YC-1-induced antiproliferation in HUVECs (Fig. 1, B and C). Dibutyl cGMP completely inhibited YC-1 induced cell prolif-eration but only in high concentrations. Taken together, these results demonstrate that YC-1 inhibits VEGF- and bFGF-induced HUVECs proliferation and that this inhibi-tion does not involve effects on cyclic GMP-dependent or nitric oxide-mediated pathways.
Effect of YC-1 on VEGF- and bFGF-Induced Cell Mi-gration and Tube Formation of HUVECs. Next, we used well established assays to assess the potential regulatory role of YC-1 on cell migration and differentiation. YC-1 (3–30M) significantly inhibited, in a dose-dependent manner, VEGF-and bFGF-induced cell migration in a transwell assay (Fig. 2A). However, HUVECs treated with 10 ng/ml VEGF and bFGF reorganized and subsequently formed capillary-like structures (Fig. 2B). YC-1 (3–30M) caused a concentration-dependent blockage of the capillary tubes and did not inhibit cell viability (MTT assay). These results demonstrate that YC-1 has the ability to block VEGF- and bFGF-induced in vitro angiogenesis.
Effect of YC-1 on VEGF- and bFGF-Induced ERK1/2 and PI3-K Activation in HUVECs. As mentioned above, the activation of both ERK1/2 and PI3-K pathways is re-quired for the proliferative and migratory effects of VEGF and bFGF on endothelial cells (Cross and Claesson-Welsh, 2001). Therefore, we evaluated the possibility that the inhib-itory effect of YC-1 might be mediated through its ability to interfere with VEGF- and bFGF-induced activation of these intracellular pathways. To determine whether YC-1 could modulate these active signaling pathways that are involved in cell functions, HUVECs were incubated with increasing amounts of YC-1 in vitro and analyzed for changes in acti-vated Akt and ERK1/2. First, we assessed the effect of YC-1 (1 and 10M) on ERK1/2 phosphorylation (Fig. 3A).
VEGF-and bFGF-induced ERK1/2 phosphorylation (10 ng/ml) was strongly inhibited by YC-1 in a concentration-dependent manner. Densitometric analysis indicated that VEGF- and bFGF-induced ERK1/2 phosphorylation was reduced by 20 to 40% in the presence of 1M YC-1 and by 50 to 60% in the presence of 10 M YC-1 (Fig. 3B). Akt is a protein kinase recruited to the membrane and activated by phosphorylation Fig. 1. Effect of YC-1 on VEGF- and bFGF-induced cell proliferation. A,
effect of YC-1 (0.01–1M) on growth factor (10 ng/ml VEGF or bFGF) HUVEC growth was examined using [3H]thymidine incorporation to as-sess proliferation. B and C, cells were treated with or without YC-1 (1 M) in the absence or presence of the indicated agents, and growth factors were added for 24 h. After the incubation period, the cell prolif-eration was assayed using [3H]thymidine incorporation assay. Data rep-resent the mean⫾ S.E.M. of six independent experiments (each per-formed in triplicate).⫹, P ⬍ 0.001 versus basal group; ⴱ, P ⬍ 0.05; ⴱⴱ, P ⬍ 0.01;ⴱⴱⴱ, P ⬍ 0.001 versus control group.
as a direct consequence of PI3 kinase activity (Cantley, 2002). We next evaluated Akt phosphorylation levels to test whether YC-1 is also capable of inhibiting angiogenic growth factor-induced PI3-K activation. As shown in Fig. 3C, YC-1 (10 and 30M) significantly suppressed the phosphorylation of Akt in a concentration-dependent manner. Total Akt ex-pression was not affected by YC-1 treatment. The ratio be-tween phosphorylated Akt and total Akt was quantified for each of the points in this experiment, and the results are shown in Fig. 3D. These data suggest that YC-1 is able to block VEGF- and bFGF-induced ERK1/2 and Akt signaling pathways in endothelial cells.
Effect of YC-1 on VEGF- and bFGF-Induced PKC␣ Activation in HUVECs. In the previous study, YC-1 signif-icantly inhibited VEGF- and bFGF-induced HUVEC prolif-eration. However, VEGF and bFGF seemto exert their mito-genic effects through activation of a PKC␣-dependent pathway in human endothelial cells (Kent et al., 1995; Well-ner et al., 1999). We next evaluated whether YC-1 reduction
in angiogenic activity is associated with reduced PKC␣ ac-tivity. Cells were treated with YC-1 (0.1–10 M) for 1 h followed by 10 ng/ml VEGF and bFGF for 30 min and 2 h, respectively. As shown in Fig. 4, YC-1 significantly sup-pressed VEGF- and bFGF-induced PKC␣ translocation from cytosol to membrane in a concentration-dependent manner. These data suggest that YC-1 is able to block VEGF- and bFGF-induced PKC␣ signaling pathways in endothelial cells. Effect of YC-1 on Neovascular Formation in Vivo. To determine whether YC-1 is capable of blocking VEGF- and bFGF-induced angiogenesis in vivo, we used an experimental model in which angiogenesis is induced by factors embedded in a pellet of Matrigel, which was injected subcutaneously in mice; the degree of vascularization was evaluated after 7 days. VEGF and bFGF (150 ng/ml) significantly induced an-giogenic responses compared with Matrigel alone (Fig. 5, A and B), and YC-1 (1–100 mg/kg/day) significantly inhibited the angiogenic response in a dose-dependent manner. Micro-scopic examination showed that the addition of VEGF and bFGF to the Matrigel induced cellularity and the formation of cords, tubules, and several blood-filled channels containing red blood cells. In contrast, Matrigel pellets without angio-genic stimuli had only a few infiltrating, single, elongated cells. VEGF- and bFGF-induced angiogenesis was signifi-cantly reduced in mice treated with VEGF or bFGF plus YC-1 in a dose-dependent manner. Quantification of angiogenesis by hemoglobin content showed that the addition of VEGF or bFGF to the Matrigel could induce an increased angiogenic response compared with the basal group (Matrigel alone) (Fig. 5C). However, YC-1 also inhibits the VEGF- and bFGF-induced hemoglobin content in a dose-dependent manner. These results clearly indicate that YC-1 is a potent antian-giogenic molecule in vivo.
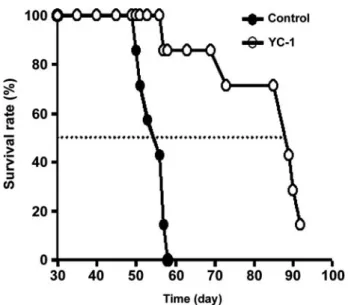
Effect of YC-1 on Antitumor Activity in Vivo. Antitu-mor activity of YC-1 (10 mg/kg/day) was investigated in nude mice starting on 1 day after implantation of an A549 tumor cell line. YC-1 showed a broad spectrum and high degree of in vivo antitumor activity (Fig. 6). The average life span was prolonged by 180%; five of the seven mice were long-term survivors (Fig. 6); no cytotoxicity was produced (MTT assay in cultured cancer cells; data not shown). The results indi-cated that YC-1 antitumor activity in the transplantable murine tumor models was due to antiangiogenic activity.
Discussion
Angiogenesis, the process of blood-vessel growth, is impor-tant during both normal development and tumor growth and metastasis. The inhibition of angiogenesis controls the ex-pansion and metastasis of these tumors (Hanahan and Folk-man, 1996). In the present study, we demonstrated that YC-1, an activator of soluble guanylyl cyclase (Ko et al., 1994), has antiangiogenic properties in vitro and in vivo that can suppress tumor growth. In an in vitro model system, we demonstrated that YC-1 markedly and dose dependently in-hibited VEGF- and bFGF-induced endothelial cell prolifera-tion, migraprolifera-tion, and tube formation. VEGF and bFGF are major angiogenic factors associated with induction and main-tenance of the neovasculature in tumors (Dow and DeVere, 2000; Karkkainen and Petrova, 2000). Half-maximal inhibi-tion was ⬍3 M. However, under the same experimental conditions, YC-1 inhibition of the specific endothelial cell Fig. 2. Effect of YC-1 on VEGF- and bFGF-induced cell migration and
tube formation. A, cells were allowed to migrate for 24 h with indicated concentration of YC-1 and 10 ng/ml growth factor (VEGF or bFGF) in the lower chamber to stimulate chemotaxis. Data are means of three inde-pendent experiments performed in triplicate.⫹, P ⬍ 0.001 versus basal group;ⴱ, P ⬍ 0.05; ⴱⴱⴱ, P ⬍ 0.001 versus control group. B, endothelial cell cord formation assays in which HUVECs in M199 medium are grown on Matrigel for 24 h in the presence or absence of indicated concentration of YC-1. To better visualize cord formation and identify the cell, viability was assayed using the MTT assay method as described under Materials
response to VEGF and bFGF was much stronger than inhi-bition of normal cell growth of endothelial cells. Previous studies demonstrated that YC-1 activates sGC through an NO-independent pathway (Ko et al., 1994; Yu et al., 1995) or through reducing the activity of phosphodiesterase (Friebe et al., 1998; Galle et al., 1999; Koglin et al., 2002) and that most of the actions of YC-1 are cGMP mediated. On the other hand, YC-1 can enhance the activation of sGC by NO or NO donors (Friebe et al., 1998; Rothermund et al., 2000; Friebe and Koesling, 2003). However, our experiments demon-strated that ODQ (an sGC inhibitor) andL-NAME (a nitric oxide synthase inhibitor) did not reverse YC-1-induced anti-proliferation in HUVECs. Therefore, we suggest that YC-1
inhibits VEGF- and bFGF-induced endothelial cells functions by a mechanism that is neither cGMP-dependent nor NO-mediated.
In preliminarily studies, we demonstrated that treatment with high concentrations (⬎50M) of YC-1 results in growth inhibition of hepatocellular carcinoma cells (HA22T) (Wang et al., 2005) or apoptosis of prostate cancer cells (PC-3) and leukemia cancer cells (HL-60 and CCRF-CEM) in culture (data not shown). Thus, the growth-inhibitory action of YC-1 at low concentration is considered to be a more specific effect on endothelial cells that are stimulated by angiogenic growth factors. Therefore, YC-1 may possess novel molecular prop-erties that interfere with common angiogenic signaling path-Fig. 3. Effect of YC-1 on ERK1/2 and Akt phosphorylation.
Cells were incubated in the absence or presence of YC-1 for 1 h, and vehicle or angiogenic growth factor (VEGF or bFGF; 10 ng/ml) was added to the cells for another 15 min. Cells were harvested for the detection of phosphorylated-ERK1/2 and total phosphorylated-ERK1/2 (A) and phosphorylated-Akt and Akt using Western blotting (C). B and D, densitometric analysis for ratio between phosphorylated and total ERK1/2 or Akt expression. Values are expressed as mean⫾ S.E.M. (n⫽ 5). ⫹, P ⬍ 0.001 compared with basal; ⴱ, P ⬍ 0.05;ⴱⴱ, P ⬍ 0.01; ⴱⴱⴱ, P ⬍ 0.001 compared with control.
ways triggered by growth factor stimulation in endothelial cells.
As noted above, the activation of ERK1/2, PI3-K and PKC␣ pathways are required for the proliferative and migratory effects of VEGF and bFGF on endothelial cells (Cross and Claesson-Welsh, 2001). However, YC-1 significantly inhibits angiogenic factor-induced ERK1/2 and Akt activities as well as PKC␣ translocation from cytosol to membrane compart-ments in a concentration-dependent manner. Furthermore, the previous study demonstrates possible molecular mecha-nisms by which YC-1, at concentrations between 5 and 50 M, inhibits FBS-induced proliferation of human vascular endothelial cells in vitro (Hsu et al., 2003). It is possible that the effect is mediated through induction of a cyclin-depen-dent kinase inhibitor, p21 or p27, but YC-1 did not induce any significant changes in cyclins or CDKs. However, in this study, our data show that YC-1 (0.01–1 M) did not affect HUVEC proliferation stimulated by FBS. Under the same experimental conditions, YC-1 inhibited the mitogenic activ-ity of VEGF and bFGF, but not FBS in the same
concentra-tion range. Taken together, the data suggest that YC-1 is most likely to inhibit cell proliferation of HUVEC through VEGF- and bFGF-mediated signaling, including ERK1/2, Akt, and PKC pathways that are requisite for the angiogenic activities of VEGF and bFGF in endothelial cells.
Data from the in vivo Matrigel plug implantation animal model indicated that YC-1 markedly inhibited angiogenic growth factor-induced new vessel formation in a dose-depen-dent manner. Considering that angiogenesis is essential for tumor growth, the antitumor activity of YC-1 may be related to its antiangiogenic activities. Subsequently, we used the high angiogenic tumor A549, a nonsmall cell lung cancer, to assess YC-1 antitumor activity. We monitored body weights of mice every three dramatically loss from 20 g to 12 to 13 g. The results showed that YC-1 prolonged animal survival rate (⬎180%) after tumor cell implantation without producing cytotoxicity. However, Chun et al. (2001) and Yeo et al. (2003) demonstrated that YC-1 is an inhibitor of HIF-1␣, which halts tumor growth (by blocking tumor angiogenesis) and tumor adaptation to hypoxia. YC-1 (ⱖ5 ⫻ 10⫺6 M) sup-Fig. 4. Effect of YC-1 on localization
of PKC-␣ in HUVECs. Cells were in-cubated in the absence or presence of YC-1 for 1 h, and vehicle or angiogenic growth factor (VEGF or bFGF; 10 ng/ ml) was added to the cells for another 30 min or 2 h, respectively. A, cells were harvested for the detection of PKC-␣ distribution between cytosolic and membrane fractions using West-ern blotting. B, densitometric analy-sis showed the membrane fraction av-erage of the ratio with respect to ␣-tubulin level from five individual experimental groups. #, P⬍ 0.05 com-pared with basal;ⴱ, P ⬍ 0.05; ⴱⴱ, P ⬍ 0.01; ⴱⴱⴱ, P ⬍ 0.001 compared with control.
pressed HIF-1␣ protein level and expression of several HIF-1␣-regulated genes (VEGF, aldolase A, and enolase 1) in cancer cells under hypoxic conditions. YC-1 in our study showed efficacy in endothelial cells by inhibiting VEGF- and bFGF-induced angiogenic functions, such as proliferation (IC50 ⬍ 1 ⫻ 10⫺7M), migration, and differentiation (both
IC50⬍ 3 ⫻ 10⫺6M), more than it inhibited the expression of
HIF-1␣-regulated genes in cancer cells (ratio of 2- to 20-fold greater inhibition). Previous studies demonstrated that in-traperitoneal injection of YC-1 (30 mg/kg) abrogates tumor growth and is associated with the suppression of tumor an-giogenesis in animal models (Yeo et al., 2003). Furthermore, we showed that oral administration of YC-1 (ID50⫽ 3 mg/kg)
inhibited VEGF- and bFGF-induced new vessel formation in the Matrigel plug implantation model. These antiangiogenic activities of YC-1 in vivo may be explained by their direct inhibition of angiogenic factor-induced cell proliferation, mi-gration, and differentiation in endothelial cells. Taken to-gether, we believe that YC-1 is worth investigating further for clinical applications in cancer therapy.
In conclusion, our results demonstrated that YC-1 inhib-ited angiogenic responses in vitro and in vivo by blocking angiogenic factor signaling pathways, such as ones involving ERK1/2, Akt, and PKC. Inhibition of tumor activity by YC-1 is related to its antiangiogenic activity, which correlates with blockage of VEGF- and bFGF-induced endothelial cell prolif-Fig. 5. Effect of YC-1 on neovascular
for-mation in vivo. A, antiangiogenesis effect of YC-1 in in vivo mouse Matrigel-plus assay. The experimental procedures are described under Materials and Methods. Matrigel without growth factors (VEGF or bFGF; 150 ng/ml) did not show any migration or invasion of endothelial cells. However, with Matrigel containing growth factor, many blood vessels appeared in the gel on mice subcutaneous. Note the significant dose-de-pendent inhibition of the formation of blood vessel in the gel after orally administration of YC-1 for 7 days. B, histological analysis (hematoxylin and eosin staining) of the ef-fect of YC-1 on in vivo angiogenesis. Matri-gel containing growth factors in vehicle-treated mice demonstrated a high degree of cellularity and the presence of blood-con-taining vessels (⫻100). C, quantitation of active vasculature inside the Matrigel by measurement of hemoglobin content. Each value represents the mean of at least five animals, and similar results were obtained in two different experiments.⫹, P ⬍ 0.001 versus basal group;ⴱⴱⴱ, P ⬍ 0.001 versus control group.
eration, migration, and tube formation. Taken together, our results suggest that YC-1 is a potent angiogenesis inhibitor with the potential to become a useful agent in the treatment of human cancer and other angiogenesis-dependent diseases.
References
Bussolino F, Mantovani A, and Persico G (1997) Molecular mechanisms of blood vessel formation. Trends Biochem Sci 22:251–256.
Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science (Wash DC)
296:1655–1657.
Chien WL, Liang KC, Teng CM, Kuo SC, Lee FY, and Fu WM (2003) Enhancement of long-term potentiation by a potent nitric oxide-guanylyl cyclase activator, 3-(5-hydroxymethyl-2-furyl)-1-benzyl-indazole. Mol Pharmacol 63:1322–1328. Chun YS, Yeo EJ, Choi E, Teng CM, Bae JM, Kim MS, and Park JW (2001)
Inhibitory effect of YC-1 on the hypoxic induction of erythropoietin and vascular endothelial growth factor in Hep3B cells. Biochem Pharmacol 61:947–954. Cross MJ and Claesson-Welsh L (2001) FGF and VEGF function in angiogenesis:
signalling pathways, biological responses and therapeutic inhibition. Trends
Phar-macol Sci 22:201–207.
Dow JK and DeVere White RW (2000) Fibroblast growth factor 2: its structure and property, paracrine function, tumor angiogenesis and prostate-related mitogenic and oncogenic functions. Urology 55:800 – 806.
Ferrara N (2001) Role of vascular endothelial growth factor in regulation of physi-ological angiogenesis. Am J Physiol Cell Physiol 280:C1358 –C1366.
Fidler IJ and Ellis LM (1994) The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell 79:185–188.
Folkman J (1990) What is the evidence that tumors are angiogenesis dependent?
J Natl Cancer Inst 82:4 – 6.
Folkman J, Merler E, Abernathy C, and Williams G (1971) Isolation of a tumor factor responsible on angiogenesis. J Exp Med 133:275–288.
Friebe A and Koesling D (2003) Regulation of nitric oxide-sensitive guanylyl cyclase.
Circ Res 93:96 –105.
Friebe A, Mullershausen F, Smolenski A, Walter U, Schultz G, and Koesling D (1998) YC-1 potentiates nitric oxide- and carbon monoxide-induced cyclic GMP effects in human platelets. Mol Pharmacol 54:962–967.
Friebe A, Schultz G, and Koesling D (1996) Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme. EMBO (Eur Mol Biol Organ) J 15:6863– 6868.
Friesel R and Maciag T (1999) Fibroblast growth factor prototype release and fibroblast growth factor receptor signaling. Thromb Haemost 82:748 –754. Galle J, Zabel U, Hubner U, Hatzelmann A, Wagner B, Wanner C, and Schmidt HH
(1999) Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity. Br J Pharmacol 127:195–203. Hanahan D and Folkman J (1996) Patterns and emerging mechanisms of the
angiogenic switch during tumorigenesis. Cell 86:353–364.
Hsu HK, Juan SH, Ho PY, Liang YC, Lin CH, Teng CM, and Lee WS (2003) YC-1 inhibits proliferation of human vascular endothelial cells through a cyclic GMP-independent pathway. Biochem Pharmacol 66:263–271.
Jaffe EA, Hoger LW, Nachman RL, Becker CG, and Minich CR (1973) Culture of human endothelial cells derived from umbilical veins. J Clin Investig 52:2745– 2756.
Karkkainen MJ and Petrova TV (2000) Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene 19:5598 –5605. Kent KC, Mii S, Harrington EO, Chang JD, Mallette S, and Ware JA (1995) Re-quirement for protein kinase C activation in basic fibroblast growth factor-induced human endothelial cell proliferation. Circ Res 77:231–238.
Klein S, Roghani M, and Rifkin DB (1997) Fibroblast growth factors as angiogenesis factors: new insights into their mechanism of action. EXS 79:159 –192. Ko FN, Wu CC, Kuo SC, Lee FY, and Teng CM (1994) YC-1, a novel activator of
platelet guanylate cyclase. Blood 84:4226 – 4233.
Koglin M, Stasch JP, and Behrends S (2002) BAY 41–2272 activates two isoforms of nitric oxide-sensitive guanylyl cyclase. Biochem Biophys Res Commun 292:1057– 1062.
Pan SL, Guh JH, Chang YL, Kuo SC, Lee FY, and Teng CM (2004) YC-1 prevents sodium nitroprusside-mediated apoptosis in vascular smooth muscle cells.
Cardio-vasc Res 61:152–158.
Pan SL, Guh JH, Huang YW, Chern JW, Chou JY, and Teng CM (2003) Identifica-tion of apoptotic and antiangiogenic activities of terazosin in human prostate cancer and endothelial cells. J Urol 69:724 –729.
Rothermund L, Friebe A, Paul M, Koesling D, and Kreutz R (2000) Acute blood pressure effects of YC-1-induced activation of soluble guanylyl cyclase in normo-tensive and hypernormo-tensive rats. Br J Pharmacol 130:205–208.
Wang JP, Chang LC, Raung SL, Hsu MF, Huang LJ, and Kuo SC (2002) Inhibition of superoxide anion generation by YC-1 in rat neutrophils through cyclic GMP-dependent and -inGMP-dependent mechanisms. Biochem Pharmacol 63:577–585. Wang SW, Pan SL, Guh JH, Chen HL, Huang DM, Chang YL, Kuo SC, Lee FY, and
Teng CM (2005) YC-1 [3-(5⬘-hydroxymethyl-2⬘-furyl)-1-benzyl indazole] exhibits a novel antiproliferative effect and arrests the cell cycle in G0-G1 in human hepa-tocellular carcinoma cells. J Pharmacol Exp Ther 312:917–925.
Wellner M, Maasch C, Kupprion C, Lindschau C, Luft FC, and Haller H (1999) The proliferative effect of vascular endothelial growth factor requires protein kinase C-alpha and protein kinase C-zeta. Arterioscler Thromb Vasc Biol 19:178 –185. Yeo EJ, Chun YS, Cho YS, Kim J, Lee JC, Kim MS, and Park JW (2003) YC-1: a
potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst
95:516 –525.
Yu SM, Cheng ZJ, Guh JH, Lee FY, and Kuo SC (1995) Mechanism of anti-proliferation caused by YC-1, an indazole derivative, in cultured rat A10 vascular smooth-muscle cells. Biochem J 306:787–792.
Address correspondence to: Dr. Che-Ming Teng, Pharmacological Institute,
College of Medicine, National Taiwan University, 1 Jen-Ai Road, Section 1, Taipei, Taiwan. E-mail: cmteng@ha.mc.ntu.edu.tw
Fig. 6. Effect of YC-1 on antitumor activity in vivo. A549 cells were
transplanted through the chest wall into the left pleural space of nude mice (i.pl.) on day 0. YC-1 (10 mg/kg) oral treatment was begun on day 1 and continued every day. Each value represents the ratio of survival animal, and similar results were obtained in two different experiments.