CASE REPORT
Pseudomonas stutzeri Necrotizing Pneumonia in
Pre-existing Pulmonary Tuberculosis
Kuo-Hsi Lin
1, Chia-Ming Chen
2, Jui-Hsing Wang
3and Mao-Wang Ho
3Abstract
Pseudomonas stutzeri (P. stutzeri) is a Gram-negative, non-fermenting rod. It is a rare pathogen; therefore, its isolation is often associated with colonization or contamination. We herein describe the first reported case of necrotizing pneumonia caused by P. stutzeri in a non-HIV infected patient with previously undiagnosed pulmonary tuberculosis. The isolate was found to be antibiotic resistant, which led to the failure of the initial treatment. This case highlights the unique presentation of necrotizing pneumonia caused by P. stutzeri and the importance of emerging antimicrobial resistance in P. stutzeri.
Key words: Pseudomonas stutzeri, necrotizing pneumonia (Intern Med 53: 2543-2546, 2014)
(DOI: 10.2169/internalmedicine.53.2247)
24 breaths/min; body temperature, 39.4 . His level of con-
Introduction
Pseudomonas stutzeri (P. stutzeri) is a Gram-negative, non-fermenting, environmental bacterium that does not typi- cally cause human infection. This microorganism has been reported in causing infections in patients with various under- lying medical conditions (1). In previous reports, infections caused by P. stutzeri were usually responsive to treatments
with-lactam, and the treatment outcomes were usually
good (1-4). We herein report a case of P. stutzeri-induced necrotizing pneumonia in a patient with previously undiag- nosed pulmonary tuberculosis. The antimicrobial resistance of P. stutzeri led to the initial unresponsiveness to treatment.
Case Report
A 79-year-old man, who had a smoking history of 30 packs/day for years, with chronic obstructive pulmonary dis- ease (COPD) was admitted to the emergency department due to progressive dyspnea along with a one-week history of fever. On the physical examinations, he was 168 cm tall and weighed 65 kg. His vital signs were as follows: blood pressure, 108/65 mmHg; pulse, 104 beats/min; respiration,
sciousness was alert. His auscultation finding indicated coarse crackles.
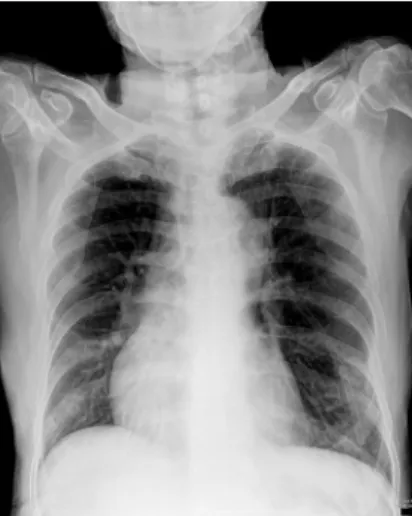
The laboratory investigation data on admission (day 1) is listed in the Table. The chest X-ray demonstrated increased vascular marking over both lung fields with peribronchial thickening but without infiltration shadow (Fig. 1).
Ampicillin-sulbactam (1.5 g every 6 hours) was initially prescribed for the treatment of the acute exacerbation of COPD. After 3 days of treatment, the antibiotic therapy was changed to levofloxacin (750 mg once daily) due to persis- tent respiratory distress and infiltration progression in the left lower lung. The patient became afebrile, but left lower lung pneumonia was still present despite the levofloxacin treatment. The antibiotic therapy was changed to vancomy- cin (1 g twice daily) and meropenem (1 g every 8 h) (Fig. 2). Sputum acid-fast staining was performed on hospi- tal day 5 as the chest X-ray showed increased infiltration in the left lower lung field (Fig. 3). The acid-fast staining re- vealed acid-fast bacilli in the sputum while the Mycobacte- rium tuberculosis DNA polymerase chain reaction (PCR) (COBAS TaqMan Mycobacterium tuberculosis (MTB) Test, Roche, Basel, Switzerland) revealed a positive result. The patient was started on anti-mycobacterial therapy with
Section of Infectious Disease, Department of Internal Medicine, Tungs' Taichung MetroHarbor Hospital, Taiwan, Department of Internal Medi- cine, Changhua Hospital, Ministry of Health and Welfare, Taiwan and Section of Infectious Disease, Department of Internal Medicine, China Medical University Hospital, Taiwan
Received for publication December 4, 2013; Accepted for publication April 18, 2014 Correspondence to Dr. Mao-Wang Ho, D7905@mail.cmuh.org.tw
Intern Med 53: 2543-2546, 2014 DOI: 10.2169/internalmedicine.53.2247
Figure 2. The hospital course and duration of treatments. AMPC/SBT: ampicillin-sulbactam, LVFX: levofloxacin, VCM: vancomycin, MEPM: meropenem, INH+RFP+EB+PZA: iso- niazid, rifampin, ethambutol and pyrazinamide, IPM/cs: imi-
Figure 1. The chest X-ray on admission revealed increased vascular marking over both lung fields with peribronchial thickening but without infiltration shadow.
penem/cilastatin
Table. The Laboratory Data on Admission (day 1) and during an Episode of Jaundice (day 16)
Parameter analyzed
Blood cells count
White blood cell Hemoglobin Platelet Segment ymphocyte Monocyte Band form Biochemistry
Blood urea nitrogen Creatinine Sodium Potassium Result(day 1) 14,900 92×103/ 63 % 16 % 3% 17 % 21 m 1.59 m 134 mmol/ 4.6 mmol/ Result(day 16) 17,900 / 12.6 89×103/ 90.8 % 4.4 % 4.6 % 0% 23 m 0.84 m 145 mmol/ 3.17 mmol/
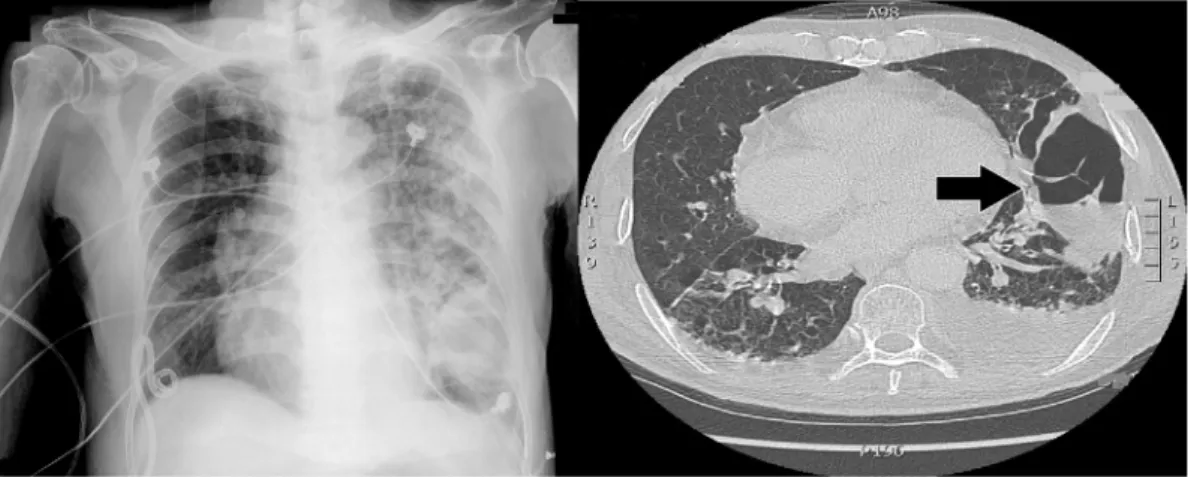
Figure 3. The chest X-ray (day 5), following sputum acid- fast staining, demonstrated increased infiltration in the left lower lung field.
rifampin (600 mg once daily), ethambutol (1,200 mg once daily), pyrazinamide (1,500 mg once daily) and isoniazid (300 mg once daily). The treatment was discontinued five days later due to a jaundice episode. The biochemical data
AST AT Total Bilirubin Direct Bilirubin C-reactive protein Albumin Cortisol HBs-Ag HCV-Ab HIV-Ab 28 11 1.29 m 15.45 m 2.2 >60 32 16 4.3 m 1.85 m 7.69 m 1.8 (-) (-) (-)
following the jaundice episode (day 16) is listed in the Ta- Arterial blood Gas (under room air condition)
ble. The screen for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus was negative. The patient did not have purulent sputum until 18 days after admission and much purulent sputum was expectorated. Chest com- puted tomography (CT) revealed left lung necrotizing pneu- monia with air-fluid level (Fig. 4). The sputum culture
pH PaO2 PaCO2 HCO3 7.35 86.9 mmHg 55.5 mmHg 30.2 m
yielded P. stutzeri, identified by the biochemistry testing, two days later. According to Clinical and Laboratory Stan- dards Institutes (CLSI) breakpoints, the isolate was suscepti- ble to amikacin, imipenem, piperacillin and cefepime and resistant to gentamycin, ciprofloxacin, levofloxacin and cef- tazidime (disc diffusion method). The antibiotic therapy was therefore changed to imipenem-cilastatin (500 mg every 8
h). Because of persistent respiratory distress, an endotracheal tube was inserted and the patient was transferred to the in- tensive care unit (ICU). Repeated sputum cultures from the endotracheal aspirates also grew P. stutzeri four days later. No other bacterium was ever isolated. The patient's clinical condition improved after one more week of treatment with imipenem-cilastatin. The chest X-ray also showed decreased 2544
Intern Med 53: 2543-2546, 2014 DOI: 10.2169/internalmedicine.53.2247
Figure 4. Left lung pneumonia progressed to necrotizing pneumonia with air-fluid level (arrow) (day 18).
infiltration of the left lower lung. The endotracheal tube was removed, and the patient was transferred to the regular ward. Anti-mycobacterial medication was gradually re- sumed. The patient had respiratory distress and desaturation 12 days after extubation due to sputum impaction. The pa- tient and his family refused further intubation. He developed respiratory failure and expired 32 days after admission.
Discussion
P. stutzeri is an aerobic, non-fermenting, Gram-negative rod with polar monotrichous flagella. P. stutzeri is a ubiqui- tous saprophyte, normally found in soil, water and hospital environments, that rarely causes human infection. However, patients with various underlying medical conditions had been reported to have infections caused by P. stutzeri (1). Pneumonia and hemodialysis-related blood stream infection were the most frequently reported infections (1). Empyema, septic arthritis, osteomyelitis and soft tissue infection has also been reported (1, 2). Patients may also get infected through trauma or medical procedures (3). We herein re- ported a case of P. stutzeri-induced necrotizing pneumonia in a patient with previously undiagnosed pulmonary tubercu- losis.
In this case, necrotizing pneumonia progressing from community acquired pneumonia is the most probably etiol- ogy. The quantity of expectorated purulent sputum increased due to the abscess cavity communicating with the bronchi- oles, while the antibiotic treatment became more effective. Repeated sputum cultures before and after the endotracheal intubation all yielded P. stutzeri only, thus it was considered as the causative pathogen.
An underlying Mycobacterium infection may cause lung parenchyma destruction and promote necrosis of the lung caused by P. stutzeri. In previous reports, patients with P. stutzeri infection may have had underlying diseases, such as liver cirrhosis, COPD, malignancy, uremia and HIV infec- tion (1, 2, 4, 5). Patients with these underlying diseases, such as our patient, were also at risk for Mycobacterium in-
fection. Previous cases of underlying Mycobacterium infec- tion superimposed by P. stutzeri infection have only been re- ported in HIV-infected patients (4, 5). Our case indicated that this infection in non-HIV infected patients is possible, and P. stutzeri should be considered as a true pathogen in patients at risk.
In previous reports, P. stutzeri isolates were susceptible to various antibiotics, such as piperacillin, third-generation cephalosporins, imipenem, fluoroquinolones and aminogly- cosides, and the response to treatment was usually good (1-5). Lee et al. reported that a few P. stutzeri isolates
with metallo--lactamase were resistant to third-generation cephalosporins and some fluoroquinolones (6). Nicolosi et al. also reported a P. stutzeri isolate causing pneumonia that was resistant to fluoroquinolones, piperacillin and imipenem (7). The P. stutzeri isolate from our patient showed resistance to third-generation cephalosporin and fluoroquinolones. This resistance also initially contributed to
deterioration of pneumonia. Metallo--lactamase was not checked in this isolate, therefore, we do not know if the re- sistance is due to the inactivation of-lactam. The emerging antimicrobial resistance of P. stutzeri could potentially lead to antibiotic treatment failure. Therefore, determine the an- timicrobial profile of the isolate and changing the treatment accordingly is necessary.
In conclusion, our case highlights a unique presentation of necrotizing pneumonia caused by P. stutzeri that rarely causes human infection. P. stutzeri should therefore be con- sidered a true pathogen in patients with underlying medical conditions, such as liver cirrhosis, COPD, uremia, malig- nancy and HIV infection. The emerging antimicrobial resis- tance of P. stutzeri could lead to treatment failure. The anti- biotic sensitivity of the organism therefore should be checked for those patients who do not respond to initial treatment.
The authors state that they have no Conflict of Interest (COI).
References
Intern Med 53: 2543-2546, 2014 DOI: 10.2169/internalmedicine.53.2247
Hosp Epidemiol 27: 630-632, 2006.
4. Roig P, Orti A, Navarro V. Meningitis due to Pseudomonas stutz- eri in a patient infected with human immunodeficiency virus. Clin 1. Reisler RB, Blumberg H. Community-acquired Pseudomonas
stutzeri vertebral osteomyelitis in a previously healthy patient: case
report and review. Clin Infect Dis 29: 667-669, 1999.
2. Campos-Herrero MI, Bordes A, Rodríguez H, Perera A, González
B, Conde A. Pseudomonas stutzeri community-acquired pneumo- nia associated with Empyema: case report and review. Clin Infect Dis 25: 325-326, 1997.
3. Yee-Guardino S, Danziger-Isakov L, Knouse M, Bingaman W, Sa-
bella C, Goldfarb J. Nosocomially acquired Pseudomonas stutzeri brain abscess in a child: case report and review. Infect Control
Infect Dis 22: 587-588, 1996.
5. Loyse A, Storring R A, Melzer M. Pseudomonas stutzeri pneumo-
nia in an HIV seropositive patient. J Infect 53: 75-76, 2006.
6. Lee NY, Yan JJ, Lee HC, Liu KH, Huang ST, Ko WC. Clinical
experiences of bacteremia caused by metallo--lactamase- producing gram-negative organisms. J Microbiol Immunol Infect
37: 343-349, 2004.
7. Nicolosi D, Nicolosi VM, Cappellani A, Nicoletti G, Blandino G.
Antibiotic susceptibility profiles of uncommon bacterial species causing severe infections in Italy. J Chemother 21: 253-260, 2009.
2014 The Japanese Society of Internal Medicine http://www.naika.or.jp/imonline/index.html