Plant and Soil 230: 135–143, 2001.
© 2001 Kluwer Academic Publishers. Printed in the Netherlands. 135
Cell wall peroxidase activity, hydrogen peroxide level and NaCl-inhibited
root growth of rice seedlings
Chuan Chi Lin & Ching Huei Kao
1Department of Agronomy, National Taiwan University, Taipei, Taiwan, Republic of China.1Corresponding author∗ Received 20 August 1999. Accepted in revised form 10 November 2000
Key words: hydrogen peroxide, NaCl, Oryza sativa, peroxidase, root growth
Abstract
The changes in cell-wall peroxidase (POD) activity and H2O2 level in roots of NaCl-stressed rice seedlings and their correlation with root growth were investigated. Increasing concentrations of NaCl from 50 to 150 mM progressively reduced root growth and increased ionically bound cell-wall POD activity. NaCl had no effect on covalently bound cell-wall POD activities. The reduction of root growth by NaCl is closely correlated with the increase in H2O2level. Exogenous H2O2 was found to inhibit root growth of rice seedlings. Since ammonium and proline accumulation are associated with root growth inhibition caused by NaCl, we determined the effects of NH4Cl or proline on root growth, cell-wall POD activity and H2O2level in roots. External application of NH4Cl or proline markedly inhibited root growth, increased cell-wall POD activity and increased H2O2level in roots of rice seedlings in the absence of NaCl. An increase in cell-wall POD activity and H2O2level preceded inhibition of root growth caused by NaCl, NH4Cl or proline. NaCl or proline treatment also increased NADH-POD and diamine oxidase (DAO) activities in roots of rice seedlings, suggesting that NADH-POD and DAO contribute to the H2O2 generation in the cell wall of NaCl- or proline-treated roots. NH4Cl treatment increased NADH-POD activity but had no effect on DAO activity, suggesting that NADH-POD but not DAO is responsible for H2O2generation in cell wall of NH4Cl-treated roots.
Abbreviations: DAO – diamine oxidase; DW – dry weight; FW – fresh weight; POD – peroxidase
Introduction
Peroxidase (POD, EC 1.11.1.7) influences plant growth through lignin synthesis (Siegel, 1953), oxid-ative coupling reactions involving phenolics that are esterified to cell wall polysaccharides (Fry, 1986), formation of isodityrosine bridges that are believed to crosslink structural protein molecules (Fry, 1986). An inverse relationship between growth rate and POD activity has been reported in many plant systems (Carpita and Gilbeaut, 1993; Chen and Kao, 1995; Fry, 1986; Gardiner and Cleland, 1974; Lee and Lin, 1995;). Thus POD is generally believed to be a putative wall rigidification enzyme (Cosgrove, 1997).
∗ FAX NO: +886-2-2362-0879. E-mail: kaoch@ccms.ntu.edu.tw
The inhibition of plant growth by salinity is a widespread problem in agricultural practice. How-ever, the mechanisms underlying this inhibition are not yet clear (Munns, 1993; Rengel, 1992). Neu-mann et al. (1994) demonstrated that root growth inhibition caused by salinity was associated with wall stiffening. Recently, we have demonstrated that an in-crease in ionically bound POD activity is associated with growth inhibition of rice seedling roots caused by NaCl (Lin and Kao, 1999). The ionically bound fraction of POD, removable from homogenized tis-sues with high ionic strength buffers, has been equated with the cell wall fraction. However, Mader et al. (1986) suggested that at least some ionically bound POD activity might be an artifact of homogenization. Therefore, to test the hypothesis that an increase in cell-wall POD activity is associated with growth
in-136
Figure 1. Effects of NaCl on root growth and cell-wall POD activities in roots of rice seedlings. Root growth and cell wall POD activities were
determined after 5 days of treatment. Vertical bars represent standard errors (n=4).
hibition of seedling roots of rice, data on POD activity extracted from the cell walls are required.
H2O2 is a necessary substrate for a cell wall stiffening process catalyzed by POD (Elstner and Heupel, 1976; Hohl et al., 1995; Schopfer, 1994). Recently, Schopfer (1996) demonstrated that H2O2 in-hibited auxin-mediated growth of maize coleoptiles. The formation of H2O2 by isolated cell walls from horseradish has been reported (Elstner and Heupel, 1976). Using a sensitive tissue-print assay, Schop-fer (1994) demonstrated that H2O2is localized in the cell wall of pea epicotyls. H2O2causes a rapid cross-linking of cell-wall polymers (Bradley et al., 1992; Schopfer, 1996). Therefore, a sufficient supply of H2O2 is required for ensuring complete stiffening of the cell wall. The present investigation was designed to study the changes in cell-wall POD activity and H2O2 level in roots of NaCl-stressed rice seedlings and their correlation with root growth.
Materials and methods
Rice (Oryza sativa L., cv. Taichung Native 1) seeds were sterilized with 2.5% sodium hypochlorite for 15 min and washed extensively with distilled water. These seeds were then germinated in a Petri dish
Figure 2. Effects of NaCl on H2O2 levels in roots of rice
seed-lings. H2O2was determined after 5 days of treatment. Vertical bars
represent standard errors (n=4).
(20 cm) containing distilled water at 37◦C under dark condition. After 1-day incubation, uniformly germin-ated seeds were selected and transferred to a Petri dish (9.0 cm) containing two sheets of Whatman No.1
fil-137
Figure 3. Effects of exogenous application of H2O2on root growth
and H2O2levels in roots of rice seedlings. Root growth and H2O2
levels were determined after 5 days of treatment. Vertical bars represent standard errors (n=4).
ter paper moistened with 10 ml of distilled water or test solutions. Each Petri dish contained 20 germinated seeds. Each treatment was replicated four times. The germinated seeds were allowed to grow at 27 ◦C in darkness and 3 ml of distilled water or test solutions was added to each Petri dish on day 3 of the growth. Fresh weight and dry weight of roots were measured at the times indicated.
Cell walls were prepared by homogenizing roots in ice-cold phosphate buffer (50 mM, pH 5.8) using a pestle and mortar. The homogenate was centrifuged at
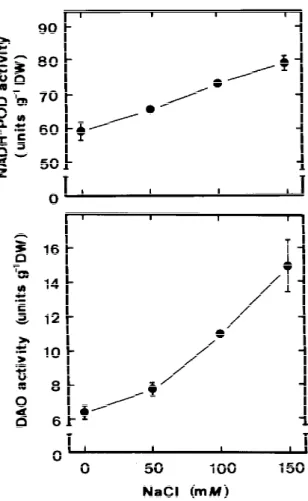
Figure 4. Effects of NaCl on NADH-POD and DAO activities in
roots of rice seedlings. NADH-POD and DAO activities were de-termined after 5 days of treatment. Vertical bars represent standard errors (n=4).
1000 g, and washed at least four times with 50 mM phosphate buffer (Lee and Lin, 1995). The pellet was collected and used as a cell wall fraction.
POD ionically bound to the cell walls was ex-tracted with 1 M NaCl. Cell walls were prepared as described above and incubated in 1 M NaCl for 2 h with shaking at 30◦C and centrifuged at 1000 g. The supernatant was considered as the ionic cell wall fraction. POD covalently bound to the cell walls was extracted as follows: Cell walls previously subjec-ted to the saline extraction procedure were incubasubjec-ted in an enzyme preparation containing 0.5% cellulase (EC 3.2.1.4, Sigma) and 2.5% pectinase (EC 3.2.1.15, Sigma), both from Aspergillus niger, in 0.1 M sodium acetate buffer, pH 5.0 (Sanchez et al., 1989). The incubation was carried out for 24 h with shaking at 25◦C. The suspension was then centrifuged at 1000 g
138
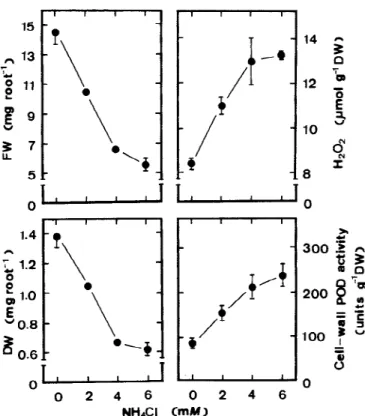
Figure 5. Effects of NH4Cl on root growth, cell-wall POD activities and H2O2levels in roots of rice seedlings. Root growth, cell-wall POD
activities and H2O2levels were determined after 5 days of treatment. Vertical bars represent standard errors (n=4).
for 10 min. The supernatant was considered to be the covalently bound cell wall fraction.
POD activities were measured using a modification of the procedure described by Curtis (1971). The as-say medium contained 0.05 M phosphate buffer (pH 5.8), 7.2 mM guaiacol, 11.8 mM H2O2 and 0.1 ml enzyme extract in a final assay volume of 3.0 ml. The reaction was initiated by the addition of H2O2 and the change in absorbance at 470 nm was measured. Activity was calculated using the extinction coefficient (26.6 mM−1 cm−1 at 470 nm) for tetraguaiacol. One unit of POD was defined as the amount of enzyme that causes the formation of 1 µmol tetraguaiacol per min. NADH-POD activities in the ionic cell wall frac-tion were determined according to the method of Ishida et al. (1987). The assay mixture contained 50 µM NADH in Na-acetate buffer (30 mM, pH 6.5), 5 mM MnCl2and 20 µM p-coumaric acid. The reaction was started by adding the enzyme, and the decrease of absorbance at 340 nm by oxidizing NADH was meas-ured at 25◦C. One unit of NADH-POD was defined as 1 nmol NADH oxidized per min.
Diamine oxidase (DAO), which is involved in polyamine catabolism, oxidizes putrescine with the
formation of 11-pyrrolinetogether with H2O2and am-monia (Smith, 1985). DAO activities in the ionic cell wall fraction were measured by the method of Naik et al. (1981). The incubation mixture contained 50 mM phosphate buffer (pH 7.8), 10 mM putresine, 0.1 mM pyridoxal phosphate and enzyme extract in a total volume of 4 ml. After incubation at 30◦C for 1 h, the reaction was terminated using 1 ml 20% (w/v) trichloroacetic acid. After 30 min, the incubation mix-ture was centrifuged at 5000 g for 15 min. One ml of ninhydrin mixture (250 mg ninhydrin in 6 ml acetic acid and 4 ml phosphoric acid) was added to the su-pernatant. Colour was developed at 100◦C for 30 min. After adding 1 ml of acetic acid absorbance was meas-ured at 510 nm. In controls, trichloroacetic acid added prior to the enzyme solution. One unit of DAO was defined as an increase of 1 A510per h.
The H2O2level was colorimetrically measured as described by Jana and Choudhuri (1981). H2O2was extracted by homogenizing 10 roots with 3 ml of phosphate buffer (50 mM, pH 6.8). The homogenate was centrifuged at 6000 g for 25 min. To determine H2O2 levels, 3 ml of extracted solution was mixed with 1 ml of 0.1% titanium chloride (Aldrich) in 20%
139
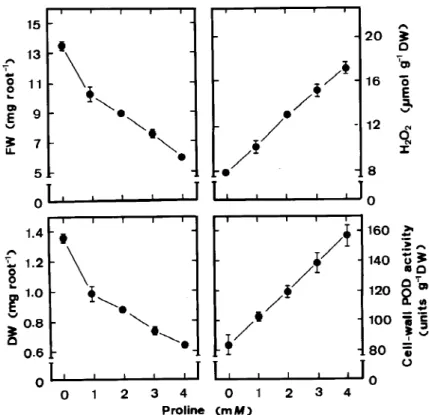
Figure 6. Effects of proline on root growth, cell-wall POD activities and H2O2levels in roots of rice seedlings. Root growth, cell wall-POD
activities and H2O2levels were determined after 5 days of treatment. Vertical bars represent standard errors (n=4).
(v/v) H2SO4and the mixture was then centrifuged at 6000 g for 15 min. The intensity of the yellow colour of the supernatant was measured at 410 nm. H2O2 level was calculated using the extinction coefficient 0.28 µmol−1cm−1.
For all measurements, each treatment was repeated four times. All experiments described here were re-peated at least three times. Similar results and identical trends were obtained each time. The data reported here are from a single experiment.
Results and discussion
Root growth was followed by measuring FW and DW of roots. Figure 1 shows the effect of NaCl on root growth of rice seedlings. Increasing concentrations of NaCl from 50 to 150 mM progressively decreased root growth.
Both ionically bound and covalently bound cell-wall PODs were present in cell cell-walls of rice roots (Figure 1). The reduction of root growth with in-creasing NaCl concentrations is correlated with an increase in ionically bound cell-wall POD activity
(Figure 1). The effect of different concentrations of NaCl on covalently bound cell- wall POD activit-ies was also examined. No appreciable changes in covalently bound cell-wall POD activities occurred between 0 and 150 mM NaCl. Thus, ionically bound POD in the cell wall preparations was assayed in all subsequent experiments.
It has been postulated that the action of POD loc-ated in the cell walls would be to confer rigidity to the cell walls and prevent later expansion involved in growth (Carpita and Gilbeaut, 1993; Fry, 1986; Gardiner and Cleland, 1974; MacAdam et al., 1992). Thus, NaCl-induced inhibition in root growth of rice seedlings is likely due to cell wall stiffening process related to the formation of cross-linking among cell wall polymers. This process appears to involve oxidat-ive coupling, dependent on H2O2(Fry, 1986). In fact, H2O2has been demonstrated to cause a rapid cross-linking of cell wall polymers (Bradley et al., 1992; Schopfer, 1996). If POD regulates cell wall stiffening by catalyzing the oxidative cross-linking of cell wall polymers, there must be a sufficient supply of H2O2. Thus, it is of great interest to know whether NaCl in-creases the level of H2O2 in roots of rice seedlings.
140
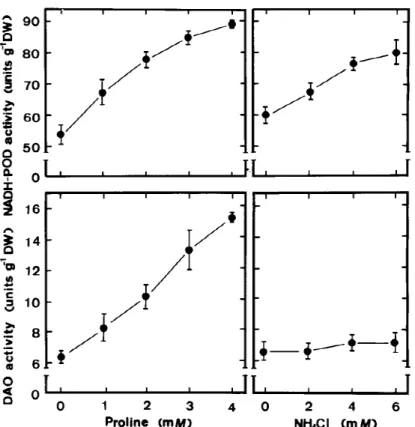
Figure 7. Effects of NH4Cl or proline on NADH-POD and DAO activities in roots of rice seedlings. NADH-POD and DAO activities were
determined after 5 days of treatment. Vertical bars represent standard errors (n=4).
Figure 2 shows the effect of NaCl concentrations on H2O2levels in the roots of rice seedlings. Increasing concentrations of NaCl from 50 to 150 mM progress-ively increased H2O2levels in roots. If H2O2plays a role in regulating NaCl-inhibited root growth of rice seedlings, exogenous application of H2O2is expected to inhibit root growth. It is indeed the case (Fig-ure 3). Increasing concentrations of H2O2 from 2.5 to 10 mM progressively increased endogenous H2O2 levels in roots of rice seedlings and decreased FW and DW. Clearly, an increase in H2O2 levels is import-ant in regulating NaCl-inhibited root growth of rice seedlings.
Generation of H2O2in the cell walls has been pre-viously proposed since it is a necessary substrate for the formation of cross-linking among cell wall poly-mers (Elstner and Heupel, 1976) and cell wall-bound malate dehydrogenase and NADH-POD activities de-voted to H2O2production have been detected (Gold-berg et al., 1987; Gross, 1977; Mader et al., 1980). As expected, increasing NADH-POD activities were found to increase with increasing NaCl concentrations in rice seedling roots (Figure 4). Concerning the
ori-gin of the NADH required in the formation of H2O2, it has been postulated that a cell wall-bound malate dehydrogenase provides this electron donor (Gross, 1977). However, we have not succeeded in detecting cell wall-bound malate dehydrogenase activity in roots of rice seedlings. Frahry and Schopfer (1998) also failed to detect cell wall-bound malate dehydrogenase activity in intact soybean roots.
DAO is widespread in the Leguminosae family (Smith, 1985) and has been reported in barley (Cogoni et al., 1990) and maize (Suzuki and Hagiwara, 1993). This enzyme, which is involved in polyamine catabol-ism, oxidizes putrescine with the formation of 11 -pyrroline together with H2O2 and ammonia (Smith, 1985). DAO activity is mainly located in the cell wall (Angelini and Federico, 1989; Angelini et al., 1990) and perhaps plays a role in regulating putrescine levels (Smith, 1985) or providing H2O2 required for per-oxidative reactions that occur in the cell walls for the formation of cross-linking (Angelini and Federico, 1989; Angelini et al., 1990). It is most likely that DAO is another source leading to H2O2generation in NaCl-inhibited root growth of rice seedlings. To test this,
141
Table 1. Changes in root growth, cell-wall POD activity, H2O2level, DAO activity and NADH-POD activity in
roots of rice seedlings treated with NaCl. Rice seeds were germinated in distilled water for 2 days and then were transferred to distilled water and NaCl (150 mM), respectively. The data represent mean value± standard errors,
n=4 Treatment Time, h 0 4 8 12 16 FW H2O 7.1±0.42 7.6±0.71 8.1±0.35 8.6±0.10 9.4±0.23 (mg root−1) NaCl 7.5±0.28 7.7±0.47 8.1±0.20 8.5±0.36 DW H2O 0.67±0.04 0.72±0.03 0.77±0.03 0.82±0.01 0.89±0.02 (mg root−1) NaCl 0.71±0.03 0.73±0.05 0.77±0.02 0.80±0.03 Cell-wall POD H2O 38.3±0.82 36.7±1.8 38.9±2.5 38.0±1.1 39.0±1.4 (units g−1DW) NaCl 41.1±5.9 49.6±3.8 57.3±2.0 65.1±3.4 H2O2 H2O 6.6±0.39 6.7±0.62 6.6±0.16 7.7±0.95 7.7±0.20 (µmol g−1DW) NaCl 7.0±0.58 8.7±0.72 9.2±0.46 10.4±0.12 DAO H2O 6.6±0.66 5.9±0.82 6.6±0.71 7.3±0.76 7.6±0.57 (units g−1DW) NaCl 7.0±0.68 8.2±0.47 8.6±0.20 9.5±0.36 NADH-POD H2O 54.3±3.2 52.3±1.7 54.0±2.4 55.6±0.52 59.8±3.7 (units g−1DW) NaCl 54.1±2.9 58.5±0.55 60.3±1.7 66.4±4.1
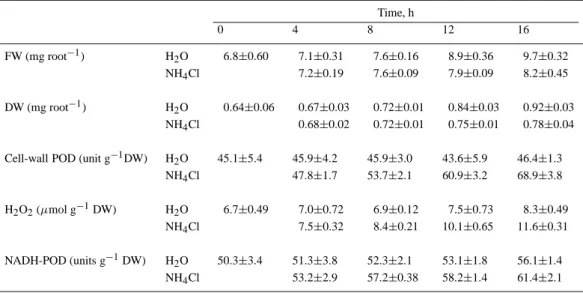
Table 2. Changes in root growth, cell-wall POD activity, H2O2level and NADH-POD activity in roots of rice seedlings
treated with NH4Cl. Rice seeds were germinated in distilled water for 2 days and then were transferred to distilled water
and NH4Cl (4 mM), respectively. The data represent mean values± standard errors, n=4
Time, h 0 4 8 12 16 FW (mg root−1) H2O 6.8±0.60 7.1±0.31 7.6±0.16 8.9±0.36 9.7±0.32 NH4Cl 7.2±0.19 7.6±0.09 7.9±0.09 8.2±0.45 DW (mg root−1) H2O 0.64±0.06 0.67±0.03 0.72±0.01 0.84±0.03 0.92±0.03 NH4Cl 0.68±0.02 0.72±0.01 0.75±0.01 0.78±0.04
Cell-wall POD (unit g−1DW) H2O 45.1±5.4 45.9±4.2 45.9±3.0 43.6±5.9 46.4±1.3
NH4Cl 47.8±1.7 53.7±2.1 60.9±3.2 68.9±3.8
H2O2(µmol g−1DW) H2O 6.7±0.49 7.0±0.72 6.9±0.12 7.5±0.73 8.3±0.49
NH4Cl 7.5±0.32 8.4±0.21 10.1±0.65 11.6±0.31
NADH-POD (units g−1DW) H2O 50.3±3.4 51.3±3.8 52.3±2.1 53.1±1.8 56.1±1.4
NH4Cl 53.2±2.9 57.2±0.38 58.2±1.4 61.4±2.1
we determined the activities of DAO in rice seedling roots in response to various concentrations of NaCl (Figure 4). As expected, increasing concentrations of NaCl from 50 to 150 mm progressively increased DAO activities. This result is consistent with our pre-vious work in which we showed that NaCl treatment
resulted in a decline in the levels of putrescine in roots of rice seedlings (Lin and Kao, 1995).
To test the causal relationship between root growth reduction, cell-wall POD activity, H2O2 level, DAO activity and NADH-POD activity caused by NaCl, 2-day-old seedlings were transferred to distilled
wa-142
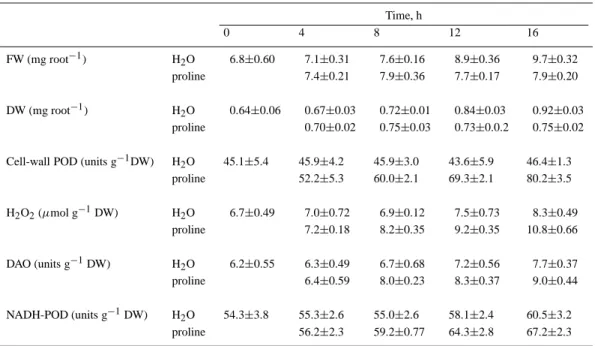
Table 3. Changes in root growth, cell-wall POD activity, H2O2level, DAO activity and NADH-POD activity in roots of rice
seedlings treated with proline. Rice seeds were germinated in distilled water for 2 days and then were transferred to distilled water and proline (4 mM), respectively. The data represent mean values± standard errors, n=4
Time, h 0 4 8 12 16 FW (mg root−1) H2O 6.8±0.60 7.1±0.31 7.6±0.16 8.9±0.36 9.7±0.32 proline 7.4±0.21 7.9±0.36 7.7±0.17 7.9±0.20 DW (mg root−1) H2O 0.64±0.06 0.67±0.03 0.72±0.01 0.84±0.03 0.92±0.03 proline 0.70±0.02 0.75±0.03 0.73±0.0.2 0.75±0.02
Cell-wall POD (units g−1DW) H2O 45.1±5.4 45.9±4.2 45.9±3.0 43.6±5.9 46.4±1.3
proline 52.2±5.3 60.0±2.1 69.3±2.1 80.2±3.5 H2O2(µmol g−1DW) H2O 6.7±0.49 7.0±0.72 6.9±0.12 7.5±0.73 8.3±0.49 proline 7.2±0.18 8.2±0.35 9.2±0.35 10.8±0.66 DAO (units g−1DW) H2O 6.2±0.55 6.3±0.49 6.7±0.68 7.2±0.56 7.7±0.37 proline 6.4±0.59 8.0±0.23 8.3±0.37 9.0±0.44 NADH-POD (units g−1DW) H2O 54.3±3.8 55.3±2.6 55.0±2.6 58.1±2.4 60.5±3.2 proline 56.2±2.3 59.2±0.77 64.3±2.8 67.2±2.3
ter and NaCl, respectively, for 4, 8, 12 and 16 h. Changes in root growth, cell-wall POD activity, H2O2 level, DAO activity and NADH-POD activity were then monitored. As indicated in Table 1, an increase in cell-wall POD activity and H2O2 level preceded inhibition of root growth caused by NaCl. The ob-servations that an increase in DAO and NADH-POD activities coincides with an increase in H2O2 level in roots caused by NaCl (Table 1) suggest that DAO and NADH-POD are the sources for the generation of H2O2 in the cell walls. Since H2O2can rapidly pass from the cytoplasm to the cell wall (Allan and Fluhr 1997), a cytoplasmic origin of released H2O2cannot be excluded.
It is known that ammonium strongly inhibits the growth of many plants (Haynes and Goh, 1978). Exo-genous application NH4Cl was also found to reduce root growth of rice seedlings (Lin and Kao, 1996a). It has also been shown that NaCl was effective in stimu-lating the accumulation of ammonium in roots of rice seedlings and that accumulation of ammonium in roots preceded inhibition of root growth caused by NaCl (Lin and Kao, 1996a). If the increases in cell-wall POD activity and H2O2level are important in regu-lating growth reduction of roots caused by NaCl, the exogenous application of NH4Cl would be expected to increase cell-wall POD activity and H2O2level in roots of rice seedlings. Figure 5 shows that addition of
NH4Cl inhibits root growth, increases cell-wall POD activity and increases H2O2level in roots.
In previous work, we have shown that proline ac-cumulation is associated with root growth inhibition of rice seedlings caused by NaCl (Lin and Kao, 1996b). In the present study, we also demonstrated that proline treatment resulted in an inhibition of root growth, an increase in cell-wall POD activity and an increase in H2O2 level (Figure 6). The data in Figure 7 clearly show that DAO and NADH-POD are responsible for the generation of H2O2 in the cell wall of proline-treated roots, whereas NADH-POD but not DAO is the source for the generation of H2O2 in NH4Cl-treated roots. Furthermore, we also observed that an increase in cell-wall POD activity and H2O2level preceded in-hibition of root growth caused by NH4Cl or proline and an increase in H2O2level coincided with an in-crease in NADH-POD or DAO activity (Tables 2 and 3).
The observations that rice seedlings treated with NH4Cl or proline, which resulted in an increase in cell-wall POD activity and H2O2 level in roots, re-duced root growth in the same way that NaCl did, further support our suggestion that cell-wall POD and H2O2 are likely participated in the regulation of root growth reduction of rice seedlings under NaCl condition.
143
Acknowledgement
This work was supported by the National Science Council of the Republic of China (NSC 89-2313-B-002-009).
References
Allan A C and Fluhr R 1997 Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9, 1559– 1572.
Angelini R and Federico R 1989 Histochemical evidence of polyam-ine oxidation and hydrogen peroxide production in the cell wall. J. Plant Physiol. 135, 212–217.
Angelini R, Manes F and Federico R 1990 Spatial and functional correlation between diamine-oxidase and peroxidase activities and their dependence upon de-etiolation and wounding in chick-pea stems. Planta 182, 89–96.
Bradley D J, Kjellbom P and Lamb C J 1992 Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: A novel, rapid defense response. Cell 70, 21–30. Carpita N C and Gilbeaut D M 1993 Structural models of primary
cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 3, 1–30.
Chen S L and Kao C H 1995 Cd induced changes in proline level and peroxidase activity in roots of rice seedlings. Plant Growth Regul. 17, 67–71.
Cogoni A, Piras C, Farei R, Melis A and Floris G 1990 Hordeum
vulgare seedlings amine oxidase. Purification and properties.
Plant Physiol. 93, 818–821.
Cosgrove D J 1997 Assembly and enlargement of the primary cell wall in plants. Annu. Rev. Cell Dev. Biol. 13, 171–201. Curtis C R 1971 Disc electrophoretic comparisons of proteins
and peroxidases from Phaseolus vulgaris leaves infected with
Agrobacterium tumefaciens. Can. J. Bot. 49, 333–337.
Elstner E F and Heupel A 1976 Formation of hydrogen peroxide by isolated cell walls from horseradish (Armoracia lapathifolia Gilib). Planta 193, 283–289.
Federico R and Angelini R 1986 Occurrence of diamine oxidase in the apoplast of pea epicotyls. Planta 167, 300–303.
Frahry G and Schopfer P 1998 Hydrogen peroxidase production by roots and its stimulation by exogenous NADH. Physiol. Plant. 103, 395–404.
Fry S C 1986 Cross-linking of matrix polymers in the growing cells of angiosperms. Annu. Rev. Plant Physiol. 37, 165–186. Gardiner M G and Cleland R 1974 Peroxidase changes during the
cessation of elongation in Pisum sativnm stems. Phytochemistry 13, 1095–1098.
Goldberg R, Liberman M, Mathieu C, Pierron M and Catesson A M 1987 Development of epidermal cell wall peroxidases along the mung been hypocotyl: possible involvement in the cell wall stiffening process. J. Exp. Bot. 38, 1378–1390.
Gross G G 1977 Cell wall-bound malate dehydrogenase from horseradish. Phytochemistry 16, 319–321.
Haynes R I and Goh K M 1978 Ammonium and nitrate nutrition of plants. Biol. Rev. 53, 465–510.
Hohl M, Greiner H and Schopfer P 1995 The cryptic growth response of maize coleoptile and its relationship to H2O2
-dependent cell wall stiffening. Physiol. Plant. 94, 491–498.
Ishida A, Ookubo K and Ono K 1987 Formation of hydrogen perox-ide by NAD(P)H oxidation with isolated cell wall-associated per-oxidase from cultured liverwort cells, Marchantia polymorpha L. Plant Cell Physiol. 28, 723–726.
Jana S and Choudhuri M A 1981 Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat. Bot. 12, 345–354.
Lee T-M and Lin Y-H 1995 Changes in soluble and cell wall-bound peroxidase activities with growth in anoxia-treated rice (Oryza
sativa L.) coleoptiles and roots. Plant Sci. 106, 1–7.
Lin C C and Kao C H 1995 Levels of endogenous polyamines and NaCl-inhibited growth of rice seedlings. Plant Growth Regul. 17, 15–20.
Lin C C and Kao C H 1996a Disturbed ammonium assimilation is associated with inhibition of roots in rice seedlings caused by NaCl. Plant Growth Regul. 18, 233–238.
Lin C C and Kao C H 1996b Proline accumulation is associated with inhibiton of rice seedling root growth caused by NaCl. Plant Sci. 114, 121–128.
Lin C C and Kao C H 1999 NaCl induced changes in ionically bound peroxidase activity in roots of rice seedlings. Plant Soil 216, 147– 153.
MacAdem J W, Sharp R E and Nelson C J 1992 Peroxidase activity in the leaf elongation zone of tall fescue. II. Spatial distribution of apoplastic peroxidase activity in genotypes differing in length of elongation zone. Plant Physiol. 99, 879–885.
Mader M, Ongemach J and Schloss P 1980 The role of peroxidase isoenzyme groups of Nicotiana tabaccum in hydrogen peroxide formation. Planta 147, 467–471.
Mader M, Nessel A and Schloss P 1986 Cell compartmentation and specific roles of isoenzymes. In Molecular and Physiological Aspects of Plant Peroxidases. Ed. H Greppin, C Penel and T Gaspar). pp 247–260. Univ. of Geneva Press, Geneva.
Munns R 1993 Physiological responses limiting plant growth in sa-line soils: Some dogma and hypothesis. Plant Cell Environ. 16, 15–24.
Naik B I, Goswami R G and Srivastawa S K 1981 A rapid and sensitive colorimetric assay of amine oxidase. Anal. Biochem. 111, 146–148.
Neumann P M, Azaizeh H and Leon D 1994 Hardening of root cell walls: Growth inhibitory responses to salinity stress. Plant Cell Environ. 17, 303–309.
Rengel Z 1992 The role of calcium in salt toxicity. Plant Cell Environ. 15, 625–632.
Sanchez O J, Pan A, Nicolas G and Labrador E 1989 Relation of cell wall peroxidase activity with growth in epicotyls of Cicer
arietinum. Effect of calmodulin inhibitors. Physiol. Plant. 75,
275–279.
Schopfer P 1994 Histochemical demonstrations and localization of H2O2in organs of higher plants by tissue printing on
nitrocellu-lose paper. Plant Physiol. 104, 1269–1275.
Schopher P 1996 Hydrogen peroxide-mediated cell-wall stiffening
in vitro in maize coleoptiles. Planta 199, 43–49.
Siegel S M 1953 On the biosynthesis of lignins. Physiol. Plant. 6, 134–139.
Smith T A 1985 Di- and polyamine oxidases of higher plants. Biochem. Soc. Trans. 13, 319–322.
Suzuki Y and Hagiwara M 1993 Purification and characterization of diamine oxidase from Zea mays shoot. Phytochemistry 33, 995–998.