行政院國家科學委員會專題研究計畫 期中進度報告
氧化性低密度脂蛋白之結構及其臨床應用研究(2/3)
計畫類別: 個別型計畫 計畫編號: NSC93-2314-B-002-031- 執行期間: 93 年 02 月 01 日至 94 年 01 月 31 日 執行單位: 國立臺灣大學醫學院內科 計畫主持人: 李源德 共同主持人: 周綠蘋,王水深,李啟明,張博淵,許秀卿 計畫參與人員: 陳靜宜 報告類型: 精簡報告 報告附件: 國際合作計畫研究心得報告 處理方式: 本計畫可公開查詢中 華 民 國 93 年 12 月 1 日
行政院國家科學委員會專題研究計畫期中進度報告
計畫名稱:氧化性低密度脂蛋白之結構及其臨床應用研究(2/3) 計畫類別:個別型計畫 計畫編號:NSC 93-2314-B-002-031 執行期間:93 年 2 月 1 日至 94 年 1 月 31 日 執行單位:國立台灣大學醫學院內科 計畫主持人:李源德教授 共同主持人:許秀卿、張博淵、周綠蘋、李啟明、王水深 計畫參與人:陳靜宜 摘要 本計畫延續第一年成果,持續自粥狀動脈硬化斑塊中萃取 oxLDL,或取自循 環血液中的 LDL 經由 FPLC 分離 LDL 子類。這些來自不同狀態不同氧化程度的 LDL,在經 trypsin 消化之後,以 MALDI-TOF 質譜儀來確定受氧化修飾的區域。然 因國人之粥狀硬化斑塊取得有困難度,特別是量的限制,通常要匯集許多病人的檢 體才得以進行分析。目前除了持續收集病人檢體,並尋求靈敏度較高功能較吻合需 求的質譜儀。本機構已陸續購入 SELDI, MALDI-TOF, MALDI-QTOF, LC-QTOF 四 種不同功能的質譜儀,MALDI-TOF 鑑定出的蛋白質,可將它的抗體 coating 於 chip, 再用 SLEDI 進行大量篩選,以作為臨床的應用;MALDI-QTOF 則可進行 peptide 定 序;而 LC-QTOF 則可以在奈米的等級下進行極微量的蛋白質後修飾位置之研究。 藉由這些先進設備的陸續設置完成,即可達成確認氧化修飾區域的既定目標。此外,本年度亦著手研究 oxLDL 對細胞功能性的調控影響,乃是藉由 proteomic 方法研究體外銅氧化 LDL 與血液分離出的 LDL 對人類臍帶靜脈內皮細胞 (human umbilical vein endothelial cells, HUVEC) 蛋白質之調控,並比較兩種處理方式對
定,並將探討其與粥狀硬化形成之關聯性,作為未來研究的新方向。
步驟:HUVEC 與 serum-free medium, 正常 LDL,銅氧化 LDL (氧化 2 或 24 小 時) 及血液分離出的 LDL (L1, L3, and L5) 培養 24 小時後,監測 MCP-1 產量以作為 細胞發炎的標準。同時將細胞蛋白質經二維電泳分析得 protein profiles,再以 MALDI-TOF 質譜儀鑑定蛋白質。 結果:不同濃度的 24 小時氧化 LDL 作用對 HUVEC 細胞數與 MCP-1 濃度並 沒有明顯的影響。HUVEC 與不同氧化程度的 LDL 培養 24 小時,細胞存活數與 MCP-1 產量並無變化。但經血液分離出的 LDL 與 HUVEC 作用 24 小時則可發現 L5 的細胞數量較 L1 與 L3 少,而 MCP-1 則較高,顯示 L5 可以引起較強的免疫發
炎反應及具有較強的 atherogenic 能力。初步結果顯示 protein profiles 不盡相似,可 能是因為銅氧化 LDL 及血液分離出的 LDL 對 HUVEC 調控的方式不同,不過仍有 待進一步的量化統計。經由質譜儀鑑定出有調控的蛋白質大多與氧化還原相關;以 peroxiredoxins 與 heat shock proteins 為大宗。
經由 proteomic 方法鑑定出的蛋白質,為探討粥狀動脈硬化的形成,提供了新 的研究方向。我們將針對特定蛋白質加以調控,以觀察它在粥狀硬化斑塊中扮演的 角色。
Abstract
The objective of this study was to study the protein profiles of HUVEC regulated
by oxLDL and LDL circulating subfraction by proteomic approaches. In addition, the
candidate proteins which were regulated by oxidized LDL and LDL subfractions were
identified. Human umbilical vein endothelial cells (HUVECs) were incubated with
serum-free medium, native LDL (100 ug/mL), oxLDL (2hr- and 24 hr-oxidized; 100
ug/mL), LDL circulating subfraction (L1, L3 and L5; 50 ug/mL) for 24 hrs, respectively.
MCP-1 was measured as an indicator of inflammation. Cytosolic proteins were applied
differences of cell viability and MCP-1 production were observed on dose responses of
24hr- oxLDL treatment. Various grades of copper oxidation of LDL had the same
effects on MCP-1production and cell viability. Circulating subfraction L5 had a highest
inflammatory reaction and caused lower cell viability, suggested that L5 had a higher
atherogenic capability than L1 and L3 did. The protein profiles of oxLDL and LDL
subfraction were different in the preliminary test. However, more replications and
statistical analysis are required to make the conclusion. For those proteins had been
identified at present, most of them were redox-related proteins, peroxiredoxins and heat
shock proteins were the majority.
Introduction
Low-density lipoprotein (LDL) oxidation plays a crucial role in atherogenesis.
Studies shown that oxidized LDL induced the proinflammation (interleukine-8 (IL8) and
monocyte chemotactic protein 1 (MCP-1); Sonoki et al., 2002); stimulated the expression
of adhesion molecules (vascular cell adhesion molecule-1, VCAM-1) (Takei et al., 2001);
inhibited the proliferation and nitric oxide synthesis in endothelial cells (Chen et al.,
2000a; Nuszkowski et al. 2001).; induced apoptosis of endothelial cells (Santa and Walsh,
1998). Also, oxidized LDL bound with high affinity to scavenger receptors on the
macrophage plasma membrane, induced macrophage proliferation (Biwa et al. 2000) and
foam cell formation (Itabe 2003).
A circulating subfraction of electronegative LDL (LDL (-)) was another type of
modified LDL. Studies shown that patients with diabetes mellitus and/or
hypercholesterolemia had increased serum levels of LDL(-) than normal subjects (Vidie
et al., 1998; Yano et al., 2004). LDL (-) contributed to atherogenesis via several
mechanisms, including proinflammatory, proapoptotic and anti-angiogenesis properties.
alpha (TNF-alpha)-induced inflammatory responses with corresponding increases in
VCAM-1 expression. In addition, LDL (-) involved in early phases of leukocyte
recruitment by inducing the production of chamomiles (IL8 and MCP-1)
(Sanchez-Quesada et al., 2004). LDL (-) impaired angiogenesis and increased apoptosis
by decreasing DNA synthesis and intracellular fibroblast growth factor 2 production.
Many researches have studied the role of oxLDL on atherogenesis by using
catalyst-oxidized LDL in vitro. However, the representation of oxLDL as circulating
LDL in physical environment is still doubtable. Since very few studies have compared
the mechanism of oxLDL and LDL subfraction on endothelial cells. The objective of
this study was to elucidate the differences between oxLDL and LDL subfraction by study
the protein profiles of HUVEC. In addition, the proteins which were regulated by
oxidized LDL and LDL circulating subfractions were identified by proteomic approach.
Materials and Methods Cell culture
Umbilical cord was obtained from pregnant females with the caesarian section in
National Taiwan University Hospital. HUVEC was isolated from umbilical cord using
0.1 % collagenase. Cells were grown in M199 medium supplemented with 10 % fetal
calf serum, 50 ug/mL endothelial cell growth supplement, 5 IU/mL heparin (Leo,
Ballerup, Denmark), cocktail of antibiotics (100 IU/mL penicillin, 0.1 mg/mL
streptomycin, 0.25 ug/mL amphotericin B) in a humidified atmosphere containing 5%
CO2. The cells were used between 3-5 passages.
Isolation and oxidation of LDL
LDL was isolated from human plasma using density-gradient ultracentrifugation.
In brief, blood sample was collected into tubes containing EDTA from healthy volunteers
1.063 g/mL by adding NaBr. The solution was centrifuged at 58,000 rpm for 11.5 hrs,
and the supernant was LDL. LDL was incubated in 10 umol CuSO4/ L PBS at 37℃ for
various periods. After the incubation, reaction was terminated by EDTA (0.5 mg/mL)
and stored in 4℃. Precautions were taken to prevent endotoxin contamination.
LDL subfraction
LDL was chromatograhed with an ion-exchange FPLC system (Pharmacia Biotech
Co) through a UnoQ12 column (BioRad) that had been equilibrated with buffer A (0.02
mol/L Tris-Cl, pH8.0, containing 1 mmol/L EDTA). Subfractions were eluted by use of
a multistep gradient of buffer B (1 mol/L NaCl in buffer A). Samples equilibrated with
buffer A were eluted with a linear gradient program at a flow rate of 2 mL/min.
Effluent was monitored at 280 nm and protected from ex vivo oxidation with 5 mmol/L
EDTA. Protein concentrations were detected by the Lowry method. Thiobarbituric
acid- reactive substances (TBARS), agarose electrophoresis and formation of conjugated
diene at absorbance 234 nm were assayed as a measure of oxidative lipid modification
was measured.
Protocol
Cell cultures grown to 90% confluence were starving for 24 hrs. After starvation,
cells were incubated with serum-free medium, native LDL (100 ug/mL), oxLDL (2hr-
and 24hr- oxidized; 100 ug/mL), LDL subfraction (L1, L3 and L5; 50 ug/mL) for 24 hrs,
respectively. MCP-1 was measured as an indicator of inflammation.
ELISA
To measure the secretion of MCP-1, ELISA was performed in medium by use of an
immunoassay kit, and MCP-1 was estimated spectrophotometrically at 450 nm.
Two-dimension electrophoresis
Cytosolic protein was extracted by lysis buffer(8 M urea, 4% CHAPS, 40 mM Tris
assayed using 2-D quant kit (Amersham Bioscience, Piscataway, NJ). Protein (1 mg)
was added to rehydration solution (7M urea, 2 M thiourea, 4% CHAPS, 10M DTT, 0.5%
Triton X-100, 0.4% carrier ampholytes and a trace of bromophenol blue). Strips were
rehydrated for 6 hrs and then IEF was performed for a total 24500 Vhr. 10%
SDS-PAGE was applied to the second dimension electrophoresis. After electrophoresis,
gels were silver stained and analyzed using ImageMaster 2D Elite 3.10 software
(Amersham Bioscience, Piscataway, NJ).
In-gel digestion and mass spectrometer
Protein spots were destained with 50% acetonitril and air dried. The dried gel was
then rehydrated in trypsin solution and incubated for 20 hrs at 37℃. After the peptides
were eluted with TFA of different concentrations, the peptide mixtures were analyzed by
using Voyager-DE time-of-flight (MADLI-TOF) mass spectrometer (Perseptive
Biosystems, Framingham, MA) with delayed extraction and a reflector. MS/MS spectra
were searched automatically against the protein database by using MOSCOT software
(http://www.matrixscience.com/). Multiple peptides from each protein generally were
detected, adding confidence to the protein identifications.
Results and discussions
Figure 1 is immunostained HUVEC by von Willebrand Factor (VWF). The cells
identified by VWF confirmed them as HUVEC in this study. Schwachula et al. (1994)
characterized the immunophenotype of HUVEC and fibroblast, and found CD31 and
VWF were HUVEC- specific proteins. Therefore, VWF was used in this study as an
identification marker for HUVEC.
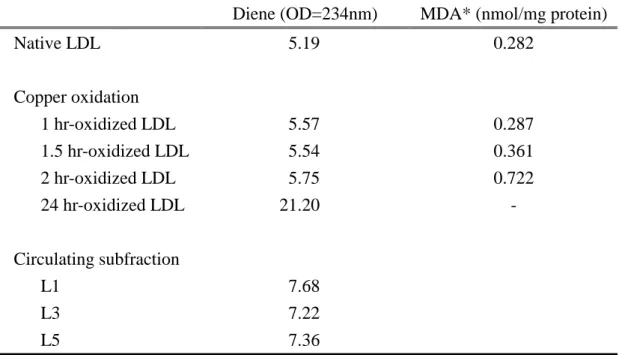
Table 1 was results of formation of conjugated dienes and TBARS. There were
slightly increase in diene and TBARS between 0 and 2 hrs of oxidation; and thereafter,
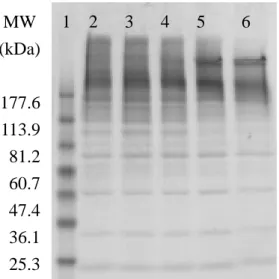
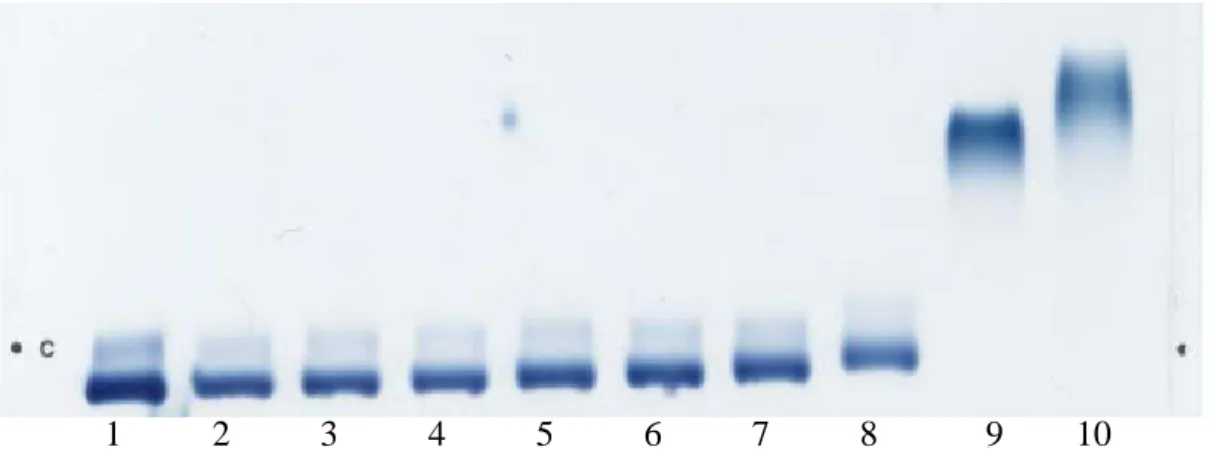
SDS-PAGE gel and agarose electrophoresis (Figure 2 and 3). As the oxidative period
increased, LDL degraded more in SDS-PAGE gel. In addition, LDL was more
electronegative when severity of oxidation increased. Therefore, 2 hr was the critical
point for LDL oxidation by copper. Diene for circulating subfractions showed no
differences on lipid peroxidation among L1, L3 and L5 (Table 1).
Dose response of oxLDL in HUVEC culture was shown on Table 2. There were
no differences observed among the treatments, suggested these concentrations did not
cause serious damages.
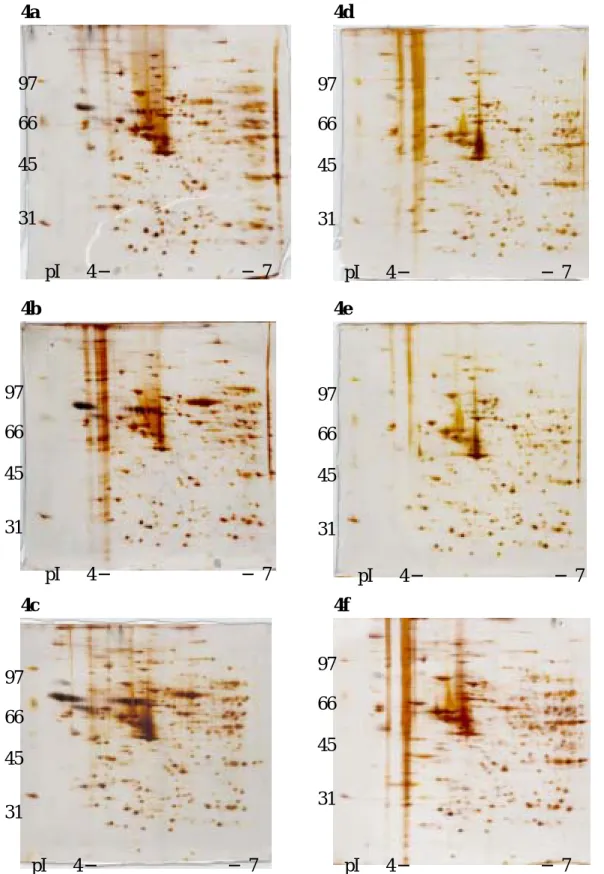
Protein profiles of HUVEC treated with oxLDL and LDL subfraction were shown
on Figure 4. Protein profiles were different between oxidized LDL in vitro and LDL
subfraction. HUVEC treated with LDL subfraction induced more protein modifications.
However, it required more replications and statistical analysis on protein expression to
make a conclusion.
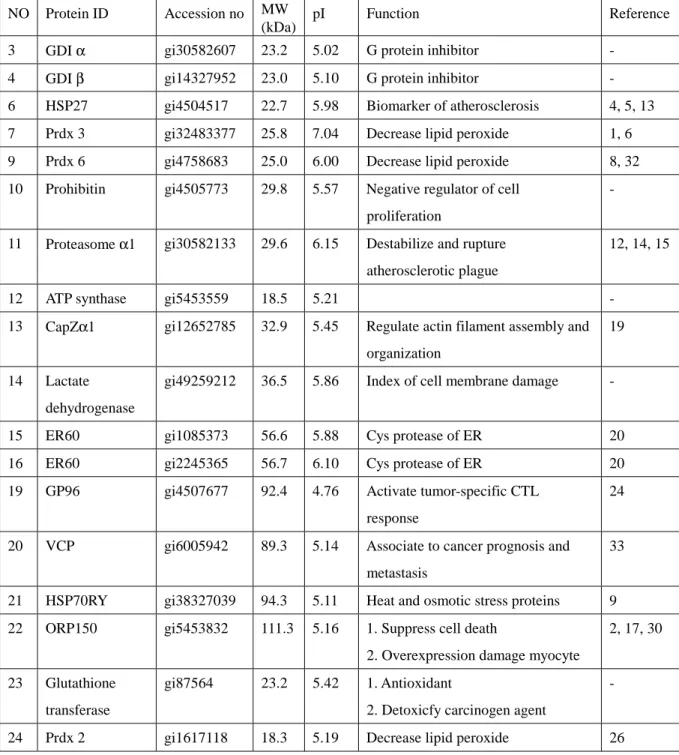
Figure 6 was the protein profiles for HUVEC identified and Table 5 was the list of
identified proteins by mass spectrometer at present. Most of them were redox relative
proteins (peroxiredoxin 2 (Prdx2), peroxiredoxin 3 (Prdx3), peroxiredoxin 6 (Prdx6),
glutathione transferase) and family of heat shock proteins (GP96, HSP70RY, HSP27,
ORP150).
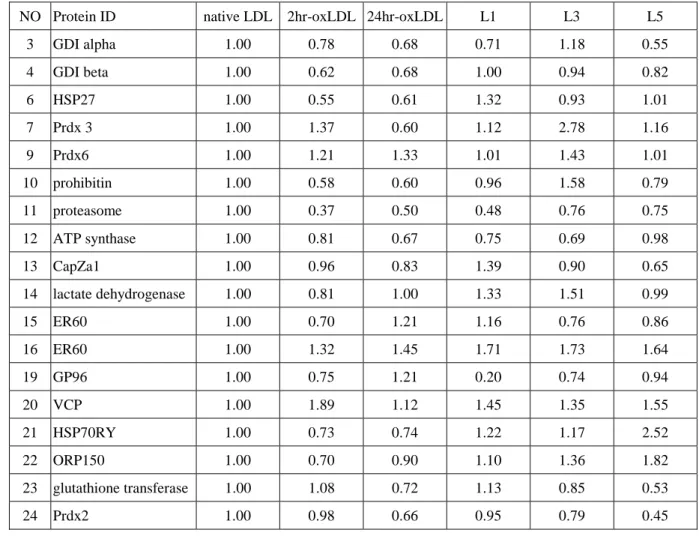
Protein expressions were quantified as the ratio of native LDL treatment (Table 6).
Comparing with 24hr-oxLDL, L5 had higher expressions of HSP70RY, ORP150, and
lower expression of glutathione transferase. Treatment of 24hr-oxLDL detected a
higher expression of Prdx6, and lower expressions of Prdx3 and HSP27. However, L5
did not show any differences. Since this was the result of preliminary test, more
replications are required.
Peroxiredoxins are thiol-specific antioxidant (TSA) family of proteins, which
metal-catalyzed oxidation system. Prdxs had involved in atherogenesis by several ways:
blocking inflammatation in endothelial cells (Shau et al., 1998); reducing plasma lipid
hydroperoxide levels and decreasing aortic root lesions (Wang et al., 2004); behaving like
glutathione peroxidase and helping recruit macrophages (Chen et al., 2000b); causing
apoptosis (Chen et al., 2004).
The relation between HSPs and atherosclerosis has been studied widely. HSP is a
potential biomarker for atherosclerosis (Martin-Ventura et al., 2004). Over-expression
of HSPs protected the intact heart against myocardial injury by reducing infarction size
(Efthymiou et al., 2004; Griffin et al., 2004), decreased apoptosis of cardiac cells against
thermal or hypoxic stress (Brar et al., 1999, Suzuki et al., 2000).
Both of GP96 and Oxygen regulated protein 150 (ORP150) are glucose- related
proteins. GP96 combined non-specific signals for the innate immune system and
specific signals for the adaptive immune system, thus played a crucial role in
proinflammation of atherogenesis (Schild and Rammensee, 2000). Suppression of
ORP150 expression attenuated survival of mononuclear phagocytes (Tsukamoto et al.,
1996), and caused a lower survival rate of mice (Bando et al., 2004).
CapZα1, a key component for F-actin capping, was involved in smooth muscle cell function (McGregor et al., 2004). ER60, cysteine protease in ER, is one member of
major histocompatibility complex (MHC) class I. ER60 induce the defense system
against intracellular pathogens by assembling in the ER with chaperones, and binding to
the transporter associated with antigen processing (Morrice and Powis, 1998).
The ubiquitin-proteasome system (UPS) had different functions on early/mid/late
stages of atherosclerosis. A high activity of UPS in coronary artery was detected in the
early stage of atherosclerosis (Hermann et al., 2003). UPS enhanced the proliferation of
smooth muscle cells and the formation of foam cell at the progression of atherosclerosis
activating the formation of T cells (Hannson 2001).
Reference
1. Araki M, Nanri H, Ejima K, Murasato Y, Fujiwara T, Nakashima Y, Ikeda M. 1999. Antioxidant function of the mitochondrial protein SP-22 in the cardiovascular system. J Biol Chem.;274:2271-2278.
2. Bando Y, Tsukamoto Y, Katayama T, Ozawa K, Kitao Y, Hori O, Stern DM,
Yamauchi A, Ogawa S. 2004. ORP150/HSP12A protects renal tubular epithelium from ischemia-induced cell death. FASEB J. 18:1401-1403.
3. Biwa T, Sakai, M, Matsumura T, Kobori S, Kaneko K, Miyazaki A, Hakamata H, Horiuchi S, and Shichiri M. 2000. Sites of action of protein kinase C and phosphoatidylinositol 3-kinase are distinct in oxidized low density
lipoprotein-induced macrophage proliferation. J. Biol. Chem. 275, 5810-5816. 4. Brar BK, Stephanou A, Wagstaff MJ, Coffin RS, Marber MS, Engelmann G,
Latchman DS. 1999. Heat shock proteins delivered with a virus vector can protect cardiac cells against apoptosis as well as against thermal or hypoxic stress. J Mol Cell Cardiol. 31:135-146.
5. Brophy CM, Molinaro JR, Dickinson M.. 2000. The macromolecular associations of heat shock protein-27 in vascular smooth muscle. Surgery. 128:320-326.
6. Chang TS, Cho CS, Park S, Yu S, Kang SW, Rhee SG. 2004. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J Biol Chem. 279:41975-41984.
7. Chen CH, Jiang W, Via DP, Luo S, Li TR, Lee YT, Henry PD. 2000a. Oxidized low-density lipoproteins inhibit endothelial cell proliferation by suppressing basic fibroblast growth factor expression. Circulation. 101:171-177.
8. Chen JW, Dodia C, Feinstein SI, Jain MK, FisherAB. 2000b. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities, J. Biol. Chem. 275: 28421–28427.
9. Dyer KD, Lavigne MC, Rosenberg HF. 1994. Hsp70RY: further characterization of a novel member of the hsp70 protein family. Biochem Biophys Res Commun. 203:577-581.
10. Efthymiou CA, Mocanu MM, de Belleroche J, Wells DJ, Latchmann DS, Yellon DM. 2004. Heat shock protein 27 protects the heart against myocardial infarction. Basic Res Cardiol. 99:392-394.
11. Griffin TM, Valdez TV, Mestril R. 2004. Radicicol activates heat shock protein expression and cardioprotection in neonatal rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 287:H1081-1088.
12. Hansson GK. 2001. Immune mechanisms in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 21:1876–1890.
13. Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, Weber LA,
Gerthoffer WT. 1999. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol Chem. 274:24211-24219.
14. Herrmann J, Ciechanover A, Lerman LO, Lerman A. 2004. The ubiquitin- proteasome system in cardiovascular diseases-a hypothesis extended. Cardiovasc Res. 61:11-21.
15. Hermann J, Gulati R, Napoli C, Woodrum JE, Lerman LO, Rodriguez-Porcel M, Sica V, Simari RD, Ciechanover A, Lerman A. 2003. Oxidative stress-related increase in ubiquitination in early coronary atherogenesis. FASEB J. 17:1730-1732. 16. Itabe H. 2003. Oxidized low-density lipoproteins: what is understood and what
remains to be clarified. Biol. Pharm. Bull. 26: 1-9.
17. Kobayashi T, Yura T, Yanagi H. 2002. The increment of anti-ORP150
autoantibody in initial stages of atheroma in high-fat diet fed mice. J Vet Med Sci. 64:177-180.
18. Martin-Ventura JL, Duran MC, Blanco-Colio LM, Meilhac O, Leclercq A, Michel JB, Jensen ON, Hernandez-Merida S, Tunon J, Vivanco F, Egido J. 2004.
Identification by a differential proteomic approach of heat shock protein 27 as a potential marker of atherosclerosis. Circulation. 110:2216-2219.
19. McGregor E, Kempster L, Wait R, Gosling M, Dunn MJ, Powell JT. 2004. F-actin capping (CapZ) and other contractile saphenous vein smooth muscle proteins are altered by hemodynamic stress: a proteonomic approach. Mol Cell Proteomics. 3: 115-124.
20. Morrice NA, Powis SJ. 1998. A role for the thiol-dependent reductase ERp57 in the assembly of MHC class I molecules. Curr Biol. 8:713-716.
21. Nuszkowski A, Grabner R, Marsche G, Unbehaun A, Malle E, Heller R. 2001. Hypochlorite-modified low-density lipoprotein inhibits nitric oxide synthesis in endothelial cells via an intracellular dislocalization of endothelial nitric oxide synthase. J. Biol. Chem. 276: 14212-14221.
22. Sanchez-Quesada JL, Benitez S, Ordonez-Llanos J. 2004. Electronegative low-density lipoprotein. Curr Opin Lipidol. 15:329-335.
23. Sata M, Walsh K. 1998. Endothelial cell apoptosis induced by oxidized LDL is associated with the down-regulation of the cellular caspase inhibitor FLIP. J Biol Chem. 1998 273:33103-33106.
24. Schild H, Rammensee H. 2000. GP96-the immune system’s swiss army knife. Nature Immunology 1:100-101.
25. Schwachula A, Riemann D, Kehlen A, Langner J. 1994. Characterization of the immunophenotype and functional properties of fibroblast-like synoviocytes in comparison to skin fibroblasts and umbilical vein endothelial cells. J
26. Shau H, Huang AC, Faris M, Nazarian R, de Vellis J, Chen W. 1998. Thioredoxin peroxidase (natural killer enhancing factor) regulation of activator protein-1
function in endothelial cells. Biochem Biophys Res Commun. 249:683-686. 27. Sonoki K, Yoshinari M, Iwase M, Iino K, Ichikawa K, Ohdo S, Higuchi S, Iida M.
2002. Glycoxidized low-density lipoprotein enhances monocyte chemoattractant protein-1 mRNA expression in human umbilical vein endothelial cells: relation to lysophosphatidylcholine contents and inhibition by nitric oxide donor. Metabolism. 51:1135-1142.
28. Suzuki K, Sawa Y, Kagisaki K, Taketani S, Ichikawa H, Kaneda Y, Matsuda H. 2000. Reduction in myocardial apoptosis associated with overexpression of heat shock protein 70. Basic Res Cardiol. 95:397-403.
29. Takei A, Huang Y, Lopes-Virella MF. 2001. Expression of adhesion molecules by human endothelial cells exposed to oxidized low density lipoprotein. Influences of degree of oxidation and location of oxidized LDL. Atherosclerosis. 2001 154:79-86. 30. Tsukamoto Y, Kuwabara K, Hirota S, Ikeda J, Stern D, Yanagi H, Matsumoto M,
Ogawa S, Kitamura Y. 1996. 150-kD oxygen-regulated protein is expressed in human atherosclerotic plaques and allows mononuclear phagocytes to withstand cellular stress on exposure to hypoxia and modified low density lipoprotein. J Clin Invest. 98:1930-1941.
31. Vedie B, Jeunemaitre X, Megnien JL, Myara I, Trebeden H, Simon A, Moatti N. 1998. Charge heterogeneity of LDL in asymptomatic hypercholesterolemic men is related to lipid parameters and variations in the ApoB and CIII genes. Arterioscler Thromb Vasc Biol. 18:1780-1789.
32. Wang X, Phelan SA, Petros C, Taylor EF, Ledinski G, Jurgens G, Forsman-Semb K, Paigen B. 2004. Peroxiredoxin 6 deficiency and atherosclerosis susceptibility in mice: significance of genetic background for assessing atherosclerosis.
Atherosclerosis. 77:61-70.
33. Yamamoto S, Tomita Y, Nakamori S, Hoshida Y, Iizuka N, Okami J, Nagano H, Dono K, Umeshita K, Sakon M, Ishikawa O, Ohigashi H, Aozasa K, Monden M. 2004. Valosin-Containing Protein (p97) and Ki-67 Expression Is a Useful Marker in Detecting Malignant Behavior of Pancreatic Endocrine Neoplasms. Oncology. 66:468-75.
34. Yano M, Inoue M, Maehata E, Shiba T, Yamakado M, Hirabayashi Y, Taniyama M, Suzuki S. 2004. Increased electronegative charge of serum low-density lipoprotein in patients with diabetes mellitus. Clin Chim Acta. 2004 340:93-98.
35. Ziouzenkova O, Asatryan L, Sahady D, Orasanu G, Perrey S, Cutak B, Hassell T, Akiyama TE, Berger JP, Sevanian A, Plutzky J. 2003. Dual roles for lipolysis and oxidation in peroxisome proliferation-activator receptor responses to electronegative low density lipoprotein. J Biol Chem. 278:39874-39881.
Figures and Charts
Figure 1. Identification of HUVEC by VWF (1:1000).
Figure 2. Protein expression of HUVEC treated with different oxidative grades of LDL
for 24 hrs. Lane 1 was marker, lane 2 was native LDL, lane 3 was 1hr-oxidized LDL, lane 4 was 1.5hr-oxidized LDL, lane 5 was 2hr-oxidized LDL, and lane 6 was
24hr-oxidized LDL. 1 2 3 4 5 6 MW (kDa) 177.6 113.9 81.2 60.7 47.4 36.1 25.3
Figure 3. Electronegativity of HUVEC treated with different oxidative grades of LDL.
Lane 1 to 10 were native LDL, 20min-oxidzed LDL, 40 min-oxidized LDL, 60
min-oxidized LDL, 80 min-oxidized LDL, 100 min-oxidized LDL, 2 hr-oxidized LDL, 3 hr-oxidized LDL, 8 hr-oxidized LDL and 24 hr-oxidized LDL, repectively.
4a 4d
4b 4e
4c 4f
Figure 4. The protein profiles of HUVEC treated with different oxLDL. A: native
LDL; b: 2 hr-oxidized LDL; c: 24 hr-oxidized LDL; d: circulating subfraction LDL- L1; e: circulating subfraction L3; f: circulating subfraction L5. Cytosolic proteins (130ng) was loaded on pI 4-7 linear IPG strips (11 cm), with 10% vertical SDS-PAGE as the second dimension. The gel was visualized by silver staining.
pI 4─ ─ 7 97 66 45 31 pI 4─ ─ 7 97 66 45 31 pI 4─ ─ 7 97 66 45 31 pI 4─ ─ 7 97 66 45 31 pI 4─ ─ 7 97 66 45 31 pI 4─ ─ 7 97 66 45 31
Figure 6 Protein profiles of HUVEC. Cytosolic proteins (1.2mg) was loaded on pI
4-7 linear IPG strips (11 cm), with 10% vertical SDS-PAGE as the second dimension. The gel was visualized by comassie blue staining.
pI=4 5 6 7 8 1 2 4 3 5 6 7 9 11 23 24 10 12 13 14 16 15 17 18 19 20 22 21 MW (kDa) 97 66 45 31
Table 1. Formations of conjugated dienes and TBARS of different oxidative grades of
LDL by copper and LDL circulating subfraction.
Diene (OD=234nm) MDA* (nmol/mg protein)
Native LDL 5.19 0.282 Copper oxidation 1 hr-oxidized LDL 5.57 0.287 1.5 hr-oxidized LDL 5.54 0.361 2 hr-oxidized LDL 5.75 0.722 24 hr-oxidized LDL 21.20 - Circulating subfraction L1 7.68 L3 7.22 L5 7.36 *MDA: malondialdehye (MDA) was the product of TBARS test.
Table 2. Dose responses of HUVEC treated with 24 hr-oxidized LDL.
Cell no (x 105) MCP-1 (pg/105 cells)
10 ug/mL 7.5 58.4
50 ug/mL 15.0 49.4
100 ug/mL 7.9 99.0
Table 3. Cell viability and inflammatory reaction of HUVEC treated with oxidized
LDL (100ug/mL) (n=2). Cell no (x 106) MCP-1 (ng/106 cells) M199 only 3.8 2.04 Native LDL 2.8 1.92 2 hr-oxidized LDL 2.9 2.49 24 hr-oxidized LDL 3.0 2.37
Table 4. Cell viability and inflammatory reaction of HUVEC treated with LDL
circulating subfraction.
(50 ug/mL) Cell no (x 106) MCP-1 (ng/106 cells)
Native LDL 2.9 1.35
L1 1.8 2.21
L3 2.7 2.19
Table 5. Lists of protein identified by mass spectrometer.
NO Protein ID Accession no MW (kDa)
pI Function Reference 3 GDI α gi30582607 23.2 5.02 G protein inhibitor - 4 GDI β gi14327952 23.0 5.10 G protein inhibitor - 6 HSP27 gi4504517 22.7 5.98 Biomarker of atherosclerosis 4, 5, 13 7 Prdx 3 gi32483377 25.8 7.04 Decrease lipid peroxide 1, 6 9 Prdx 6 gi4758683 25.0 6.00 Decrease lipid peroxide 8, 32 10 Prohibitin gi4505773 29.8 5.57 Negative regulator of cell
proliferation
-
11 Proteasome α1 gi30582133 29.6 6.15 Destabilize and rupture atherosclerotic plague
12, 14, 15
12 ATP synthase gi5453559 18.5 5.21 - 13 CapZα1 gi12652785 32.9 5.45 Regulate actin filament assembly and
organization
19
14 Lactate dehydrogenase
gi49259212 36.5 5.86 Index of cell membrane damage -
15 ER60 gi1085373 56.6 5.88 Cys protease of ER 20 16 ER60 gi2245365 56.7 6.10 Cys protease of ER 20 19 GP96 gi4507677 92.4 4.76 Activate tumor-specific CTL
response
24
20 VCP gi6005942 89.3 5.14 Associate to cancer prognosis and metastasis
33
21 HSP70RY gi38327039 94.3 5.11 Heat and osmotic stress proteins 9 22 ORP150 gi5453832 111.3 5.16 1. Suppress cell death
2. Overexpression damage myocyte
2, 17, 30
23 Glutathione transferase
gi87564 23.2 5.42 1. Antioxidant 2. Detoxicfy carcinogen agent
-
Table 6 Quantity of protein expression. Data were shown as the ratio of native LDL.
NO Protein ID native LDL 2hr-oxLDL 24hr-oxLDL L1 L3 L5 3 GDI alpha 1.00 0.78 0.68 0.71 1.18 0.55 4 GDI beta 1.00 0.62 0.68 1.00 0.94 0.82 6 HSP27 1.00 0.55 0.61 1.32 0.93 1.01 7 Prdx 3 1.00 1.37 0.60 1.12 2.78 1.16 9 Prdx6 1.00 1.21 1.33 1.01 1.43 1.01 10 prohibitin 1.00 0.58 0.60 0.96 1.58 0.79 11 proteasome 1.00 0.37 0.50 0.48 0.76 0.75 12 ATP synthase 1.00 0.81 0.67 0.75 0.69 0.98 13 CapZa1 1.00 0.96 0.83 1.39 0.90 0.65 14 lactate dehydrogenase 1.00 0.81 1.00 1.33 1.51 0.99 15 ER60 1.00 0.70 1.21 1.16 0.76 0.86 16 ER60 1.00 1.32 1.45 1.71 1.73 1.64 19 GP96 1.00 0.75 1.21 0.20 0.74 0.94 20 VCP 1.00 1.89 1.12 1.45 1.35 1.55 21 HSP70RY 1.00 0.73 0.74 1.22 1.17 2.52 22 ORP150 1.00 0.70 0.90 1.10 1.36 1.82 23 glutathione transferase 1.00 1.08 0.72 1.13 0.85 0.53 24 Prdx2 1.00 0.98 0.66 0.95 0.79 0.45