0095-1137/11/$12.00 doi:10.1128/JCM.01774-10
Copyright © 2011, American Society for Microbiology. All Rights Reserved.
Factors Associated with Nasal Colonization of Methicillin-Resistant
Staphylococcus aureus among Healthy Children in Taiwan
䌤
Chih-Jung Chen,
1,2Kuang-Hung Hsu,
3Tzou-Yien Lin,
1,2Kao-Pin Hwang,
4Po-Yen Chen,
5and Yhu-Chering Huang
1,2*
Division of Pediatric Infectious Diseases, Department of Pediatrics, Chang Gung Memorial Hospital,1College of Medicine, Chang Gung University,2and Laboratory for Epidemiology and Department of Health Care Management,
Chang Gung University,3333 Taoyuan, Taiwan; Department of Pediatrics, China Medical University Hospital, Taichung, Taiwan4; and Department of Pediatrics, Taichung Veterans General Hospital, Taichung, Taiwan5
Received 1 September 2010/Returned for modification 17 October 2010/Accepted 2 November 2010
Methicillin-resistant Staphylococcus aureus (MRSA) has been identified as a major cause of community-associated (CA) S. aureus infections in the past decade. The main reservoir in the community for MRSA and the factors contributing to its worldwide spread remain poorly defined. Between July 2005 and June 2008, a total of 6,057 healthy children 2 to 60 months of age were screened for carriage of S. aureus and Streptococcus
pneumoniae in Taiwan. The prevalence and epidemiological factors influencing MRSA carriage were
deter-mined. MRSA strains were tested for antimicrobial susceptibility and underwent molecular characterization. The overall prevalences of MRSA and S. aureus carriage were 7.8% and 23.2%, respectively. A majority (88%) of MRSA isolates belonged to a common Asian-Pacific CA-MRSA lineage, multilocus sequence type 59, and were resistant to multiple non-beta-lactam antibiotics. The carriage rate of MRSA was higher among subjects 2 to 6 months old (P < 0.0001), residing in northern Taiwan (Pⴝ 0.0003), and enrolled later in the study (P < 0.0001). MRSA colonization was associated with the number of children in the family (adjusted odds ratio [aOR], 1.114; 95% confidence interval [CI], 1.002 to 1.240; Pⴝ 0.0463) and day care attendance (aOR, 1.530; 95% CI, 1.201 to 1.949; Pⴝ 0.0006). Breast feeding (P < 0.0001) and colonization with S. pneumoniae (P ⴝ 0.0170) were protective against MRSA colonization. We concluded that epidemic CA-MRSA strains increas-ingly colonized Taiwanese children between 2005 and 2008. The carriage rate varied significantly across different demographical features. Crowding was an independent environmental risk factor that might accel-erate CA-MRSA transmission in the community.
Two of the major changes in the epidemiology of methicil-lin-resistant Staphylococcus aureus (MRSA) in the past decade were the worldwide emergence of community-associated (CA) MRSA strains and an increasing number of CA-MRSA infec-tions in previously healthy hosts (24, 35). The CA-MRSA iso-lates were initially identified in pediatric populations and sub-sequently reported in certain adult populations, such as Native Americans, military recruits, prison inmates, drug users, men who have sex with men, and competitive sports participants (3, 12, 14, 15, 23, 31, 38). Although superficial skin and soft tissue infections remained the most common manifestation of CA-MRSA, severe diseases such as necrotizing pneumonitis, ne-crotizing fasciitis, osteomyelitis, pyomyositis, septic embolism, and venous thrombosis were not uncommon and previously caused death in healthy children (5, 13).
The causes of the increasing incidence of CA-MRSA infec-tion in previously healthy hosts are not completely understood, and the factors influencing CA-MRSA virulence remain an issue of ongoing debate (2, 9, 30). Colonization with S. aureus has been identified as an important risk factor for the devel-opment of S. aureus infections in both community and hospital
settings (17, 36). Evidence further suggests that, compared to methicillin-susceptible S. aureus (MSSA), colonization with MRSA imposes a significantly greater risk for development of subsequent infections (10). In the past few years, the preva-lence of MRSA colonization increased significantly among healthy hosts during CA-MRSA epidemics (8, 19). The car-riage of MRSA with CA genotypes was significantly higher among children than among adults (25). Furthermore, living with young children was associated with increased risk of MRSA colonization in adults (37). When combined, these observations strongly suggested young children were the major reservoir of MRSA in the community and were the main population responsible for the accelerated transmis-sion of CA-MRSA. Other host and environmental factors contributing to the worldwide spread of CA-MRSA remain poorly defined. Identification of these factors should expand our understanding of the epidemiology of CA-MRSA colo-nization and may help in developing measures for control-ling its spread.
In this study, we investigated the epidemiological factors associated with MRSA colonization among young children in the community setting. To our best knowledge, this is the largest-scale study of S. aureus colonization that targets young children and simultaneously explores a variety of influencing factors, including microbial interference from another important pediatric pathogen, Streptococcus pneu-moniae.
* Corresponding author. Mailing address: Division of Pediatric Infectious Diseases, Department of Pediatrics, Chang Gung Memo-rial Hospital, 5 Fu-Shin Street, Kweishan 333, Taoyuan, Taiwan. Phone: 886-3-3281200. Fax: 886-3-3288957. E-mail: ychuang@adm .cgmh.org.tw.
䌤Published ahead of print on 17 November 2010.
MATERIALS AND METHODS
Study design.Between July 2005 and June 2008, all 2- to 60-month-old healthy children who visited general health checkup clinics in three representative teach-ing hospitals in Taiwan were invited to participate in this study. The hospitals were located in the suburban area of northern Taiwan (Linko Medical Center of Chang Gung Memorial Hospital) and in two metropolitan areas of central (Taichung Veterans General Hospital) and southern (Kaohsiung branch, Chang Gung Memorial Hospital) Taiwan. These hospitals all provided primary to ter-tiary care to children of all ages, and the medical costs of the visits were covered by the compulsory national health insurance system in Taiwan. Therefore, access to the hospitals was not affected by socioeconomic status. Children were enrolled in the study if they met the inclusion criteria (discussed below), and informed consent was obtained from their parents or legal guardians. A swab (BBL CultureSwab Plus; Becton Dickinson and Company) of both anterior nares and another flexible swab (Venturi Transystem; Copan Innovation) from deep in the nasopharyngeal space were obtained from each participant. The swabs were used for isolation of both S. aureus and S. pneumoniae. For the standardization of specimen collection, the sampling procedure was performed by a well-trained nurse at each study site. The nasal and nasopharyngeal swabs were immediately placed in the transport medium and transported to the clinical microbiology laboratory in each institute for detection of S. aureus and S. pneumoniae by standard methods. Identification of S. pneumoniae was confirmed by optochin sensitivity and bile solubility. S. aureus was identified by coagulase testing, and oxacillin susceptibility was assessed by the disc diffusion method according to Clinical and Laboratory Standards Institute 2006 guidelines (7). This study was approved by the institute review boards from the three sampled hospitals.
Demographics and anthropometric variables of subjects. Children with chronic renal failure, dialysis, indwelling devices, thalassemia major, chronic cardiovascular diseases, chronic lung disease, nephrotic syndrome, liver cirrhosis, diabetes mellitus, congenital immunodeficiency, human immunodeficiency virus infection, asplenia, or malignancy or receiving immunosuppressant agents were excluded from the study. Prematurity without complication was not seen as an underlying condition, and those subjects were eligible for the study. A standard-ized questionnaire interview of the parents or primary caregivers was used to collect the information needed in this study. In addition to the demographic data (date of birth, gender, and history of breast feeding), we also collected environ-mental factors and health conditions of the subjects. These variables were hy-pothesized to be potential factors associated with MRSA colonization. The environmental factors included house size, number of children in the family, frequency of hand washing, passive smoking, sleeping alone or with parents, kindergarten or day care attendance, and the identity of the main caregiver during the day. The children’s health-related factors included vaccination history for seasonal influenza and S. pneumoniae, history of acute otitis media, history of recent upper respiratory tract infection, and recent (⬍2 weeks) antibiotic use.
The administered questionnaires were collected from the three study sites. The data were digitalized and cleaned in a computer core laboratory before we proceeded with statistical analysis.
Molecular characterization of MRSA isolates.All S. aureus isolates were sent to the Linko Medical Center of Chang Gung Memorial Hospital for further microbiological characterization. A random sample of MRSA isolates (1 out of 2 consecutive isolates from central and southern Taiwan and 3 out of 4 consec-utive isolates from northern Taiwan) were prospectively selected for molecular characterization. Pulsed-field gel electrophoresis (PFGE) with SmaI digestion was used in this study to fingerprint the MRSA isolates and was performed
according to procedures described by Huang et al. (22). The genotypes were designated in alphabetical order, as in the previous studies (17–19); any new genotype identified was designated consecutively. PFGE patterns with fewer than four band differences from an existing genotype were defined as subtypes of that genotype.
SCCmec typing of isolates was done using a multiplex PCR strategy described previously (19). The control strains for staphylococcal chromosomal cassette mec (SCCmec) types I, II, III, and IVa, kindly provided by Keiichi Hiramatsu, were as follows: type I, NCTC10442; type II, N315; type III, 85/2082; and type IVa,
JCSC4744. SCCmec typing for type VTwas determined by using a particular
primer described elsewhere (20), and strain TSGH-17, kindly provided by Chi-Chien Wang, was used as a control.
The presence of Panton-Valentine leukocidin (PVL) genes was determined by a PCR technique described by Lina et al. (27). Some isolates with representative PFGE patterns were selected and underwent multilocus sequence typing (MLST) (http://www.mlst.net) (11). The allelic profiles were assigned through a comparison of the sequence at each locus with the sequences of the known alleles in the S. aureus MLST database and were defined as sequence types accordingly. Statistics.The comparison of categorical variables between study groups was performed with a chi-square test, or with Fisher’s exact test where appropriate, while the differences between study groups in the numerical variables were tested by a two-sample t test. Multiple logistic regression analysis was applied to explore factors associated with MRSA and MSSA colonization. Statistical significance
was defined as a P value of⬍0.05 in the tests. The data were analyzed with SAS
software version 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Characteristics of study subjects. From July 2005 to June 2008, a total of 6,057 children aged from 2 to 60 months (mean
age, 25.0 months⫾ 17.2 months) were enrolled in the study.
The majority of the participants were male (54.5%), and 7.3% of the 6,057 children had a birth history of prematurity. The
age distribution (P⫽ 0.9799), gender (P ⫽ 0.5047), and
pre-maturity (P⫽ 0.0653) did not differ significantly for the
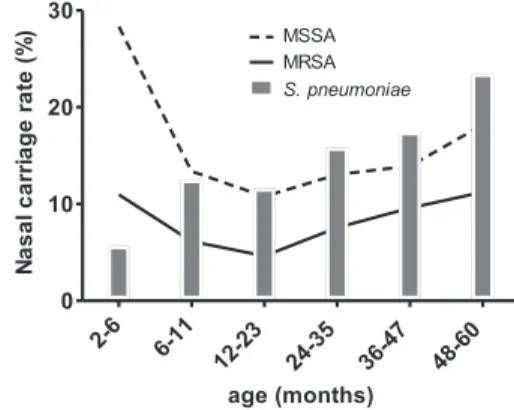
sub-jects across the three regions of Taiwan. Nasal carriage of MRSA and MSSA was detected in 473 (7.8%) and 931 (15.4%) subjects, respectively (Table 1). Infants 2 to 6 months old had the highest incidence of S. aureus carriage but the lowest inci-dence of S. pneumoniae carriage (Fig. 1). The rate of S. aureus carriage declined to a nadir in the second year of life but gradually increased between 2 and 5 years of age. The carriage of S. pneumoniae generally increased with age.
The colonization rates of S. aureus were different among the three areas, with the highest incidence in northern Taiwan (27.4%) and the lowest incidence in central Taiwan (20.3%) (Table 1). The geographical variation was observed in both
MRSA (P⫽ 0.0004) and MSSA (P ⫽ 0.0006) carriage. There
FIG. 1. Age-specific distribution of MRSA, MSSA, and S.
pneu-moniae carriage in children younger than 6 years old.
TABLE 1. Nasal carriage of MSSA, MRSA, and S. pneumoniae in Taiwanese children aged 2 to 60 months from July 2005 to
June 2008 in northern, central, and southern Taiwan
Area of Taiwan No. of subjects
No. (%) witha:
MSSA MRSA S. pneumoniae
Northern 2,017 361 (17.9) 192 (9.5) 261 (12.9) Southern 2,023 286 (14.1) 156 (7.7) 281 (13.9) Central 2,017 284 (14.1) 125 (6.2) 315 (15.6)
Total 6,057 931 (15.4) 473 (7.8) 857 (14.1)
aComparison of colonization rates among the three areas (northern, southern,
and central Taiwan) by chi-square test: MSSA, P⫽ 0.0006: MRSA, P ⫽ 0.0004;
were also geographical differences in S. pneumoniae coloniza-tion. The highest incidence of S. pneumoniae colonization oc-curred in central Taiwan, while northern Taiwan had the low-est incidence.
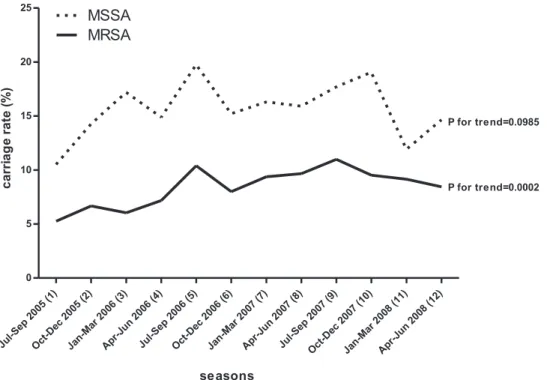
When these rates were graphically compared across the pe-riod of study (Fig. 2), an increasing trend of MRSA carriage
was identified (P⫽ 0.0002). The increasing temporal trend was
highly significant in central and southern Taiwan (P⫽ 0.0129
and P⬍ 0.0001, respectively), but not in northern Taiwan (P ⫽
0.6983). The incidence of MSSA carriage varied greatly, from 10.5% to 19.8%, at different seasons but did not develop a
significant trend during the same period for any area (P ⫽
0.1758 for northern Taiwan, P ⫽ 0.1870 for central Taiwan,
and P⫽ 0.8076 for southern Taiwan).
Association between S. aureus and S. pneumoniae coloniza-tion.S. pneumoniae colonization was identified in 857 (14.1%) subjects of this study. Cocolonization with S. pneumoniae and S. aureus was found in 122 (2%) subjects. A negative correla-tion was found between S. aureus and S. pneumoniae coloni-zation. Children with S. aureus colonization had a positivity rate of 8.7% for S. pneumoniae colonization, while a positivity rate of 15.8% for S. pneumoniae colonization was identified
among children without S. aureus colonization (P⬍ 0.0001).
The trend was retained when further stratified by MRSA and MSSA (Table 2). The colonization rates of S. pneumoniae also differed significantly among MRSA and MSSA carriers (12.3%
versus 6.9%; P⫽ 0.0008).
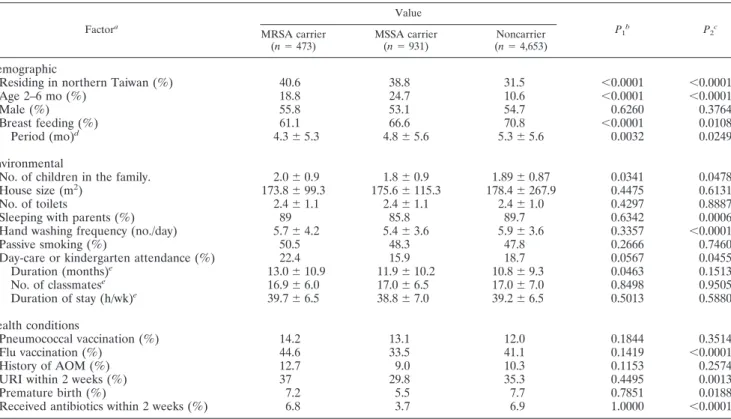
Epidemiological factors associated with MRSA and MSSA carriage.The variables correlated with MRSA and MSSA car-riage by means of univariate analysis are displayed in Table 3. MSSA colonization in children appeared to be influenced more readily than MRSA colonization by various health and environmental factors in the univariate analysis. For instance,
sleeping with parents, lower frequency of hand washing, influ-enza vaccination, premature birth, upper respiratory tract in-fections, and use of antibiotics within the previous 2 weeks were all associated with decreased incidence of MSSA coloni-zation but did not influence colonicoloni-zation by MRSA. Further, a greater number of children in the family and day care atten-dance both increased the risk of MRSA colonization but were associated with decreased incidence of MSSA colonization.
Multiple logistic regression analysis has demonstrated fac-tors including residing in northern Taiwan, subject enrollment at a later time in the study, age of 2 to 6 months, a greater number of children in the family, and day care attendance as independent factors for MRSA carriage. Subjects with a his-tory of breast feeding and colonization by S. pneumoniae had lower MRSA carriage rates (Table 4). In addition to the sig-nificant demographic factors found in the MRSA model, use of antibiotics within the previous 2 weeks and prematurity were two significant variables associated with decreased incidence of MSSA colonization.
FIG. 2. Temporal trend of MRSA and MSSA nasal colonization in Taiwanese children from July 2005 to June 2008.
TABLE 2. Association of S. aureus and S. pneumoniae colonization in Taiwanese children
S. pneumoniae
colonization
No. (%) with S. aureus:
MRSAa MSSAa Noncarrier Total
Yes 58 (12.3) 64 (6.9) 735 (15.8) 857 (14.1) No 415 (87.7) 867 (93.1) 3,918 (84.2) 5,200 (85.9)
Total 473 931 4,653 6,057
a
Comparison of S. pneumoniae colonization rates between S. aureus carriers
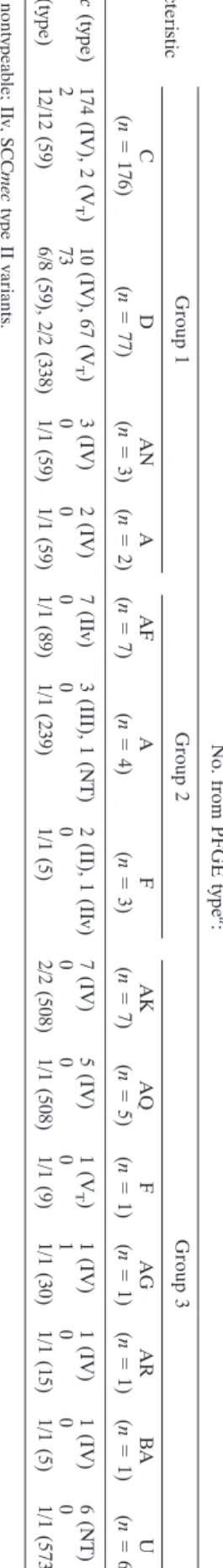
Molecular analysis and antimicrobial susceptibilities of MRSA isolates. Of 473 MRSA isolates, 294 (62.2%) were randomly selected for determination of PFGE types, SCCmec types, and the presence of PVL genes. The strains included 74.5% (northern Taiwan), 53.8% (southern Taiwan), and 53.6% (central Taiwan) MRSA isolates. A total of 12 PFGE types with 71 subtypes were identified. The strains were further divided into 3 groups (Table 5). Group 1 contained isolates of four PFGE types belonging to the dominant Asian-Pacific CA-MRSA
lin-eage, ST59, or its single-locus variant, ST338. In this group, two
major clones characterized as PFGE type C/SCCmec IV/PVL⫺,
and PFGE type D/SCCmec VT/PVL⫹ were predominant and
identified in 59.2% and 22.8% of the 294 isolates, respectively. Group 2 contained 14 isolates carrying SCCmec II or III, which belonged to two pandemic hospital-associated (HA)-MRSA genotypes, ST5 and ST239, and a less commonly re-ported genotype, ST89. Isolates in group 3 consisted of 7 minor PFGE types and constituted 6 unrelated MLST types. Except
TABLE 3. Univariate analysis of epidemiologic factors associated with MRSA and MSSA nasal carriage in Taiwanese children from July 2005 to June 2008
Factora Value P1b P2c MRSA carrier (n⫽ 473) MSSA carrier (n⫽ 931) Noncarrier (n⫽ 4,653) Demographic
Residing in northern Taiwan (%) 40.6 38.8 31.5 ⬍0.0001 ⬍0.0001
Age 2–6 mo (%) 18.8 24.7 10.6 ⬍0.0001 ⬍0.0001
Male (%) 55.8 53.1 54.7 0.6260 0.3764
Breast feeding (%) 61.1 66.6 70.8 ⬍0.0001 0.0108
Period (mo)d 4.3⫾ 5.3 4.8⫾ 5.6 5.3⫾ 5.6 0.0032 0.0249
Environmental
No. of children in the family. 2.0⫾ 0.9 1.8⫾ 0.9 1.89⫾ 0.87 0.0341 0.0478
House size (m2) 173.8⫾ 99.3 175.6⫾ 115.3 178.4⫾ 267.9 0.4475 0.6131
No. of toilets 2.4⫾ 1.1 2.4⫾ 1.1 2.4⫾ 1.0 0.4297 0.8887
Sleeping with parents (%) 89 85.8 89.7 0.6342 0.0006
Hand washing frequency (no./day) 5.7⫾ 4.2 5.4⫾ 3.6 5.9⫾ 3.6 0.3357 ⬍0.0001
Passive smoking (%) 50.5 48.3 47.8 0.2666 0.7460
Day-care or kindergarten attendance (%) 22.4 15.9 18.7 0.0567 0.0455
Duration (months)e 13.0⫾ 10.9 11.9⫾ 10.2 10.8⫾ 9.3 0.0463 0.1513 No. of classmatese 16.9⫾ 6.0 17.0⫾ 6.5 17.0⫾ 7.0 0.8498 0.9505 Duration of stay (h/wk)e 39.7⫾ 6.5 38.8⫾ 7.0 39.2⫾ 6.5 0.5013 0.5880 Health conditions Pneumococcal vaccination (%) 14.2 13.1 12.0 0.1844 0.3514 Flu vaccination (%) 44.6 33.5 41.1 0.1419 ⬍0.0001 History of AOM (%) 12.7 9.0 10.3 0.1153 0.2574
URI within 2 weeks (%) 37 29.8 35.3 0.4495 0.0013
Premature birth (%) 7.2 5.5 7.7 0.7851 0.0188
Received antibiotics within 2 weeks (%) 6.8 3.7 6.9 1.0000 ⬍0.0001
aAOM, acute otitis media; URI, upper respiratory tract infection.
bP
1, statistical test between MRSA carriers and noncarriers.
cP
2, statistical test between MSSA carriers and noncarriers, either the chi-square test or a two-sample t test.
dData derived from those with a history of breast feeding.
eData derived from those attending day care.
TABLE 4. Risk factors for MRSA and MSSA carriage in Taiwanese children by multiple logistic regression analysis
Factor
MRSA carrier vs. noncarrier MSSA carrier vs. noncarrier
Adjusted OR (95%
confidence interval) P
Adjusted OR (95%
confidence interval) P
Colonization by S. pneumoniae 0.697 (0.518–0.938) 0.0170 0.436 (0.331–0.573) ⬍0.0001 Residing in northern Taiwan 1.447 (1.187–1.763) 0.0003 1.405 (1.205–1.639) ⬍0.0001 Timing of subject enrollmenta 1.059 (1.031–1.087) ⬍0.0001
Age 2–6 mo 2.236 (1.725–2.898) ⬍0.0001 2.631 (2.133–3.245) ⬍0.0001
Breast feeding 0.650 (0.532–0.794) ⬍0.0001 0.767 (0.657–0.897) 0.0009
No. of children in family 1.114 (1.002–1.240) 0.0463 0.966 (0.886–1.052) NSb
Day care attendance 1.530 (1.201–1.949) 0.0006 1.173 (0.955–1.440) NS
Received antibiotics (⬍2 weeks) 0.606 (0.417–0.880) 0.0036
Premature birth 0.696 (0.511–0.946) 0.0198
a
A continuous variable was created by dividing the period of study into 12 study seasons (3 months/season). The seasons were coded from 1 (July to September 2005) to 12 (April to June 2008) as shown in Fig. 2.
b
for isolates of genotype U, all isolates in this group carried
SCCmec IV or VT. Genotype U belonged to ST573, a
single-locus variant of ST1, and harbored an untypeable SCCmec. PVL was identified in one isolate of ST30 in this group.
The analysis of the temporal trend for each genotype dis-closed no significant change during the period of the study, except for genotype U in group 3. The genotype carrying an untypeable SCCmec was not detected in any isolates in the first year, appeared in the second year (1.7%), and accounted for 10.9% of the isolates tested in the third year of study (P for the
trend,⬍0.0001). The genotype distributions were not
associ-ated with other epidemiological factors, including the geo-graphic areas and nasopharyngeal carriage of S. pneumoniae. The MRSA isolates exhibited extremely high rates of resis-tance to erythromycin (92.4%), clindamycin (85.6%), and
mul-tiple (ⱖ2) non-beta-lactam antibiotics (88.8%). Resistance to
trimethoprim-sulfamethoxazole (TMP-SMX), fusidic acid, doxycycline, and glycopeptides was rare and was identified in 2.5%, 0.8%, 3.6%, and 0% of isolates, respectively.
DISCUSSION
Pooled data incorporating 8,350 patients from 10 studies of MRSA carriage (mostly in the United States) between 1998 and 2002 showed a MRSA prevalence of 1.3% (34). A much
lower rate (ⱕ0.24%) was estimated among persons without
health care-associated risks. In contrast to the data from the meta-analysis, results from this study indicated a strikingly high incidence of MRSA carriage (7.8%) among healthy Taiwanese children from 2005 to 2008, with rates ranging from 6.2% to 9.5% in different geographic areas. The carriage rate in chil-dren reported here was also significantly higher than in the Taiwanese adult populations surveyed during the same period
(3.8%; P⬍ 0.0001) (37). This observation may suggest unique
epidemiology for MRSA in Taiwanese children or, more likely, a universal increase in MRSA carriage in the past decade. We previously identified a sharp rise in the MRSA carriage rate in children living in northern Taiwan from 1.9% in 2001 and 2002 to 9.5% in 2005 and 2006 (20, 21). The carriage rate appeared to plateau at 9.5% in this study. The incidence of MRSA carriage in southern Taiwan also increased from 3.3% in 2001 to 6.7% in 2005 and 2006, and further to 7.7% by 2008 (20, 28). A lower rate of MRSA carriage was identified in central Tai-wan, but it still increased from 4.8% in 2005 and 2006 to 6.2% in 2005 to 2008 (20).
Two major clones with multidrug resistance belonging to the ST59 lineage accounted for the increasing incidence of MRSA colonization. The two clones also predominated in the clinical CA-MRSA isolates that have been identified in nearly 60% of CA S. aureus infections in Taiwan (18). These observations clearly revealed an accelerated, island-wide transmission of MRSA in the community. Children harboring MRSA played an important role in this changing epidemiology and could be one of the major forces boosting the incidence of CA-MRSA infections in previously healthy individuals.
The factors associated with CA-MRSA carriage in children have never been comprehensively surveyed. In this study, we collected samples from different geographic areas over a 3-year span, which allowed us to simultaneously analyze the demo-graphic, environmental, and host factors influencing MRSA
TABLE 5. Molecular characterizations of 294 MRSA nasal isolates from healthy Taiwanese children from July 2005 to June 2008 Characteristic No. from PFGE type a : Group 1 Group 2 Group 3 C (n ⫽ 176) D (n ⫽ 77) AN (n ⫽ 3) A (n ⫽ 2) AF (n ⫽ 7) A (n ⫽ 4) F (n ⫽ 3) AK (n ⫽ 7) AQ (n ⫽ 5) F (n ⫽ 1) AG (n ⫽ 1) AR (n ⫽ 1) BA (n ⫽ 1) U (n ⫽ 6) SCC mec (type) 174 (IV), 2 (V T ) 10 (IV), 67 (V T ) 3 (IV) 2 (IV) 7 (IIv) 3 (III), 1 (NT) 2 (II), 1 (IIv) 7 (IV) 5 (IV) 1 (V T ) 1 (IV) 1 (IV) 1 (IV) 6 (NT) PVL ⫹ 2 7 3 0000 0 0 0 0 100 0 MLST (type) 12/12 (59) 6/8 (59), 2/2 (338) 1/1 (59) 1/1 (59) 1/1 (89) 1/1 (239) 1/1 (5) 2/2 (508) 1/1 (508) 1/1 (9) 1/1 (30) 1/1 (15) 1/1 (5) 1/1 (573 b ) a NT, nontypeable; IIv, SCC mec type II variants. b ST573 is a single-locus variant of ST1.
colonization. The interplay between S. aureus and another common pediatric pathogen, S. pneumoniae, was also explored, while the other epidemiological factors were controlled. It was intriguing that, except for the demographic factors, there were no host or environmental factors that increased the risk of MSSA colonization. Furthermore, a variety of factors, includ-ing recent exposure to antibiotics and premature birth history, surrogates for exposure to health care facilities and antimicro-bial agents, were associated with a decreasing incidence of MSSA carriage. MRSA appeared not to be affected by these factors. The findings indicated a contributing role of the emer-gence of antibiotic resistances in MRSA transmission within the community. Moreover, crowded environments, such as liv-ing with a greater number of children and attendliv-ing day care, significantly increased the risk of MRSA colonization. These observations may explain the increasing incidence of MRSA colonization in the past decade while the MSSA colonization rate remained stable during the same period.
The innate immunity of the host has been implicated in the mechanisms of S. aureus colonization (26, 32). Our finding that a history of breast feeding and the feeding period after birth affected the colonization rates of MRSA and MSSA may be explained by the involvement of host humoral immunity. The factor was not previously reported to be associated with S. aureus colonization. Further studies are needed to explore the roles of active and passive immunity in CA-MRSA nasal col-onization.
The inverse relationship between S. aureus and S. pneu-moniae colonization has been previously observed among both healthy children and children with acute illness (2, 29). The mechanisms of microbial interference were not completely understood and may involve the bactericidal effect of S. pneumoniae against S. aureus mediated by the production of hydrogen peroxide (33). The inhibiting effect on S. aureus colonization seemed restricted to hosts without HIV-1 infec-tions and specific to S. pneumoniae serotypes included in the 7-valent pneumococcal conjugate vaccine (1, 29). Our analysis further indicated that colonization by S. pneumoniae had a significantly greater impact on the colonization rate of MSSA
than on that of MRSA (odds ratio [OR], 1.61; P⫽ 0.0165),
which supported the idea that the epidemic CA-MRSA was less susceptible to environmental factors. However, the finding was in contrast to a report from South Africa investigating a group of children with a high rate (67.3%) of HIV-1 infection and hospitalization for severe pneumonia (29). There was a negative correlation between S. pneumoniae and MRSA, but not MSSA. Multiple factors may be responsible for these ob-servations, including the patients’ ethnicities, HIV infection, and the presence of severe pneumonia. The MRSA isolates in the current study were from healthy children and belonged to the epidemic CA-MRSA clone, which may behave differently than the MRSA strains identified in the South African patients with high rates of HIV-1 infection.
Most of the MRSA isolates (82%) in the current study belonged to one of two major clones, characterized as PFGE
C/SCCmec IV/PVL⫺and PFGE D/SCCmec VT/PVL⫹. These
isolates were from a single genetic lineage, ST59, and demon-strated high rates of resistance to several non-beta-lactam
an-tibiotics. The PFGE D/SCCmec VT/PVL⫹clone, though
ac-counting for a smaller part (22.8%) of the colonizing
isolates in this survey, were identified in a majority (69%) of clinical CA-MRSA isolates in one prospective study in chil-dren between 2004 and 2005 (18). In the same study, the
PFGE C/SCCmec IV/PVL⫺clone was responsible for only
8.8% of the clinical isolates. The discrepancy of clonal dis-tributions in clinical and colonizing isolates was also iden-tified in other studies (6, 37). The finding suggested greater virulence of the clone positive for PVL than of its sister clone negative for PVL. Another study is under way to investigate the virulence of CA-MRSA by comparing the two major clones in Taiwan.
Although they accounted for only 7.5% of all MRSA iso-lates, emerging CA genotypes (carrying type IV, V, or untype-able SCCmec in group 3) appeared to be increasingly identified during the period of the study. Genotype U, belonging to ST573 and carrying an untypeable SCCmec, was of particular interest due to its rapid increase in nasal colonization. The finding indicated that the overall increase of MRSA carriage in Taiwanese children was in part due to the appearance of emerging MRSA clones in the community. The impact of the emerging clones remained unclear and requires further molec-ular study of clinical CA-MRSA isolates.
The high rates of multidrug resistance of CA-MRSA in the current study were not observed in several prevalent CA-MRSA clones in other areas of the world (2). The character-istics of the CA-MRSA strains complicated the treatment of CA S. aureus infections and limited the choice of antimicrobial agents in putative S. aureus infections in previously healthy children (4). However, most isolates from this study were sus-ceptible to TMP-SMX and fusidic acid, which appeared to be an attractive alternative choice for empirical therapy of puta-tive CA S. aureus infections in Taiwanese children with non-invasive diseases.
There were several limitations in this study. First, the nose and pharynx are not the only sites of S. aureus colonization. The rate of S. aureus colonization may be underestimated without simultaneous swabs of other body sites (e.g., axillae, groin, and anus). Second, some epidemiological factors influ-encing MRSA and MSSA carriage may not have been col-lected. For instance, we did not sample the household mem-bers whose status of S. aureus carriage may have influenced the study subjects (16). Information related to exposure to health care facilities was not available for the other household mem-bers; however, we believe this should not be an important factor in the study. The type II or III staphylococcal chromo-somal cassette mec elements, presumed to be characteristic of HA-MRSA isolates, were carried by very few MRSA isolates (4.8%) (Table 5) in this study. This finding suggested that the dominant HA-MRSA isolates have very limited, if any, spread in the community. Third, subjects were recruited from three main hospitals; therefore, the prevalence of MRSA and MSSA carriage may not be generalized to the entire pediatric popu-lation in Taiwan. We noted that the MRSA carriage rate var-ied significantly from site to site. However, the impact of these geographic differences and temporal trends was controlled in the multivariate analysis of epidemiological risks. The signifi-cance of the identified factors associated with MRSA and MSSA carriage should be valid in and applicable to the general population of preschool age children in Taiwan.
incidence of MRSA colonization in preschool age Taiwanese children between 2005 and 2008. The carriage rate varied with age and within different geographic areas. Crowded environ-ments increased the risk of MRSA colonization, whereas a history of breast feeding and colonization by S. pneumoniae were protective from MRSA colonization in healthy children. Compared to MSSA, MRSA appeared to be less susceptible to environmental factors, including microbial interference by S. pneumoniae. This characteristic can contribute to the increas-ing incidence of CA-MRSA colonization and infections in pre-viously healthy hosts and may partly explain the worldwide spread of this “superbug.”
ACKNOWLEDGMENTS
We thank Keiichi Hiramatsu and Chi-Chien Wang for providing control strains of MRSA for SCCmec typing.
The work was supported by grants from Chang Gung Memorial Hospital (CMRPG450121, CMRPG450122). The funding organiza-tion had no role in the design and conduct of the study and the collection, analysis, and interpretation of the data.
REFERENCES
1. Bogaert, D., et al. 2004. Colonisation by Streptococcus pneumoniae and
Staphylococcus aureus in healthy children. Lancet 363:1871–1872.
2. Boyle-Vavra, S., and R. S. Daum. 2007. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab. Invest. 87:3–9.
3. Centers for Diseases Control and Prevention. 2003. Outbreaks of commu-nity-associated methicillin-resistant Staphylococcus aureus skin infections— Los Angeles County, California, 2002–2003. MMWR Morb. Mortal. Wkly. Rep. 52:88.
4. Chen, C. J., et al. 2007. Experience with linezolid therapy in children with osteoarticular infections. Pediatr. Infect. Dis. J. 26:985–988.
5. Chen, C. J., et al. 2007. Clinical features and molecular characteristics of invasive community-acquired methicillresistant Staphylococcus aureus in-fections in Taiwanese children. Diagn. Microbiol. Infect. Dis. 59:287–293. 6. Chen, F. J., et al. 2005. Methicillin-resistant Staphylococcus aureus in Taiwan.
Emerg. Infect. Dis. 11:1760–1763.
7. Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial disk susceptibility tests; approved standard, 9th ed. CLSI, Wayne, PA.
8. Creech, C. B., II, D. S. Kernodle, A. Alsentzer, C. Wilson, and K. M. Edwards. 2005. Increasing rates of nasal carriage of methicillin-resistant
Staphylococcus aureus in healthy children. Pediatr. Infect. Dis. J. 24:617–621.
9. Deleo, F. R., M. Otto, B. N. Kreiswirth, and H. F. Chambers. 2010. Community-associated meticillin-resistant Staphylococcus aureus. Lancet
375:1557–1568.
10. Ellis, M. W., D. R. Hospenthal, D. P. Dooley, P. J. Gray, and C. K. Murray. 2004. Natural history of community-acquired methicillin-resistant
Staphylo-coccus aureus colonization and infection in soldiers. Clin. Infect. Dis. 39:
971–979.
11. Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol.
38:1008–1015.
12. Gilbert, M., et al. 2006. Outbreak in Alberta of community-acquired (USA300) methicillin-resistant Staphylococcus aureus in people with a his-tory of drug use, homelessness or incarceration. CMAJ 175:149–154. 13. Gonzalez, B. E., et al. 2005. Severe Staphylococcal sepsis in adolescents in
the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics 115:642–648.
14. Groom, A. V., et al. 2001. Community-acquired methicillin-resistant
Staphy-lococcus aureus in a rural American Indian community. JAMA 286:1201–
1205.
15. Herold, B. C., et al. 1998. Community-acquired methicillin-resistant
Staph-ylococcus aureus in children with no identified predisposing risk. JAMA
279:593–598.
16. Huang, Y. C., et al. 2009. Association of Staphylococcus aureus colonization in parturient mothers and their babies. Pediatr. Infect. Dis. J. 28:742–744. 17. Huang, Y. C., Y. H. Chou, L. H. Su, R. I. Lien, and T. Y. Lin. 2006.
Methicillin-resistant Staphylococcus aureus colonization and its association with infection among infants hospitalized in neonatal intensive care units. Pediatrics 118:469–474.
18. Huang, Y. C., C. F. Ho, C. J. Chen, L. H. Su, and T. Y. Lin. 2008. Compar-ative molecular analysis of community-associated and healthcare-associated methicillin-resistant Staphylococcus aureus isolates from children in northern Taiwan. Clin. Microbiol. Infect. 14:1167–1172.
19. Huang, Y. C., C. F. Ho, C. J. Chen, L. H. Su, and T. Y. Lin. 2007. Nasal carriage of methicillin-resistant Staphylococcus aureus in household contacts of children with community-acquired diseases in Taiwan. Pediatr. Infect. Dis. J. 26:1066–1068.
20. Huang, Y. C., K. P. Hwang, P. Y. Chen, C. J. Chen, and T. Y. Lin. 2007. Prevalence of methicillin-resistant Staphylococcus aureus nasal colonization among Taiwanese children in 2005 and 2006. J. Clin. Microbiol. 45:3992– 3995.
21. Huang, Y. C., L. H. Su, C. J. Chen, and T. Y. Lin. 2005. Nasal carriage of methicillin-resistant Staphylococcus aureus in school children without iden-tifiable risk factors in northern Taiwan. Pediatr. Infect. Dis. J. 24:276–278. 22. Huang, Y. C., et al. 2004. Molecular epidemiology of clinical isolates of
methicillin-resistant Staphylococcus aureus in Taiwan. J. Clin. Microbiol.
42:307–310.
23. Kazakova, S. V., et al. 2005. A clone of methicillin-resistant Staphylococcus
aureus among professional football players. N. Engl. J. Med. 352:468–475.
24. King, M. D., et al. 2006. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA300 clone as the predominant cause of skin and soft-tissue infections. Ann. Intern. Med. 144:309–317.
25. Kuehnert, M. J., et al. 2006. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J. Infect. Dis. 193:172–179. 26. Li, M., et al. 2009. Staphylococcus aureus mutant screen reveals interaction of
the human antimicrobial peptide dermcidin with membrane phospholipids. Antimicrob. Agents Chemother. 53:4200–4210.
27. Lina, G., et al. 1999. Involvement of Panton-Valentine leukocidin-producing
Staphylococcus aureus in primary skin infections and pneumonia. Clin.
In-fect. Dis. 29:1128–1132.
28. Lu, P. L., et al. 2005. Risk factors and molecular analysis of community methicillin-resistant Staphylococcus aureus carriage. J. Clin. Microbiol. 43: 132–139.
29. McNally, L. M., et al. 2006. Lack of association between the nasopharyngeal carriage of Streptococcus pneumoniae and Staphylococcus aureus in HIV-1-infected South African children. J. Infect. Dis. 194:385–390.
30. Otto, M. 2010. Basis of virulence in community-associated methicillin-resis-tant Staphylococcus aureus. Annu. Rev. Microbiol. 64:143–162.
31. Pan, E. S., et al. 2003. Increasing prevalence of methicillin-resistant
Staph-ylococcus aureus infection in California jails. Clin. Infect. Dis. 37:1384–1388.
32. Peschel, A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10:179–186.
33. Regev-Yochay, G., K. Trzcinski, C. M. Thompson, R. Malley, and M. Lip-sitch.2006. Interference between Streptococcus pneumoniae and
Staphylo-coccus aureus: in vitro hydrogen peroxide-mediated killing by StreptoStaphylo-coccus pneumoniae. J. Bacteriol. 188:4996–5001.
34. Salgado, C. D., B. M. Farr, and D. P. Calfee. 2003. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin. Infect. Dis. 36:131–139.
35. Vandenesch, F., et al. 2003. Community-acquired methicillin-resistant
Staph-ylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide
emergence. Emerg. Infect. Dis. 9:978–984.
36. von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N. Engl. J. Med. 344:11–16.
37. Wang, J. T., et al. 2009. Prevalence of and risk factors for colonization by methicillin-resistant Staphylococcus aureus among adults in community set-tings in Taiwan. J. Clin. Microbiol. 47:2957–2963.
38. Zinderman, C. E., et al. 2004. Community-acquired methicillin-resistant
Staphylococcus aureus among military recruits. Emerg. Infect. Dis. 10:941–