Ma trix Ef fect of Al kali and Al ka line Earth Metal Ha lides on Flu o res cence
Quan tum Yield of In dium in La ser-induced Flu o res cence
Flame Spec trom e try
Ching-Bin Ke ( ) and King-Chuen Lin* ( ) De part ment of Chem is try, Na tional Tai wan Uni ver sity, Tai pei 106, and
In sti tute of Atomic and Mo lec u lar Sci ences, Ac a de mia Sinica, P. O. Box 23-166, Tai pei 106, Tai wan, R.O.C.
As in ter fered with by al kali and al ka line earth metal halides added as the ma trix in an acet y lene/air flame, the flu o res cence quan tum yield of In as the analyte in a la ser-induced flu o res cence (LIF) flame spec trom e try has been thor oughly char ac ter ized. The flu o res cence quan tum yield is de ter mined by a ra tio of F to A, where F is the mea sured flu o res cence of In and A is the dif fer ence be tween the ab sorp tion sig nals re corded for the analyte and the blank so lu tions. The nor mal ized flu o res cence sig nal is treated to pre vent de vi a tions due to vari a tions of the at om iza tion ef fi ciency un der the con di tions with and with out the ma trix added. The flu o res -cence quan tum yield is mea sured to be al most in de pend ent of the ma trix con cen tra tion up to 500 ppm ( g/mL) stud ied, un der con di tions of ei ther op ti cal unsaturation or sat u ra tion. By con sid er ing a quench ing ef fect in duced by elec tronatom col li sions, the cal cu lated flu o res cence quan tum yields are con sis tent with our ob ser -va tions.
IN TRO DUC TION
The ma trix ef fects on flu o res cence and ab sorp tion flame and nonflame spec tro met ric tech niques and in duc coupled plasma (ICP) emis sion spec trom e try have been widely stud ied. A better un der stand ing of the in ter fer ence mech a nisms in volved seems to be cru cial to re duc ing ef fec -tively the ma trix in ter fer ence and mea sur ing trace analytes pre cisely, in ad di tion to op ti miz ing the op er at ing con di tions. Es sen tially the re sul tant in ter fer ence may cause such ef fects as shift ing the ion iza tion equlibrium of the analyte,1,2 in duc -ing chem i cal re ac tions,3,4 vary ing the prop er ties of analyte vol a til iza tion5,6 and at om iza tion,7,8 quench ing the analyte pop u la tion in the ex cited state,9,10 chang ing the trans port prop er ties of so lu tions into a flame or an ICP sys tem,11,12 re -form ing the spa tial dis tri bu tion of the analyte pop u la tion,13,14 caus ing broad en ing of the line shape15,16 and af fect ing the dif -fu sion of the con cen tra tion gra di ent17,18 and sys tem tem per a -ture.19,20 The phys i cal and chem i cal phe nom ena in volved are very com pli cated and thus the in ter pre ta tion is very di ver si -fied.
Ion iza tion and chem i cal ef fects are two in ter fer ence mech a nisms fre quently con sid ered in op ti cal flame spec -trom e try.1-4 The for mer pro cess re sults in a sub stan tial in -crease in the num ber of the elec trons, which may sup press the ion iza tion of the analyte be ing de tected. On the other hand,
the chem i cal re ac tion with the ma trix added may con sume the analyte and thus de press the ab sorp tion sig nal. Sev eral meth -ods, such as chro mato graphic sep a ra tion, acid i fi ca tion, and analyte ex trac tion or di lu tion,21-24 have been em ployed to re move or di min ish ma trix in ter fer ences. How ever, the phys i cal and chem i cal com plex ity ob scures a de tailed un der stand ing of the phe nom ena in volved in most cases. Thus far, re -search into the ma trix ef fect has been sub stan tially fo cused on ICP emis sion and ab sorp tion flame spec trom e try. LIF flame spec trom e try is sel dom in ves ti gated, es pe cially with re gard to the mech a nisms that the ma trix in duces to in ter fere with the analyte.
In this work, we at tempted to un der stand the ma trix ef fect on the flu o res cence quan tum yield in LIF flame spec -trom e try. Al kali and al ka line earth metal halides were used as the ma trix and the LIF re sponse of In as the analyte was de tected. In ad di tion to caus ing ex ci ta tion in ter fer ence, the ma trix ef fect in LIF may be es sen tially treated as in the ab sorp tion spec trom e try. The caused ion iza tion and chem i cal re ac -tions, con sid ered as the ma jor in ter fer ences, may change the at om iza tion ef fi ciency of the analyte. There fore, the flu o res -cence quan tum yield was treated as a ra tio of flu o res -cence to ab sorp tion in ten si ties. The nor mal ized flu o res cence be comes in de pend ent of the pop u la tion vari a tion in the ground state of the analyte. In this man ner, the flu o res cence quan tum yield was mea sured to be al most in de pend ent of the ma trix stud ied,
un der ei ther op ti cal unsaturation or sat u ra tion con di tions. A the o ret i cal pre dic tion, tak ing into ac count the quench ing pro cess of elec tronatom col li sions, is con sis tent with our ob ser -va tions.
EX PER I MEN TAL Flame Sys tem
We per formed the LIF and ab sorp tion ex per i ments in an at mo spheric flame. The ap pa ra tus is de picted in Fig. 1. A com mer cial burner as sem bly (Perkin-Elmer, Norwalk, CT, USA) with a 100 0.5 mm slot burner head was cou pled with an in ter locked gas con trol sys tem, by means of which acet y -lene and air were pre mixed prior to reach ing the burner head. The flow rate of the fuel and the air were reg u lated at 0.5 L/min and 12.5 L/min, re spec tively. The flame tem per a ture was pre vi ously de ter mined to be 2500 K.25,26 Aque ous so lu -tions of In salt were pre pared from 1 to 100 ppm ( g/mL) and nebulized at a flow rate of 4.5 mL/min into the burner head.
La ser Source
The light source for LIF de tec tion was a 10 Hz, 5-8 ns Nd:YAG la ser-pumped dye la ser (Quan ta Ray PDL-2, CA, USA), emit ting at 651.2 nm with the use of a DCM dye. The ra di a tion was then fre quency-doubled through a KDP crys tal which was housed in a wave length ex tender with a de vice of auto-tracking con trol ler (Quan ta Ray WEX, CA, USA). The re sult ing wave length at 325.6 nm was used to ex cite the In atom in the 52P3/2 52D5/2 tran si tion. The unfocused ex ci ta -tion beam was collimated with a pin hole of 5 mm2 cross sec -tion and then di rected lon gi tu di nally through the flame at 12 0.1 mm above the burner head. The la ser en ergy, prior to
reach ing the flame, was mea sured con tin u ously by a sur face ab sorb ing disk cal o rim e ter (Scientech 36-0001, CO, USA).
Re agents
An a lyt i calreagent grade salts (Merck, Darmstadt, Ger -many) were used to pre pare the analyte and ma trix so lu tions. For most ex per i ments, the analyte was pre pared at a 3 ppm In con cen tra tion, which lay in the lin ear range in the con cen tra tion de pend ence mea sure ments. The ma trix so lu tions of al -kali and al ka line earth metal chlo rides (LiCl, NaCl, RbCl, CsCl, MgCl2, CaCl2, SrCl2, and BaCl2) were pre pared at metal con cen tra tions of 500 ppm.
Nebulization Ef fi ciency Mea sure ment
We mea sured the nebulization ef fi ciency to be 0.090 0.006 for the analyte un der the con di tions with and with out 500 ppm of interferents. This rules out nebulization and trans port as im por tant con tri bu tors to the ob served ma trix be hav ior. A sim i lar con clu sion was reached in ICP ex per i -ments.13
LIF and Ab sorp tion De tec tion
The non-resonance flu o res cence in the 62S1/2 52P1/2 tran si tion at 410.1 nm was mon i tored, as In was ex cited from the 52P
3/2 to the 52D5/2 state. The sig nal was col lected per pen dic u larly rel a tive to the la ser beam axis onto a mono chro ma -tor (McPherson 270, MA, USA) via a pair of lenses of 10 and 2.5 in. The en trance and the exit slits of the mono chro ma tor were open to 500 m; the grat ing was set at 410.1 nm, al low -ing for a spec tral trans mit tance of 1 nm. The trans mit ted LIF was de tected by a photomultiplier tube (Hamamatsu R955, Hamamatsu City, Ja pan) at tached to the mono chro ma tor, and fed into a box car in te gra tor (EG&G PAR Models 4402 and 4422, Prince ton, NJ, USA) for im prove ment of the sig naltonoise ra tio. The data was then stored in a mi cro com -puter for fur ther treat ment.
The ab sorp tion spec trum of the analyte In was de tected with a photodiode, which was po si tioned along the la ser beam axis. For op ti miz ing the in stru ment sen si tiv ity and avoid ing any op ti cal sat u ra tion, the power of the in ci dent la -ser beam was ap pro pri ately at ten u ated us ing the fil ters. The ex per i men tal con di tions were oth er wise iden ti cal with those for the LIF ap pa ra tus.
Ex per i men tal Con di tions
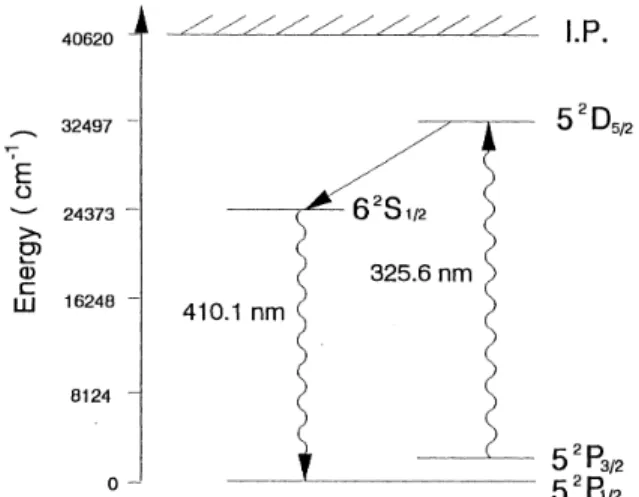
A par tial en ergy di a gram of the In atom for resonance LIF de tec tion is shown in Fig. 2. The ini tial state 52P3/2 , with an en ergy 2213 cm-1 above the ground state 52P1/2, was ther mally pop u lated. A care ful test was made to
Fig. 1. Sche matic di a gram of the ap pa ra tus for la serinduced flu o res cence and atomic ab sorp -tion de tec -tion.
en sure ac cu rate de tec tion of the sig nal. A lin ear dy namic range of the In con cen tra tion was mea sured up to 100 ppm. A 3 ppm In con cen tra tion was ac cord ingly used through out the ex per i ments. The de pend ence of the LIF sig nal on the la ser power for a 3 ppm In so lu tion was mea sured. The back ground re sponse was de ter mined as the la ser was de tuned off the res -o nance line, as sum ing that the in ter fer ence by the scat tered ra di a tion was in de pend ent of the la ser wave length. Un der the con di tions used, the flu o res cence sig nal be came op ti cally sat u rated when the la ser en ergy ex ceeded 100 J. Two par al lel ex per i ments for the ma trix ef fect on un sat u rated and sat u rated LIF were con ducted si mul ta neously. The cor re spond -ing in ci dent la ser en ergy was main tained at 60 3 J and 300
15 J, re spec tively.
RE SULTS AND DIS CUS SION
Mea sure ments of flu o res cence, ab sorp tion and quan tum yield
Based on the ap pa ra tus de scribed ear lier, LIF and ab -sorp tion spec tra of 3 ppm In with and with out ma trix added were mea sured. The flu o res cence quan tum yield is pro por tional to the ra tio of F to A, where F is the mea sured flu o res -cence of In and A is the dif fer ence be tween the ab sorp tion sig nals re corded for the analyte and the blank so lu tions.9,27 The ab sorp tion mea sure ment re flects the rel a tive ground state pop u la tion of the analyte un der the con di tions with and with out the ma trix added.
To jus tify the ma trix ef fect on the flu o res cence quan
-tum yield, a ra tio
(1)
is writ ten, where FMX and AMX de note the quan ti ties mea sured
when var i ous types of Group 1A and 2A metal chlo rides were added along with the analyte so lu tion. The rel a tive un satura -tion flu o res cence, FMX/F, and rel a tive ab sorp tion mea sure -ment, AMX/A, are shown in Fig. 3, where 500 ppm of each metalcontaining ma trix were added. The cor re spond ing rel a -tive quan tum yield, as ex pressed by eq. 1, is shown in Fig. 4. The sat u ra tion flu o res cence ra di ance was ver i fied to de pend on the to tal atomic pop u la tion den sity and the spon ta ne ous emis sion co ef fi cient of the ex cited state analyte of in ter est.28 That is, un der the sat u ra tion con di tions ex ci ta tion in ter fer -ence can be ne glected and only at om iza tion in ter fer -ence will be con sid ered. In fact, the mea sured rel a tive quan tum yield, YMX/Y, ap pears to be in de pend ent of the al kali and al ka line earth metal chlo rides added, un der ei ther unsaturation or sat u ra tion con di tions. Our re sults show an in sig nif i cant dif fer ence be tween these two op ti cal con di tions, as the ma trix con -cen tra tion is at 500 ppm, larger than the analyte con -cen tra tion by about two or ders of mag ni tude. How ever, the up per limit of ma trix con cen tra tion that does not af fect the flu o res cence quan tum yield has not been de ter mined.
In the flu o res cence mea sure ment, In is ex cited via the 52P3/2 52D5/2 tran si tion, while the LIF is mon i tored at 410.1
Fig. 2. Par tial en ergy di a gram of the In atom for the non-resonance LIF de tec tion for 62S
1/2 52P1/2
emis sion with 52P
3/2 52D5/2 ex ci ta tion. The
52D
5/2 62S1/2 tran si tion is ac ti vated by col li
-sions. / / MX MX MX Y F A Y F A
Fig. 3. Ma trix de pend ence of unsaturation flu o res -cence, F, and ab sorp tion, A, mea sure ment of a 3 ppm In so lu tion. The ab sorp tion is ex pressed by the dif fer ence in sig nals re corded for the analyte and the blank so lu tion. The F and A mea sure -ments are nor mal ized to the sig nals of In in the ab sence of ma trix. The al kali and al ka line earth metal chlo rides were pre pared at a 500 ppm metal con cen tra tion.
nm from the 62S1/2 to 52P1/2 state. The 62S1/2 state is pop u lated via collisional de ac ti va tion. The collisional de ac ti va tion rate stem ming from the 52D5/2 state may be dom i nated by the 52D5/2-62S1/2 tran si tion, with en ergy dif fer ence of 8000 cm-1, due to the ef fect of near res o nance en ergy trans fer. The frac tion of 52D5/2-62S1/2 en ergy trans fer may not be af fected by the ad di tion of ma trix, of which the con cen tra tion is neg li gi ble as com pared to the con di tion of 1 atm flame com po si -tions. Our ob ser va tion of the rel a tive quan tum yield, YMX/Y, also shows that the ma trix ef fect on thecollisional de ac ti va -tion in the 52D5/2 62S1/2 tran si tion should be neg li gi ble.
As salt ma trix interferent is added to the In so lu tion, sev eral pos si ble dis tur bances may oc cur. First, the re leased al kali or al ka line earth met als have a rel a tively low ion iza tion po ten tial, thus feasibly in duc ing the ion iza tion pro cess in the flame. Through the mass ac tion ef fect, the ex cess of elec trons re leased may shift the ion iza tion equi lib rium of the analyte, thereby sup press ing the In ion iza tion re ac tion.1,2,29 In this sense, the LIF sig nal of the analyte will be en hanced ow ing to the in crease in the neu tral In at oms avail able for ex ci ta tion. Sec ond, the re leased non-metal at oms may lead to a chem i cal re ac tion with the analyte to form an in dium monohalide com -pound, which causes sup pres sion of the LIF sig nal.3,4,30,31 Third, the ma trix it self or the re leased spe cies, in clud ing the elec trons, may in ter fere with the ex cited state of In, thereby en hanc ing the radiationless collisional de ac ti va tion.9,10,32 In this work, the nor mal ized flu o res cence sig nal is treated to pre vent de vi a tions due to vari a tions of the at om iza tion ef fi -ciency un der the con di tions with and with out the ma trix
added.
The o ret i cal pre dic tion of rel a tive quan tum yield
For com par i son with our ob ser va tions, the flu o res cence quan tum yield is es ti mated the o ret i cally. As in the emission spec trom e try,9,32 the elec tron-impact col li sions are con sid ered to be the ma jor con trib u tor to the collisional de ac -ti va -tion pro cesses in the LIF flame spec trom e try. As suming that the elec tron ve loc ity fol lows a Maxwellian dis tri bu tion and the mi cro scopic re vers ibil ity holds for the quench ing col -li sion, one ob tains the quench ing rate co ef fi cient, kq, for the
elec tron-impact col li sion:9,32
(2)
where go and g1 are the sta tis ti cal weights for the ground and
the ex cited states of In, re spec tively, me is the elec tron mass, k
is the Boltzmann con stant, T is the sys tem tem per a ture, and e is the elec tronimpact ex ci ta tion cross sec tion as the elec -tron en ergy is above the ex ci ta tion thresh old, Eex.33 By tak ing
into ac count a flame tem per a ture of 2500 K, an av er age rel a -tive ve loc ity of the elec tron of 3.1 107 cm s-1 and the re lated cross sec tion 1 Å2, ac cord ing to eq. 2, the rate co ef fi cient of elec tron-atom col li sions is es ti mated to be 3.1 10-9 cm3 s-1, only an or der of mag ni tude smaller than in ICP. Note that the cross sec tion adopted here is 3-6 times larger than those ever re ported for dif fer ent tran si tions.34
Given the elec tron num ber den sity, ne, the ra di a tive
life time, o, of the ex cited state and the quench ing rate co ef fi -cient of elec tron-atom col li sions, the quan tum yield, Y, may be cal cu lated as fol lows:9,32
(3)
Among those pa ram e ters re quired, the elec tron num ber den -sity may be de ter mined from the equi lib rium con stant for flame ion iza tion. Ac cord ing to the Saha equa tion, this ion iza -tion equi lib rium con stant for the analyte or the ma trix in the flame can be ex pressed in terms of the re lated par ti tion func -tion by33
(4)
where nA, nA+, and ne de note the num ber den si ties of at oms,
ions and elec trons, re spec tively, h is the Planck’s con stant, Qi
and QA are the elec tronic par ti tion func tion of the ion and the
atom, re spec tively, and Vi is the ion iza tion po ten tial. The ion
tem per a ture is con sid ered to be equal to the elec tron tem per a -ture un der the con di tions of ther mal equi lib rium. The value
Fig. 4. Rel a tive quan tum yield of flu o res cence mea -sure ment for a 3 ppm In so lu tion as a func tion of ma trix. The al kali and al ka line earth metal chlo rides were pre pared at a 500 ppm metal con cen -tra tion. 1 8 ( / ) 1 ex q o e e E kT k g g kT m 1 1 8 1 o e( o/ ) e 1 ex e E kT Y n g g kT m 3/ 2 3 (2 ) 2 exp( / ) A e e i i i A A n n m kT Q K V kT n h Q
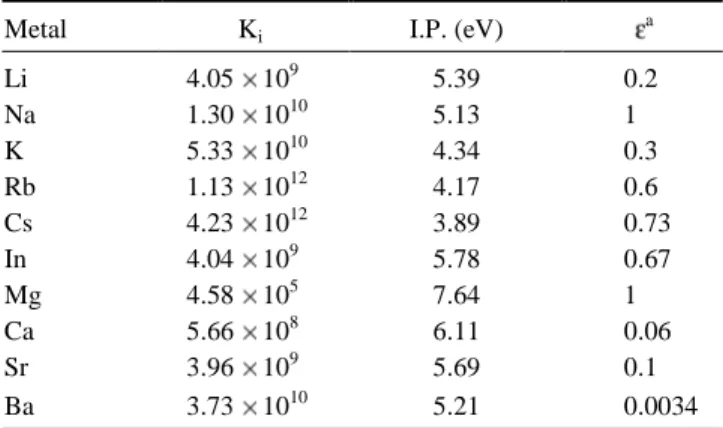
of Qi/QA is es ti mated to be 0.32, 0.5 and 2 for in dium, al kali
metal, and al ka line earth metal, re spec tively.35 Sub sti tuting into eq. 4 the ra tio, the elec tron mass, a known flame tem per -a ture, -and the rel e v-ant ion iz-a tion po ten ti-al, the re spec tive ion iza tion equi lib rium con stant for the analyte and the metal ma trix can be ob tained. The re sults are given in Ta ble 1. The value of ne may be sub se quently eval u ated un der the con di
-tions with and with out the ma trix added, pro vided that nA is
known. nA may be es ti mated by the prod uct of the at om iza tion
ef fi ciency and the to tal num ber den sity of the el e ment A in the flame, which can be readily de ter mined in terms of the so -lu tion con cen tra tion.36 The at om iza tion ef fi cien cies for in -dium, al kali metal, and the al ka line earth metal are re ferred to in ref er ences.37,38 Their val ues are also listed in Ta ble 1.
Sub sti tuting into eq. 3 the quench ing rate co ef fi cient of elec tron-atom col li sions, the ra di a tive life time of 7.69 ns for the In 52D5/2 state and the elec tron num ber den sity of 8.3 1011 cm-3 and 2.9 1010 cm-3 eval u ated with and with out ad di -tion of 500 ppm ma trix (NaCl as an ex am ple), re spec tively, the val ues of YNaCl and Y are pre dicted to be 0.99975 and
0.99999, re spec tively. Anal o gously, we es ti mated the elec -tron quench ing rate co ef fi cient re lated to the In 62S1/2 state, and in turn ob tained flu o res cence quan tum yields of 0.99970 and 0.99999 with and with out ad di tion of NaCl, re spec tively. The quan tum yield for the nonresonance LIF in a flame sys -tem is ver i fied to be in de pend ent of the stud ied con cen tra tion of the Group 1A and 2A metal halides as the ma trix. This con -clu sion is con sis tent with the ob ser va tions.
Note that the the o ret i cal pre dic tion in volves sev eral as -sump tions, such as ne glect of the quench ing pro cess by the spe cies other than elec trons, ne glect of three-body col li sions, va lid ity of lo cal ther mal equi lib rium for the elec tron ve loc ity
dis tri bu tion and va lid ity of mi cro scopic re vers ibil ity. As with ICP, the elec trons pro duced in a flame have a rel a tively large num ber and a sub stan tial collisional cross sec tion in ter act ing with the analyte. The elec tron num ber den sity of 8.3 1011 cm-3 is roughly 1-2 or ders of mag ni tude smaller than the to tal num ber den sity of ma trix nebulized. How ever, the col li sion cross sec tion by elec trons is 2-3 or ders of mag ni tude larger than that by most atomic spe cies re leased. It is rea son able to as sume that the elec tronatom col li sion is pre dom i nantly re -spon si ble for the quench ing pro cess. The other as sump tions de scribed above have been widely adopted in flame and ICP sys tems.9,32
CON CLU SION
We have stud ied the flu o res cence quan tum yield of In as in ter fered with by Group 1A and 2A metal chlo rides in an acet y lene/air flame. A nor mal ized flu o res cence of In was mea sured to char ac ter ize the flu o res cence quan tum yield, for pre vent ing pop u la tion vari a tion of ground state In at oms caused by the ad di tion of the metal chlo rides. The mea sured flu o res cence quan tum yield was al most in de pend ent of the ma trix con cen tra tion stud ied, un der ei ther op ti cal unsatura tion or sat u ra tion con di tions. The the o ret i cal pre dic tion, in -volv ing the quench ing pro cess of elec tron-atom col li sions, agrees with the ob ser va tions.
AC KNOWL EDG MENT
This work is fi nan cially sup ported by the Na tional Sci -ence Coun cil of the Re pub lic of China un der the con tract no.NSC 89-2119-M-002-007.
Re ceived Jan u ary 28, 2002.
Key Words
Ma trix ef fect; Flu o res cence quan tum yield; La ser-induced flu o res cence.
REF ER ENCES
1. Luecke, W. Spectrochim. Acta 1992, 47B, 741.
2. Grove, E. L.; Scott, C. W.; Jones, F. Talanta 1965, 12, 327. 3. Haraguchi, H.; Fuwa, K. Spectrochim. Acta 1975, 30B, 535. 4. Kantor, T. Spectrochim. Acta 1994, 49B, 1717 and 1733. Table 1. Calculated Ionization Equilibrium Constants Ki,
Ioniza-tion Potentials (I.P.), and AtomizaIoniza-tion Efficiencies , for Alkali, Alkaline Earth Metals and the Analyte In
Metal Ki I.P. (eV) a
Li 4.05 109 5.39 0.2 Na 1.30 1010 5.13 1 K 5.33 1010 4.34 0.3 Rb 1.13 1012 4.17 0.6 Cs 4.23 1012 3.89 0.73 In 4.04 109 5.78 0.67 Mg 4.58 105 7.64 1 Ca 5.66 108 6.11 0.06 Sr 3.96 109 5.69 0.1 Ba 3.73 1010 5.21 0.0034 a Ref. 37, 38.
5. Kantor, T.; Bezur, L.; Pungor, E.; Winefordner, J. D.
Spectrochim. Acta 1983, 38B, 581.
6. Fuller, C. W. Anal. Chim. Acta 1976, 81, 199.
7. Olivares, J. A.; Houk, R. S. Anal. Chem. 1986, 58, 20.
8. Hobbs, S. E.; Olesik, J. W. Appl. Spectrosc. 1991, 45, 1395. 9. Wu, M.; Hieftje, G. M. Spectrochim. Acta 1994, 49B, 149. 10. Omenetto, N. Spectrochim. Acta 1988, 43B, 63.
11. Skogerboe, R. K.; Olson, K. W. Appl. Spectrosc. 1978, 32, 181.
12. Borowiec, J. A.; Boom, A. W.; Dillard, J. H.; Cresser, M. S.; Browner, R. F.; Matteson, M. J. Anal. Chem. 1980, 52, 1054. 13. Rybarczyk, J. P.; Jester, C. P.; Yates, D. A.; Koirtyohann, S.
R. Anal. Chem. 1982, 54, 2162.
14. Kornblum, G. R.; De Galan, L. Spectrochim. Acta 1977,
32B, 445.
15. Boss, C. B.; Hieftje, G. M. Anal. Chem. 1979, 51, 895. 16. West, A. C.; Fassel, V. A.; Kniseley, R. N. Anal. Chem. 1973,
45, 2420.
17. Blades, M. W.; Holick, G. Spectrochim. Acta 1981, 36B, 861.
18. Gal ley, P. J.; Glick, M.; Hieftje, G. M. Spectrochim. Acta
1993, 48B, 769.
19. Gunter, W. H.; Visser, K.; Zeeman, P. B. Spectrochim. Acta
1982, 37B, 571.
20. Caughlin, B. L.; Blades, M. W. Spectrochim. Acta 1985, 40B, 987.
21. Bolshov, M. A.; Zybin, A. V.; Koloshnikov, V. G.; Smirenkina, I. I. Spectrochim. Acta 1988, 43B, 519. 22. Jack son, K. W.; Mahmood, T. M. Anal.Chem. 1994, 66,
252R.
23. Kachin, S. V.; Smith, B. W.; Wineforder, J. D. Appl.
Spectrosc. 1985, 39, 587.
24. Fang, Z.; Dong, L.; Xu, S. J. Anal. At. Spectrom. 1992, 7,
293.
25. Su, K. D.; Chen, C. Y.; Lin, K. C.; Luh, W. T. Appl.
Spectrosc. 1991, 45, 1340.
26. Ke, C. B.; Lin, K. C. Appl. Spectrosc. 1998, 52, 187. 27. Pearce, S. J.; De Galan, L.; Winefordner, J. D. Spectrochim.
Acta 1968, 23B, 793.
28. Winefordner, J. D. J. Chem. Educ. 1978, 55, 72.
29. Gilmutdinov, A. Kh.; Shlyachtina, O. M. Spectrochim. Acta
1991, 46B, 1121.
30. Fujiwara, K.; Haraguchi, H.; Fuwa, K. Anal. Chem. 1975,
47, 1670.
31. Haraguchi, H.; Fuwa, K. Bull. Chem. Soc. Ja pan. 1975, 48, 3056.
32. Hasegawa, T.; Smith, B. W.; Winefordner, J. D. Spectro -chim. Acta 1987, 42B, 1093.
33. Alkemade, C. Th. J.; Hol lander, T. J.; Snelleman, W.; Zeegers, P. J. Th. Metal Va pors in Flames; Pergamon Press: Ox ford, 1982; pp 13-90 (ch.2); pp 460-461 (ch.4).
34. Nepiipov, E. I. Opt. Spectrosc. 1975, 38, 186.
35. De Galan, L.; Smith, R.; Winefordner, J. D. Spectrochim.
Acta 1968, 23B, 521.
36. Winefordner, J. D.; Vickers, T. J. Anal. Chem. 1964, 36,
1939.
37. De Galan, L.; Winefordner, J. D. J. Quant. Spectrosc.
Radiat. Trans fer. 1967, 7, 251.
38. De Galan, L.; Samaey, G. F. Spectrochim. Acta 1970, 25B, 245.