Effects of corrosion environments on the surface finishing of copper
chemical mechanical polishing
M.T. Wang
a ,*, M.S. Tsai
b, C. Liu
b, W.T. Tseng
b, T.C. Chang
b, L.J. Chen
c, M.C. Chen
a aDepartment of Electronics Engineering, National Chiao-Tung University, Hsinchu, Taiwan
b
National Nano Device Laboratory, 1001 Ta Hsuell Rd., Hsinchu, Taiwan
c
Department of Submicron Technology Development, ERSO/ITRI, Hsinchu, Taiwan
Abstract
Copper chemical mechanical polishing (Cu-CMP) was investigated using slurries containing alumina abrasive and various types and concentrations of oxidizer, as well as benezotriazole (BTA) additive. We found that the corrosion rate of copper film decreases with increasing BTA concentration in 3 vol.% HNO3solution, but the passivation effect of BTA saturates as the concentration reaches about
0.01 M. On the other hand, a small amount of H2O2 in H2O2/XJFW8099 slurry enhances the corrosion rate dramatically, while an
abundance of H2O2in the slurry suppresses the corrosion reaction effectively. The polishing rate and its non-uniformity decrease with
increasing volume ratio of H2O2in H2O2/XJFW8099 slurry. A very smooth surface with a removal rate of 363.0 nm/min was obtained using
the slurry of 1/1 ratio. Depletion of H2O2 in the slurry can result in more abrasive residuals as well as a rougher surface; the surface
roughness increases from 0.3 to 0.9 nm as the H2O2/XJFW8099 ratio decreases from 1/1 to 1/4. Our results indicate that the corrosion rate,
polishing rate and surface roughness of copper films are sensitively dependent on the type and concentration of oxidizer as well as the BTA additive.1997 Elsevier Science S.A.
Keywords: Corrosion; Chemical mechanical polishing; Benzotriazole; Copper
1. Introduction
Chemical mechanical polishing (CMP) has been pro-posed as a viable technique to widen the process window and to reduce the defect density in integrated circuit (IC) manufacture [1–3]. Recently, a number of studies on copper chemical mechanical polishing (Cu-CMP) have achieved multilevel interconnections with low electrical resistance, excellent electromigration resistance, and global planariza-tion [4–9]. However, the limited passivaplanariza-tion offered by Cu oxide formed on copper surface during CMP process could result in pitting and increased surface roughness. A clear understanding of corrosion effect on polishing rate and sur-face finishing are therefore extremely important to accom-plish Cu metallization.
It has been shown that the use of benzotriazole (BTA) provides a protective and stable film on copper surfaces, which can withstand chemical and thermal environments [10]. For a polishing process using a nitric acid slurry with 6 wt.% alumina (Al2O3), it was reported that the
aver-age surface roughness increased from an initial roughness of 1.0 nm to 15.0 nm [11]. Moreover, it was found that the use of a different pad structure produced a different polishing rate of copper film for a polishing process using a commer-cially available Rodel XJFW8099 slurry, which was diluted with hydrogen peroxide (H2O2) [12]. Although these studies
have provided much valuable information on Cu-CMP, few studies have been done on the effects of corrosion environ-ments on the surface finishing of Cu-CMP process.
The purpose of this study was to investigate the effects of corrosion environments on Cu-CMP. Corrosion rate, polish-ing rate, surface roughness and micro-scratch of copper film were investigated using various types of oxidizers, such as HNO3 and H2O2. The effects of BTA in nitric-acid-based
slurry were evaluated, and various types of slurry were examined. The results of this study may be useful in control of corrosion environments in Cu-CMP processes.
2. Experimental procedures
The corrosion/polishing sample was a two-layer structure
0040-6090/97/$17.00 1997 Elsevier Science S.A. All rights reserved P I I S 0 0 4 0 - 6 0 9 0 ( 9 7 ) 0 0 5 0 0 - 2
of Cu/Ta with thicknesses of 900/30 nm, sputter deposited on a 6-in (150-mm) diameter Si wafer which was covered with a 500-nm thick thermally grown SiO2. The under layer
of 30-nm Ta was used as an adhesion promoter for the copper deposition, since copper does not adhere well on the thermal oxide. The wafer was cut into 3×3-cm2pieces for corrosion rate experiments, while the entire 150-mm wafer was used for CMP of Cu.
To investigate the effect of BTA addition to HNO3
solu-tion on the corrosion rate of copper film, we prepared two
kinds of solutions: (i) 3 vol.% HNO3 dilute solutions to
which were added various concentrations of BTA ranging from 0.001 to 0.04 M; and (ii) 0.01 M BTA solution to
which were added various volume percentages of HNO3
ranging from 0.001 to 20%. To investigate the effect of H2O2concentration on the corrosion rate of copper film, a
Rodel H2O2/XJFW8099 slurry with volume ratio ranging
from 0 to 4 was used. In this study, commercially available
semiconductor grade 30 wt.% H2O2 and 70 wt.% HNO3
were used.
For studying the effects of slurry type on the uniformity and polishing rate of Cu-CMP, three types of slurry were
prepared: HNO3/BTA, H2O2/XJFW8099 and Cabot
WA355/FE-10, as shown in Table 1. The volume ratio of H2O2/XJFW8099 relevant to polishing was varied from 1/1
to 1/4. Cu-CMP was performed with an IPEC/Planar 372M polisher using a Rodel Suba 500 pad. Table 2 gives the experimental parameters and conditions used for Cu-CMP process.
The corrosion tests were performed by dipping the 3×
3-cm2 sample pieces in various corrosion solutions without
intentional stirring. To determine the corrosion/polishing rate of copper film, a four-point probe was used to measure the sheet resistance of copper film, and the change of sheet resistance before and after the corrosion treatment or CMP process was used to determine the corrosion/polishing rate. The polishing rate of Cu-CMP was measured at 13 points across a 150-mm diameter wafer (10-mm edge exclusion). Non-uniformity of polishing rate is defined as follows: Non−uniformity(%) =100%×(Max−Min)polishing rate
2×mean value
Scanning electron microscope (SEM) was employed to
characterize the surface morphology and to measure the thickness of copper film. Atomic force microscope (AFM) was used to characterize the surface roughness before and after the polishing process.
3. Results and discussion
3.1. Passivation effect of BTA on copper corrosion
Nitric acid (HNO3) is a strong oxidizer which attacks
copper very rapidly. Benzotriazole (BTA) is a well known corrosion inhibitor for copper corrosion [13–17]. The mechanism by which BTA is effective in inhibiting corro-sion appears to be the formation of a continuous Cu-BTA complex film tightly adhered to the copper surface.
Fig. 1 shows the corrosion rate of copper as a function of
BTA concentration in a 3 vol.% HNO3solution. The
corro-sion rate of copper decreases with increasing BTA concen-tration, but the passivation effect of BTA saturates as the concentration reaches about 0.01 M. Fig. 2 shows the
corro-sion rate of copper film versus HNO3 volume percentage
with and without 0.01 M BTA. Without any BTA, the
cor-rosion rate increases as the HNO3 volume percentage
increases; with the addition of 0.01 M BTA, a very low corrosion rate was obtained unless the volume percentage was higher than 10%. These results indicate that the 0.01 M BTA is an effective corrosion inhibitor for copper in the
region of low HNO3volume percentage (,10%).
Table 1
General specifications of slurries Specifications Slurry type
(1–3 vol.%) HNO3 (0.01 M BTA) H2O2/XJFW8099 (volume ratio: 1/1–1/4) WA355/ FE-10 (0.01 M BTA) pH value 0.85 4.20 1.7
Oxidizer HNO3 H2O2 Fe(NO3)
Abrasive Al2O3 Al2O3 Al2O3
Solid contents (%) 5 18–28 3
Table 2
Experimental parameters and conditions used for Cu-CMP
Platen/carrier speed 20/42 r.p.m.
Down pressure 3.0 p.s.i.
Back pressure 1.5 p.s.i.
Slurry flow rate 150 ml/min
Temperature 29–30°C
Pad Suba 500
Pad condition None
Fig. 1. Corrosion rate of copper film for various BTA molar concentration in 3 vol.% HNO3.
3.2. Effect of BTA on Cu-CMP
Fig. 3 shows the polishing rate distribution of copper film
across a 150-mm diameter wafer using a 1–3 vol.% HNO3
slurry with and without 0.01 M BTA. As shown in Fig. 3a, a higher average polishing rate of 267.4 nm/min as well as a higher non-uniformity of 16.6% were obtained using the 1
vol.% HNO3slurry without BTA. With the addition of 0.01
M BTA, both a lower polishing rate of 249.0 nm/min and a lower non-uniformity of 3.5% were obtained. Thus, better polishing rate non-uniformity can be obtained by adding BTA in the slurry. Increasing the volume percentage of
HNO3from 1 to 3% with BTA added resulted in a slight
increase in polishing rate and nearly the same
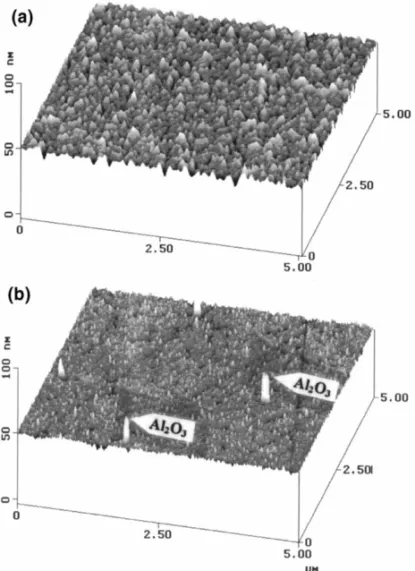
non-unifor-mity, as shown in Fig. 3b. The polished copper film had a shiny and coppery color surface, and the surface roughness was measured to be less than 1.0 nm. Fig. 4 shows the copper surface before and after polishing. Before CMP, the copper film has an average roughness of 1.4 nm, as analyzed using an atomic force microscope shown in Fig. 4a. After CMP, the surface roughness was reduced to within 1.0 nm, but a micro-scratched surface was obtained, as shown in Fig. 4b.
3.3. Effect of H2O2concentration on the corrosion rate of copper film
The corrosion rate of copper film decreases with increas-ing volume ratio of H2O2 in H2O2/XJFW8099 slurry, as
shown in Fig. 5. Without H2O2in the XJFW8099 slurry, a
very low corrosion rate of 4.0 nm/min was obtained; how-ever, a drastic increase of corrosion rate to 78.0 nm/min was obtained by adding a small amount of H2O2(3 vol.%). This
result indicates that the corrosion of copper would be enhanced dramatically when an oxidizer, such as H2O2, is
present in the environment. Increasing the H2O2/XJFW8099
volume ratio to 4 decreases the corrosion rate to 0.54 nm/ min. Instead of rapid corrosion in a slurry containing a small amount of H2O2, an abundance of H2O2in the slurry could
result in a denser and thicker oxide layer formed on copper
Fig. 2. Corrosion rate of copper film with and without addition of 0.01 M BTA at various HNO3volume percentages.
Fig. 3. Polishing rate distribution of copper film across a 150-mm diameter wafer using a slurry of (a) 1 vol.% of HNO3with and without addition of 0.01 M BTA and (b) 1 vol.% and 3 vol.% of HNO3with 0.01 M BTA.
Fig. 4. AFM micrographs showing the features of copper surface (a) before and (b) after polishing.
surface, which could act as a passivation layer to resist chemical attack. Thus, Cu-CMP should be performed in a slurry containing a sufficient amount of H2O2for passivation
of the recessed area.
3.4. Effect of H2O2concentration on Cu-CMP
Cu-CMP was performed using H2O2/XJFW8099 slurry of
various volume ratios of H2O2to XJFW8099 with the
pol-ishing conditions shown in Table 2. The polpol-ishing rate
increases with decreasing volume ratio of H2O2 in the
H2O2/XJFW8099 slurry, as shown in Fig. 6; the increasing
polishing rate also accompanied the increase of non-unifor-mity. There are two possible explanations for this observa-tion as follows. (i) Solid contents of abrasive (Al2O3)
decrease as the volume ratio of H2O2/XJFW8099 is
increased, and the lower abrasive content could result in lower polishing rate. (ii) Chemical corrosion can be sup-pressed in the slurry containing a sufficient amount of H2O2, as concluded from Fig. 5. This implies that higher
contents of H2O2 in the H2O2/XJFW8099 slurry provides
better passivation for the recessed surface of copper during the CMP process; thus, the polishing rate and non-unifor-mity are reduced.
Fig. 7 shows the morphology of the polished surface
using the H2O2/XJFW8099 slurry with different volume
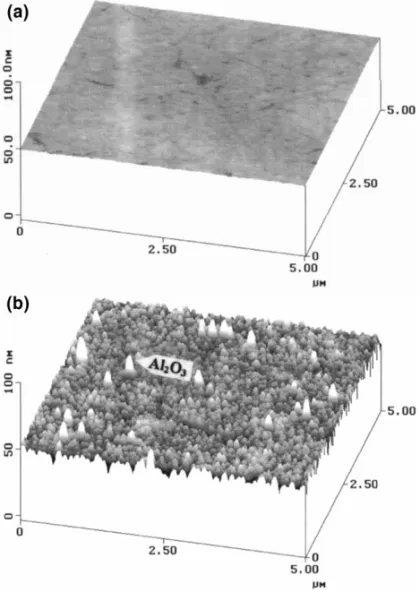
ratios of H2O2to XJFW8099. A very smooth surface with
a removal rate of 363.0 nm/min was obtained using the
slurry of 1/1 ratio, as shown in Fig. 7a. On the other hand, depletion of H2O2in slurry can result in serious corrosion of
copper thus generating a rougher surface, which in turn results in more abrasive residues, as shown in Fig. 7b. Sur-face roughness increases from 0.3 to 0.9 nm as the H2O2/
XJFW8099 ratio decreases from 1/1 to 1/4. Generally, micro-scratches with a depth ranging from 0.5 to 3.0 nm were always found even though a shiny surface was obtained.
3.5. Cu-CMP using WA355/FE-10 slurry
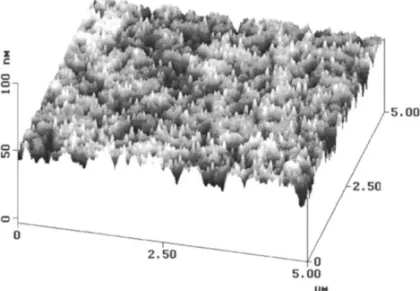
We also examined the performance of commercially available Cabot WA355/FE-10 slurry composite of ferric nitrate oxidizer and abrasives for Cu-CMP. A high level of non-uniformity, as high as 35.8%, and a worse surface were observed. With a 0.01 M BTA added to this slurry, non-uniformity can be reduced to 5.1% and a polishing rate as high as 1.22 mm/min can be achieved. However, the WA355/FE-10 slurry, either with or without BTA, produced a worse surface roughness, as characterized in Fig. 8. The polished surface has a brown color and is not shiny. The ferric-ion-base slurry appear to be unsuitable for Cu-CMP
Fig. 5. Corrosion rate of copper film at various volume ratios of H2O2/ XJFW8099.
Fig. 6. Polishing rate distribution across a 150-mm diameter wafer using H2O2/XJFW8099 slurry and Suba 500 pad.
Fig. 7. AFM micrographs showing the morphology of polished surface using H2O2/XJFW8099 slurry with H2O2to XJFW8099 volume ratios of (a) 1/1 and (b) 1/4.
process, although the addition of a higher concentration of BTA might improve the surface finishing.
4. Conclusions
The effects of corrosion environments on Cu-CMP pro-cess using various types and concentrations of oxidizer have been investigated. Our results show that the addition of 0.01 M BTA in nitric-acid-based slurry resulted in an efficient corrosion inhibition for the copper film. With 0.01 M BTA
in 1 vol.% HNO3slurry, a polishing rate of 249.0 nm/min
and non-uniformity of 3.5% were obtained. For H2O2/
XJFW8099 slurry, a small amount of H2O2 in the slurry
enhanced the corrosion rate dramatically, while an abun-dance of H2O2in the slurry suppressed the corrosion rate
effectively. The polishing rate decreased but non-uniformity was improved with increasing volume ratio of H2O2in the
slurry. Using either H2O2/XJFW8099 or TiNO3/BTA slurry,
we obtained a shiny surface with a surface roughness of less than 1.0 nm, although surface micro-scratch was observed by atomic force microscope. The WA355/FE-10 slurry, however, did not produce an adequate polished surface.
Acknowledgements
This work was supported by the National Science Council (ROC) under Contract No. NSC85-2215-E-009-068.
References
[1] F.B. Kaufman, D.B. Thompson, R.E. Broadie, M.A. Jaso, W.L. Guthrie, D.J. Pearson and M.B. Small, J. Electrochem. Soc., 138 (1991) 3460.
[2] M.A. Fury, Solid State Technol., April (1995) 47. [3] M.A. Fury, Solid State Technol., July (1995) 81.
[4] S. Lakshminarayanan, J.M. Steigerwald, D.T. Price, M. Bourgeois, T.P. Chow, R.J. Gutmann and S.P. Murarka, IEEE Electron Device Lett., EDL-15 (1994) 307.
[5] J.M. Steigerwald, D.J. Duquette, S.P. Murarka and R.J. Gutmann, J. Electrochem. Soc., 142 (1995) 2379.
[6] J.M. Steigerwald, R. Zirpoli, S.P. Murarka, D. Price and R.J. Gut-mann, J. Electrochem. Soc., 141 (1994) 2842.
[7] N. Awaya, H. Inokawa, E. Yamamaoto, Y. Okazaki, M. Miyake, Y. Arita and T. Kobayashi, IEEE Trans. Electron Devices, ED-43 (1996) 1206.
[8] C.K. Hu, B. Luther, F.B. Kaufman, J. Hummel, C. Uzoh and D.J. Pearson, Thin Solid Films, 262 (1995) 84.
[9] Z. Stavreva, D. Zeidler, M. Plo¨tner and K. Drescher, Appl. Surface Sci., 108 (1997) 39.
[10] V. Brusic, M.A. Frisch, B.N. Eldridge, F.P. Novak, F.B. Kaufman and G.S. Frankel, J. Electrochem. Soc., 138 (1991) 2253. [11] R. Carpio, J. Farkas and R. Jairath, Thin Solid Films, 266 (1995) 238. [12] J.F. Wang, A.R. Sethuraman, L.M. Cook, K. Ueno and Y. Tsuchiya,
Proceedings of VMIC 1996 June 18–20, p. 387.
[13] V. Brusic, G.S. Frankel, C.K. Hu, M.M. Plenchaty and G.C. Schwartz, IBM J. Res. Dev., 37 (1993) 173.
[14] M. Ito and M. Takahashi, Surf. Sci., 158 (1985) 609.
[15] L. Cohen, V.A. Brusic, F.B. Kaufman, G.S. Frankel, S. Motakef and B. Rush, J. Vac. Sci. Technol., A8 (1990) 2417.
[16] R.R. Thomas, V.A. Brusic and B.N. Rush, J. Electrochem. Soc., 139 (1992) 678.
[17] C.M. Crory and J.M. Rosamilia, J. Electrochem. Soc., 136 (1982) 105.
Fig. 8. AFM micrographs showing the increased surface roughness result-ing from WA355/FE-10 slurry with 0.01 M BTA.