Experimental and Theoretical Studies of Rate Coefficients for the Reaction O(
3P) +
C
2H
5OH at High Temperatures
†Chih-Wei Wu
Department of Applied Chemistry, National Chiao Tung UniVersity, Hsinchu 30010, Taiwan Yuan-Pern Lee*
Department of Applied Chemistry and Institute of Molecular Science, National Chiao Tung UniVersity, Hsinchu 30010, Taiwan, and Institute of Atomic and Molecular Sciences, Academia Sinica, Taipei 10617, Taiwan Shucheng Xu
Department of Chemistry, Emory UniVersity, Atlanta, Georgia 30322 M. C. Lin
Department of Applied Chemistry and Institute of Molecular Science, National Chiao Tung UniVersity, Hsinchu 30010, Taiwan, and Department of Chemistry, Emory UniVersity, Atlanta, Georgia 30322
ReceiVed: December 28, 2006; In Final Form: March 29, 2007
Rate coefficients of the reaction O(3P) + C2H5OH in the temperature range 782-1410 K were determined using a diaphragmless shock tube. O atoms were generated by photolysis of SO2 at 193 nm with an ArF excimer laser; their concentrations were monitored via atomic resonance absorption. Our data in the range 886-1410 K are new. Combined with previous measurements at low temperature, rate coefficients determined for the temperature range 297-1410 K are represented by the following equation: k(T) ) (2.89 ( 0.09)× 10-16T1.62exp[-(1210 ( 90)/T] cm3molecule-1s-1; listed errors represent one standard deviation in fitting. Theoretical calculations at the CCSD(T)/6-311+G(3df, 2p)//B3LYP/6-311+G(3df) level predict potential energies of various reaction paths. Rate coefficients are predicted with the canonical variational transition state (CVT) theory with the small curvature tunneling correction (SCT) method. Reaction paths associated with trans and gauche conformations are both identified. Predicted total rate coefficients, 1.60× 10-22T3.50 exp(16/T) cm3molecule-1s-1for the range 300-3000 K, agree satisfactorily with experimental observations. The branching ratios of three accessible reaction channels forming CH3CHOH + OH (1a), CH2CH2OH + OH (1b), and CH3CH2O + OH (1c) are predicted to vary distinctively with temperature. Below 500 K, reaction 1a is the predominant path; the branching ratios of reactions 1b,c become∼40% and ∼11%, respectively, at 2000 K.
Introduction
Ethanol (C2H5OH) is an important and versatile renewable energy source; it may be used as a neat fuel, as an oxygenate additive, as a fuel extender in an internal engine via combustion,1 or in a fuel cell via catalytic electrolytic reactions.2Combustion of ethanol fuel might lead to formation of toxic acetaldehyde (CH3CHO); hence detailed modeling of the oxidation processes of ethanol is important. In addition to pyrolysis of C2H5OH, the reaction
is one of the most important processes in combustion of C2H5-OH. Rate coefficients of reaction 1 have been determined in the temperature range 297-886 K by several groups.3-9 Experimental conditions, reported rate coefficients near room temperature, and Arrhenius parameters of these studies are listed
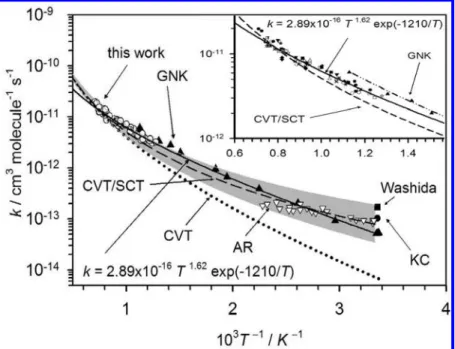
in Table 1 for comparison; the corresponding Arrhenius plots are also shown in Figure 1. Grotheer et al. (designated GNK in Figure 1) employed both discharge-flow and flash photolysis methods to investigate reaction 1 and reported the only measurement of rate coefficient for T > 450 K; rate coefficients in the range 297-886 K may be fitted with the equation
The recommended value in a literature review by Herron employed this expression.10 Owens and Roscoe employed discharge-flow technique coupled with either chemilumines-cence or mass spectrometry detection to determine k1 in the temperature range 301-439 K and reported6
In a later report, after considering effects from regeneration of ethanol from disproportionation reaction of the R-alkanol
†Part of the special issue “M. C. Lin Festschrift”.
* To whom correspondence should be addressed. E-mail: yplee@ mail.nctu.edu.tw. O(3P) + C2H5OH f products (1) k1) 9.88 × 10-19T2.46exp[-(932/T)] cm3molecule-1s-1 (2) k1) (6.95 ( 0.85) × 10-13exp[-(758 ( 204)/T)] cm3molecule-1s-1 (3)
10.1021/jp068977z CCC: $37.00 © 2007 American Chemical Society Published on Web 05/12/2007
radicals, Ayub and Roscoe revised this rate coefficient to become 1.6 times that listed in eq 3,
as shown in Figure 1 and designated as AR.7
Rate coefficients of this reaction show a non-Arrhenius behavior, as prersented in Figure 1 and indicated by eq 2. The rate coefficient is predicted to increase more rapidly at tem-peratures above 500 K, yielding an upward curved Arrhenius plot. However, even in the low-temperature range 301-439 K, rate coefficient described by eqs 4 and 2 vary by as much as 47% with activation energies varied from E/R ) 758 ( 204 to 1800 ( 60 K.6,9Experimental data for temperatures above 886 K, critical to combustion, are lacking.
There are three energetically accessible channels for this reaction at high temperature, as the oxygen atom may attack hydrogen atoms at three distinct positions,
The branching between these channels plays important roles in the formation of the end products, inhibition of flames, formation of soot, and pollution control.11,12Washida used photoionization spectrometry to show that reaction 1a account for 98-100% of the total rate of reaction 1 at 300 K.8Dutton and co-workers employed laser-induced fluorescence under crossed molecular beam conditions to determine the branching ratio for the reaction of O + C2H5OD; they found that reaction 1c is faster than
reactions 1a,b at a translational temperature of 3500 K.13 Marinov compared existing experimental data with branching ratios of reactions of oxygen atoms with methane and propane to predict the temperature dependence of the rate coefficients to be12
According to this model, reaction 1c becomes increasingly important at higher temperatures and reaches a branching ratio similar to that of reaction 1a near 1400 K. By comparison with our previous investigations of the reaction O + CH3OH,14the branching ratio of reaction 1c seems to be greater than expected, especially at low temperatures. To the best of our knowledge, no theoretical investigation on the O + C2H5OH system with high-level quantum-chemical calculations has been reported.
Because of the importance of this reaction in combustion, kinetic data at higher temperatures are needed. We have determined rate coefficients of the title reaction up to 1410 K with a diaphragmless shock tube. We also performed theoretical calculations on this reaction to compare with our experimental measurements and to understand the branching among these three H-abstraction channels at varied temperatures.
Experiments
All experiments were carried out at NCTU. The diaphrag-mless shock tube apparatus and technique have been described previously.15,16The shock tube (length 5.9 m and i.d. 7.6 cm) is coupled with a detection arrangement using atomic resonance absorption. A microwave-discharged lamp with a flowing gas mixture of∼1% O2in He served as a light source for spectral absorption of O atoms. Emission at 130.23, 130.49, and 130.60 nm, corresponding to transitions of O(3S-3P2,1,0), passes perpendicularly through the shock tube near the end before entering into a vacuum UV monochromator (reciprocal linear dispersion 4.0 nm mm-1, slit width 350 µm) before being detected with a solar-blind photomultiplier tube. The speed of the shock wave was determined with four pressure sensors connected to three time-frequency counters for measurements of intervals of arrival signals.
For kinetic measurements, O atoms were generated from SO2 by laser photolysis at 193 nm. At 193 nm, the absorption cross section of SO2is 3.4× 10-18cm2at 1100 K and 2.8× 10-18 cm2at 2000 K.17Light from the ArF excimer laser at 193 nm enters the shock tube from the quartz end-plate and passes along
TABLE 1: Summary of Reported Experimental Rate Coefficients Using Various Methods
temp/K pressure (gas)/Torr 1013k(∼298 K)a 1013Aa (E
a/R)/K methodb ref
298 532-565 (N2O), 10.5-50.5 (C2H5OH) 1.03 SP/GC Kato and Cvetanovic (KC)3
301-439 1.15-1.35 (no O2), 1.28-1.36 (excess O2) 0.88 11.2 ( 1.3 758 ( 204 DF/CL&MS Ayub and Roscoe (AR)7
298 3.70 (NO) 1.70 ( 0.30 DF/MS Washida8
298-886 1.75-2.55 (N2, NO) 0.52 c c DF/RF Grotheer et al. (GNK)9
5.5-220.4 (O2, NO) FP/RF Grotheer et al. (GNK)9
782-1410 613-2039 (Ne) 1340 ( 110d 3040 ( 80d ST/ABS this work
aIn units of cm3molecule-1s-1.bKey: SP, sensitized photolysis; GC, gas chromatography; DF, discharge flow; CL, chemiluminescence; MS, mass spectrometry; FP, flash photolysis; RF, resonance fluorescence; ST, shock tube; ABS, absorption.ck(T) ) 9.88× 10-19T2.46exp[-932/T]
cm3molecule-1s-1.dk(T) ) (2.89 ( 0.09)× 10-16T1.62exp [-(1210 ( 90)/T] cm3molecule-1s-1from combined data of this work and Grotheer et al.
Figure 1. Arrhenius plots of previously reported k1for the reaction O
+ C2H5OH: GNK (2);9AR(3);7KC (b);3Washida (9).8Fitted results
are also shown as lines of various types drawn for the temperature range of study. A combination of first character of each author’s last name is used to indicate previous reports, as listed in Table 1.
k1) (1.12 ( 0.13) × 10-12exp[-(758 ( 204)/T)] cm3molecule-1s-1 (4) O(3P) + C2H5OH f OH + CH3CHOH (1a) f OH + CH2CH2OH (1b) f OH + CH3CH2O (1c) k1a) 3.12 × 10-17T1.85exp[-(918/T)] cm3molecule-1s-1 (5) k1b) 1.56 × 10-16T1.70exp[-(2747/T)] cm3molecule-1s-1 (6) k1c) 2.62 × 10-17T2.00exp[-(2239/T)] cm3molecule-1s-1 (7)
the tube. A pulse generator was employed to trigger the photolysis laser about 50-150µs after the arrival of the incident shock wave detected with the pressure sensor located closest to the end-plate.
Before each experiment, the system was pumped below 5.0 × 10-7Torr. The temperature (T5), density (F5), and pressure (P5) in the reflected shock regime were calculated from measured velocity of the incident shock and the initial pressure, temperature, and composition of the test gas using the ideal shock-wave theory18 with Mirels’ boundary layer correc-tions.19,20Because we are interested in the temperature range 780-1400 K, we employed Ne instead of Ar as the buffer gas. We calibrated the concentration of O atoms in the shock tube with pyrolysis of N2O by assuming a 100% yield of O atoms.21 The concentration of O atoms is fitted with the equation
in which absorbance A ) ln(I0/I) is calculated with the light intensity before and after production of O atoms, denoted as I0 and I, respectively.
He (99.9995%, AGA Specialty Gases), Ne (99.999%, AGA Specialty Gases), N2O (99.999%, Scott Specialty Gases), O2 (99.995%, Scott Specialty Gases), and SO2(99.98%, Matheson) were used without further purification. C2H5OH (99.8%, Mallinckrodt, Analytical Reagent grade) was purified by passing the vapor through P2O5to remove trace water impurity. Mixtures of C2H5OH in Ne (40-442 ppm) and SO2 in Ne (192-699 ppm) were used. The concentration of C2H5OH in Ne was carefully calibrated with IR absorption in a multipass absorption cell.
Computational Methods
The geometries of reactants, intermediates, transition states, and products, including gauche- and trans-conformers, of the O + C2H5OH system were optimized at the B3LYP/6-311+G-(3df) level of theory. Single-point energies of all species were calculated with the CCSD(T)/6-311+G(3df, 2p) method,22based on the optimized geometries.
Rate coefficients for different reaction paths in the temper-ature range of T ) 300-3000 K were calculated with canonical variational transition state theory (CVT) with zero curvature tunneling corrections (ZCT) and small curvature tunneling corrections (SCT) using the POLYRATE program of Truhlar et al.23
All calculations were carried out with Gaussian 0324programs using a PC cluster and the computers at the Emerson Computa-tion Center of Emory University.
Results and Discussion
Thermal decomposition of C2H5OH at high temperature should be considered before characterizing the title reaction. The decomposition of C2H5OH not only decreases its concentra-tion but also triggers a series of secondary reacconcentra-tions involving either O atoms or other reactive intermediates. Despite extensive experimental investigations of thermal decomposition of C2H5-OH, the branching of each channel remains uncertain.25We rely on theoretical predictions of the branching ratio of pyrolysis in the modeling.26-30According to the prediction, below 10 atm and in the temperature range 700-2500 K, the dominant channel is the formation of C2H4and H2O; at the high-pressure limit and T > 1500 K, formation of CH3 + CH2OH becomes
dominant and the channel to form CH3CH2+ OH also becomes competitive. All experiments were carried out at T < 1450 K to minimize complications due to thermal decomposition of C2H5OH.
A. Rate Coefficient k1for O + C2H5OH. Experiments were
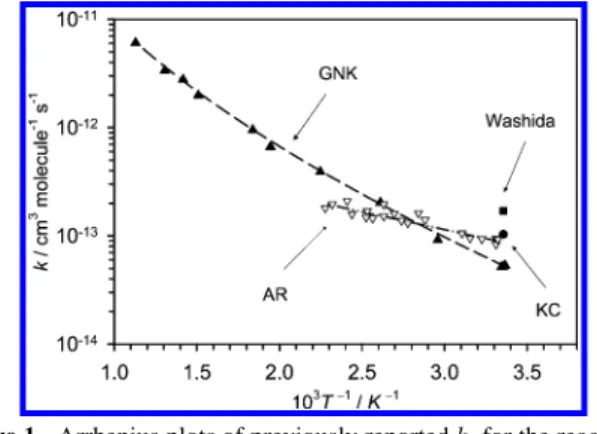
carried out under pseudo-first-order conditions with [C2H5OH]0 . [O]. Figure 2 shows a typical temporal profile recorded for the mixture containing SO2, C2H5OH, and Ne after laser photolysis at 193 nm. The concentration of O atoms at reaction period t, [O]t, is derived according to eq 8. [O]t follows an exponential decay in the initial stage. The apparent pseudo-first-order rate coefficient kIis derived with the equation
in which a and b are fitting parameters to account for small deviation from the exponential decay due to secondary reactions. The apparent bimolecular rate coefficient, k′1, is thus derived from
Comparison of k′1with the true bimolecular rate coefficient k1 provides information on the extent of interference due to secondary reactions.
At low temperature, previous experiments indicate that reaction 1 is dominated by channel (1a).8 Washida used photoionization spectrometry to show that reaction 1a account for 98-100% of the total rate of reaction 1 at 300 K. However, according to Marinov,12as temperature increases, reaction 1c becomes more important and becomes the most important channel above 1400 K. Our theoretical calculations (discussed in section C) show that reaction 1c is unimportant under our experimental conditions and the branching ratio of reaction 1b increases from∼0.15 at 1000 K to ∼0.28 at 1400 K. Because we are only probing the decay of [O] and cannot distinguish among channels (1a)-(1c) in our experiments, in our model we employed branching ratios calculated theoretically in this work to derive the total decay coefficient. Because of the small branching ratio for reactions 1b,c, the errors in these branching ratios do not affect much the value of total rate coefficient.
Photolysis of SO2at 193 nm is quite efficient in generating O atoms, thus enabling us to use smaller concentrations of SO2. In our previous experiments with CH3OH, no significant [O]/1013molecule cm-3) 4.499A - 3.208A2+ 4.356A3
(8)
Figure 2. A typical temporal profile of [O] observed after irradiation
of a sample containing SO2(300 ppm) and C2H5OH (80 ppm) in Ar. T ) 1135 K, and total density ) 1.20× 1019molecule cm-3. The thick solid line represents fitted results using the model described in text.
ln([O]t/[O]0) ) -kIt + at2- bt3 (9)
TABLE 2: Reaction Models Employed to Derive Rate Coefficients of O + C2H5OH
no. reacn rate expression ref
*1a O + C2H5OH f OH + CH3CHOH fitted
*1b O + C2H5OH f OH + CH2CH2OH k1b/k1acalcd from theory
*1c O + C2H5OH f OH + CH3CH2O k1c/k1acalcd from theory
*2 O + OH f O2+ H 2.3× 10-11exp(110 ( 100/T) 14 3 O + H2f OH +H 8.44× 10-20T2.67exp(-3167/T) 40 4 OH + OH f O + H2O 5.93× 10-20T2.4exp(1063/T) 41 5 H2O2+ H f OH + H2O 1.7× 10-11exp(-1800/T) 42 6 H2O2+ O f OH + HO2 1.6× 10-17T2exp(-2000/T) 43 7 CH3+ CH3(+M) f C2H6(+M) k ) 1.5× 10-7T-1.18exp(-329/T) 14a,b k0) 8.77 × 10-7T-7.03exp(-1389/T) 8 CH3+ H (+M) f CH4(+M) k ) 2.31× 10-8T-0.534exp(-270/T) 14a,c k0) 7.23 × 10-15T-4.76exp(-1227/T) 9 CH4+ H f CH3+ H2 3.65× 10-20T3exp(-4401/T) 44 10 CH4+ O f CH3+ OH 4.7× 10-10exp(-6506/T) 45 *11 CH3+ O f CH2O + H (1.41( 0.17)× 10-10 14 12 CH3+ OH f CH2+ H2O 1.2× 10-11exp(-1400/T) 46 13 CH3+ OH (+M) f CH3OH (+M) k∞) 1.45 × 10-10T0.1 12a,d k0) 1.59 × 10-6T-7.4exp(-315/T) 14 CH3OH + O f CH2OH + OH 8.80× 10-20T2.61exp(-941/T) 14 15 CH3OH + O f CH3O + OH 4.15× 10-23T3.64exp(-974/T) 14 16 CH2O + OH f HCO + H2O 6.47× 10-11exp(-705/T) 14 17 CH2O + H f HCO + H2 3.62× 10-16T1.77exp(-1509/T) 14 18 CH2O + O f HCO + OH 3.0× 10-11exp(-1552/T) 14 *19 C2H5OH f CH2OH + CH3 4.46× 1066T-15.18exp(-53930/T) 28 20 C2H5OH f C2H4+ H2O 2.22× 1038T-7.56exp(-38450/T) 28 21 C2H5OH + OH f C2H4OH + H2O 2.89× 10-13T0.27exp(-302/T) 12 *22 C2H5OH + OH f CH3CHOH + H2O 4.1× 10-12exp(-70 ( 200/T) 47 23 C2H5OH + OH f CH3CH2O + H2O 1.24× 10-12T0.3exp(-822/T) 12 24 C2H5OH + H f C2H4OH + H2 3.12× 10-21T3.2exp(-3598/T) 29 *25 C2H5OH + H f CH3CHOH + H2 2.98× 10-19T2.53exp(-1721/T) 29 26 C2H5OH + H f CH3CH2O + H2 9.22× 10-47T10.6exp(2244/T) 29 27 C2H5OH + CH3f C2H4OH + CH4 5.48× 10-22T3.3exp(-6185/T) 30 28 C2H5OH + CH3f CH3CHOH + CH4 3.31× 10-23T3.37exp(-3842/T) 30 29 C2H5OH + CH3f CH3CH2O + CH4 3.38× 10-24T3.57exp(-3886/T) 30 30 CH3CH2O + M f CH3CHO + H + M 1.93× 1011T-5.89exp(-12713/T) 12 31 CH3CH2O + M f CH3+ CH2O + M 2.24× 1014T-6.96exp(-11972/T) 12 32 CH3CHOH + CH3f C3H6+ H2O 3.32× 10-11 12 *33 CH3CHOH + O f CH3CHO + OH 1.66× 10-10 10 34 CH3CHOH + H f CH3+ CH2OH 3.32× 10-11 48
*35 CH3CHOH + M f CH3CHO + H + M 1.66× 10-10exp(-12575/T) 12
36 CH3CHO + O f CH3CO + OH 2.94× 10-6T-1.9exp(-1496/T) 12
37 CH3CHO + O f CH2CHO + OH 6.18× 10-11T-0.20exp(-1789/T) 12
38 CH3CHO + H f CH3CO + H2 7.74× 10-11T-0.35exp(-1503/T) 12
39 CH3CHO + H f CH2CHO + H2 3.07× 10-12T0.40exp(-2696/T) 12
40 CH2CHO f CH2CO + H 1.81× 1043T-9.61exp(-23072/T) 12 41 C2H6+ H f C2H5+ H2 8.97× 10-22T3.5exp(-2620/T) 44 42 C2H6+ O f C2H5+ OH 4.98× 10-17T2exp(-2573/T) 44 43 C2H6+ OH f C2H5+ H2O 1.2× 10-17T2exp(-435/T) 42 44 C2H5+ H f C2H4+ H2 2.08× 10-10exp(-4024/T) 49 45 C2H5+ H f CH3+ CH3 6.00× 10-11 42 46 C2H5+ H f C2H6 4.98× 10-11 12 47 C2H5+ OH f C2H4+ H2O 4.00× 10-11 43 48 C2H5+ O f CH3+ CH2O 2.16× 10-10 10 *49 C2H4+ O f CH3+ HCO (1.35 ( 0.24)× 10-17T1.88exp(-90/T) 50 50 C2H4+ O f CH2CHO + H 7.88× 10-18T1.88exp(-90/T) 50 51 C2H4+ H (+M) f C2H5(+M) k∞) 1.79 × 10-12T0.45exp(-916/T) 12a,e k0) 3.06 × 10-14T-5exp(-2237/T) 52 O + SO f S + O2 3.0× 10-11exp(-6980/T) 51 53 O + SO + M f SO2+ M 3.3× 10-26T-1.84 52 *54 O + SO2+ M f SO3+ M 4.0× 10-32exp(-1000 ( 200/T) 47 55 O + SO2f O2+ SO2 8.30× 10-12exp(-9800/T) 53 56 O + CH2OH f CH2O + OH 7.0× 10-11 54 57 CH2OH (+M) f CH2O + H (+M) k∞) 2.80 × 1014T-0.73exp(-16509/T) 54a,f k0) 9.98 × 109T-5.39exp(-18209/T) ak
0and k∞refer to low- and high-pressure limits, respectively. The Fcparameters in the Troe equation are listed separately. Unless otherwise
noted, all species are assumed to have a third body efficiency of 1.0.bF
c) (1 - 0.619) exp[-(T/73.2)] + 0.619 exp[-(T/1180)]. Enhanced third
body coefficient (relative to N2): ηAr) 0.7.cFc) (1 - 0.783) exp[-(T/74)] + 0.783 exp[-(T/2941)] + exp[-(6964/T)]. Enhanced third body
coefficient (relative to N2): ηAr) 0.7.dFc) (1 - 0.025) exp[-(T/1 × 10-15)] + 0.025 exp[-(T/8000)] + exp[-(3000/T)]. Enhanced third body
coefficient: ηH2O) 10.0; ηH2) 2.0; ηCO2) 3.0; ηCO) 2.0.eFc) exp[-(T/95)] + exp[-(200/T)]. Enhanced third body coefficient: ηH2O) 5.0;
ηH2) 2.0; ηCO2) 3.0; ηCO) 2.0. fF
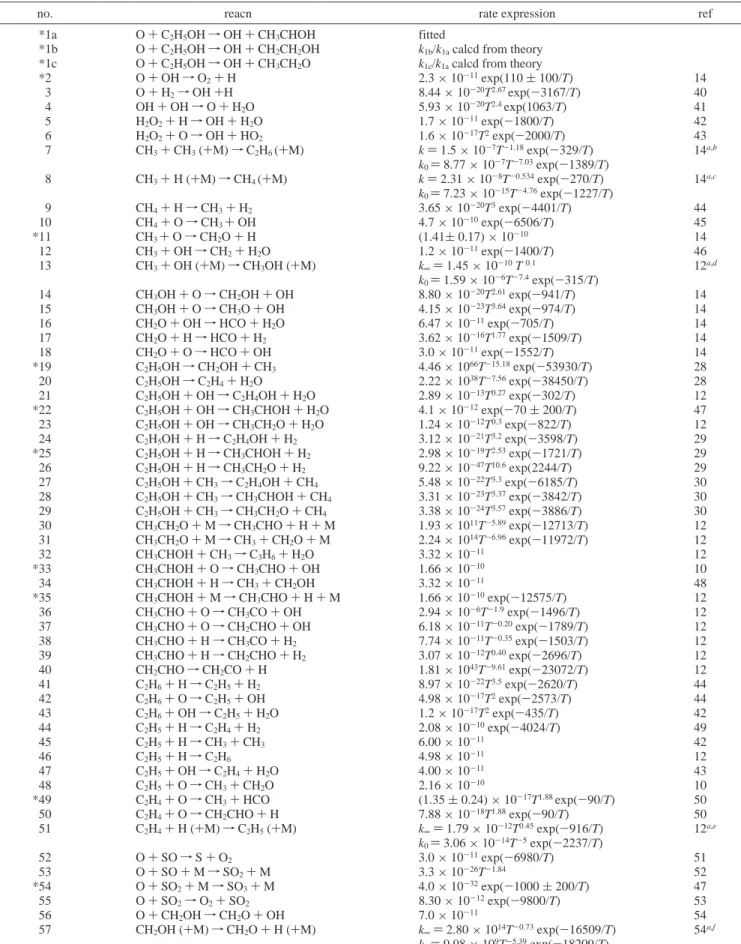
TABLE 3: Experimental Conditions and Rate Coefficients k1for the Reaction O + C2H5OHa
P1/Torr P4/Torr Ms T5/K 10-15[SO2] 10-13[O] 10-14[C2H5OH] 1012k1 k1′/ k1
80 ppm C2H5OH + 300 ppm SO2 56.39 2334 2.28 1300 2.81 2.37 7.51 (13.9 ( 0.3) 0.87 78.84 2339 2.12 1135 3.59 3.17 9.60 (8.9 ( 0.1) 1.11 70.60 2348 2.18 1196 3.33 2.94 8.91 (10.9 ( 0.2) 1.11 90.66 2302 2.03 1056 3.92 3.62 10.48 (7.7 ( 0.2) 1.25 128.40 2316 1.90 934 5.05 4.59 13.48 (5.1 ( 0.1) 1.35 166.80 2327 1.80 853 6.06 6.58 16.20 (3.5 ( 0.1) 1.93 80 ppm C2H5OH + 502 ppm SO2 178.40 2322 1.74 803 10.30 9.43 16.41 (3.1 ( 0.1) 1.78 170.00 2300 1.80 852 10.30 6.40 16.50 (3.8 ( 0.1) 1.71 173.60 2338 1.76 819 10.20 8.06 16.25 (3.4 ( 0.1) 1.94 99.50 2324 1.98 1008 6.95 5.95 11.10 (6.9 ( 0.1) 1.57 140.20 2343 7.85 894 8.87 6.94 14.18 (4.7 ( 0.1) 1.73 68.25 2369 2.18 1194 5.38 4.59 8.61 (10.7 ( 0.2) 1.26 164 ppm C2H5OH + 206 ppm SO2 57.07 2305 2.26 1275 1.92 2.18 15.37 (11.4 ( 0.3) 1.29 79.01 2310 2.12 1137 2.47 2.59 19.71 (8.2 ( 0.2) 1.04 70.79 2324 2.18 1192 2.28 2.61 18.24 (10.2 ( 0.3) 0.82 90.98 2319 2.06 1080 2.74 3.26 21.87 (7.1 ( 0.2) 1.19 128.50 2298 1.89 924 3.42 4.38 27.34 (5.2 ( 0.1) 1.13 167.30 2306 1.80 847 4.13 4.91 33.02 (3.8 ( 0.1) 1.12 126.10 2314 1.90 936 3.40 3.73 27.12 (4.7 ( 0.1) 1.46 81 ppm C2H5OH + 333 ppm SO2 91.80 2370 2.08 1095 4.52 7.44 10.94 (9.0 ( 0.2) 1.30 55.90 2345 2.31 1331 3.13 5.21 7.59 (15.6 ( 0.3) 1.12 55.03 2340 2.32 1341 3.10 4.98 7.50 (13.9 ( 0.2) 1.26 81.21 2340 2.12 1134 4.10 6.44 9.92 (9.1 ( 0.2) 1.31 71.05 2341 2.20 1214 3.76 6.29 9.10 (11.4 ( 0.2) 1.17 130.10 2346 1.90 934 5.66 9.63 13.72 (5.7 ( 0.1) 1.72 64.30 2327 2.24 1253 3.47 5.74 8.40 (14.5 ( 0.2) 0.99 81 ppm C2H5OH + 214 ppm SO2 55.07 2345 2.33 1348 2.00 3.70 7.54 (13.9 ( 0.2) 1.17 92.00 2351 2.08 1095 2.91 6.09 10.97 (8.9 ( 0.1) 1.24 70.50 2350 2.21 1227 2.41 4.66 9.09 (11.5 ( 0.2) 1.19 129.50 2349 1.93 958 3.70 9.42 13.94 (6.1 ( 0.1) 1.58 81.26 2341 1.97 999 2.40 5.42 9.04 (7.0 ( 0.1) 1.48 158.00 2382 1.85 890 4.25 11.28 16.00 (4.6 ( 0.1) 1.88 442 ppm C2H5OH + 192 ppm SO2 56.40 2340 2.20 1223 1.73 2.93 39.77 (9.0 ( 0.2) 1.10 92.96 2336 2.06 1078 2.61 4.31 60.06 (6.7 ( 0.2) 1.05 70.03 2352 2.21 1223 2.15 3.40 49.38 (8.6 ( 0.2) 1.22 144.80 2295 1.87 905 3.55 5.99 81.52 (4.7 ( 0.1) 1.46 164 ppm C2H5OH + 302 ppm SO2 71.04 2347 2.21 1225 3.42 4.02 18.56 (10.9 ( 0.2) 1.12 55.08 2340 2.32 1339 2.81 3.78 15.22 (15.1 ( 0.4) 1.14 91.28 2369 2.09 1106 4.10 5.25 22.24 (8.1 ( 0.2) 1.41 55.46 2350 2.31 1331 2.82 3.07 15.28 (12.5 ( 0.3) 1.09 149.60 2370 1.87 908 5.76 8.12 31.25 (4.5 ( 0.1) 1.63 85.96 2370 2.11 1130 3.92 4.34 21.26 (7.5 ( 0.2) 1.14 180.50 2370 1.83 876 6.75 9.26 36.58 (3.4 ( 0.1) 1.93 40 ppm C2H5OH + 206 ppm SO2 55.22 2350 2.32 1339 1.92 2.16 3.72 (12.4 ( 0.2) 1.40 90.51 2350 2.06 1078 2.72 2.79 5.28 (8.0 ( 0.2) 1.04 71.16 2335 2.04 1061 2.11 2.37 4.10 (7.4 ( 0.1) 1.57 67.84 2350 1.72 782 1.56 1.40 3.02 (3.2 ( 0.1) 1.88 55.23 2347 2.33 1344 1.92 1.98 3.73 (14.1 ( 0. 5) 1.22 68.83 2350 2.23 1246 2.29 2.31 4.44 (10.7 ( 0.3) 1.10 80.22 2416 2.17 1182 2.57 2.82 4.99 (10.3 ( 0.2) 1.04 165 ppm C2H5OH + 504 ppm SO2 59.18 2351 2.29 1307 4.97 5.92 16.22 (12.5 ( 0.3) 1.30 92.45 2348 1.97 997 6.43 7.72 20.98 (7.2 ( 0.2) 1.60 71.01 2357 2.22 1232 5.74 5.78 18.74 (13.3 ( 0.6) 1.05 89.21 2375 2.10 1117 6.75 7.15 22.02 (7.6 ( 0.2) 1.33 130.00 2350 1.93 962 8.78 10.15 28.67 (5.6 ( 0.2) 1.48 58.44 2450 2.31 1331 4.96 4.78 16.20 (12.5 ( 0.3) 1.08 154.30 2358 1.86 900 9.87 9.55 32.20 (4.3 ( 0.1) 1.86 80 ppm C2H5OH + 699 ppm SO2 58.30 2348 1.82 863 4.98 3.87 5.73 (4.3 ( 0.1) 1.53
variations in derived rate coefficients were observed for pho-tolysis of SO2at 193 and 248 nm. The absorption cross section of C2H5OH at 193 nm, 6.5× 10-19cm2,31is similar to that of CH3OH, 3.2× 10-19cm2;32hence, the effect of photolysis of C2H5OH at 193 nm is small.
Several interference reactions need to be considered. Ac-cording to modeling, at 1500 K and [C2H5OH]0) 2.66 × 1015, [SO2]0) 2.74 × 1015, and [Ne] ) 1.33× 1019molecule cm-3, less than 20% of C2H5OH decomposes within 90 µs; they proceed via the following paths:
The products CH2OH and CH3react rapidly with O atoms
Hence, subsequent reactions involving H and OH need to be considered. Reactions of O atoms with the major product of reaction 1,
also needs consideration; this reaction is responsible for production of OH in addition to the title reaction.
Because we used SO2as the source of O atoms, reactions involving SO, SO2, and SO3should also be considered:14
We modeled observed temporal profiles of [O] with a commercial kinetic modeling program FACSIMILE.33 The reactions employed in the model are listed in Table 2; the program is basically a simplified version of that employed by Marinov12with additional reactions involving S and SOx and with updated rate coefficients. The rate coefficients are obtained from listed literature unless noted. It should be noted that
inclusion of 57 reactions in the model is only for completeness. If we use a further simplified model with 10 major reactions (marked with an asterisk in Table 2), the results are within 5% of those derived with a more complete model.
Because the laser was triggered about 50-150µs after arrival of the reflected shock wave at the observation zone, pyrolysis of C2H5OH before generation of O atoms should be taken into account, especially at high temperature. We modeled these reactions in two separate periods: the first period started from the arrival of the reflected shock wave and ended with the arrival of the photolysis laser pulse, and the second period started on arrival of the photolysis laser pulse. In the first period, we used [O] ) 0 to derive concentrations of all reactants and intermedi-ates at the end of this period, which were then employed in the second period, along with experimentally observed concentration of laser-produced O atoms, to model the temporal profile of [O]. In the fitting, the branching ratio of the title reaction was calculated quantum-chemically in this work, literature values of rate coefficients of all reactions in the model except the title reaction k1 were held constant, and the bimolecular rate coefficient k1was varied to yield the best fit.
Experimental conditions and values of k1for 71 measurements in a temperature range 782-1410 K using mixtures of various concentrations of C2H5OH (40-442 ppm) and SO2(192-699 ppm) are summarized in Table 3. Ranges of reactant concentra-tions are as follows: [C2H5OH]0 ) (0.30-8.15) × 1015 molecule cm-3; [SO2]0) (1.56-10.3) × 1015molecule cm-3; [O]0 ) (1.40-11.3) × 1013molecule cm-3; [Ne] ) (6.80-22.4)× 1018molecule cm-3. There is no obvious systematic deviation for a specific set of data, supporting that our model is adequate. Values of k′1/k1, also listed in Table 3, indicate that the pseudo-first-order model in general yields rate coefficient greater by as much as 2.1 times the true value and secondary reactions should be taken into account in these cases. Typically the deviation is smaller when [C2H5OH]/[O] and the temperature are greater.
Sensitivity analysis has been performed for representative conditions near 1100, 1200, and 1300 K; the results are shown in Table 4. The rate coefficient of the title reaction is most sensitive to variations of rate coefficients of reactions 11, 25, 33, 35, and 54 at low temperatures and reactions 11, 19, 30, 33, 35, and 54 at high temperatures. In most cases, at temperatures below 1450 K the rate coefficient k1 varies by less than 20% if the rate coefficient of one of the above reactions was varied by a factor of 2. In the extreme case at temperatures near 1450 K at which pyrolysis of C2H5OH
TABLE 3: (Continued)
P1/Torr P4/Torr Ms T5/K 10-15[SO2] 10-13[O] 10-14[C2H5OH] 1012k1 k1′/ k1
80 ppm C2H5OH + 699 ppm SO2 58.61 2372 1.82 863 5.01 3.31 5.76 (3.6 ( 0.1) 1.6 8 55.37 2392 1.82 867 4.75 3.15 5.46 (4.7 ( 0.1) 1.45 58.84 2390 1.76 816 4.78 3.03 5.50 (3.8 ( 0.1) 1.83 53.06 2392 2.05 1073 5.41 4.98 6.21 (7.7 ( 0.2) 1.78 49.00 2397 2.39 1410 5.97 5.44 6.86 (19.7 ( 0.7) 1.34 51.13 2392 2.27 1287 5.90 5.31 6.77 (14.0 ( 0.5) 1.22 52.16 2392 2.36 1381 6.28 6.06 7.21 (18.7 ( 0.6) 1.55 40 ppm C2H5OH + 404 ppm SO2 55.51 2364 2.32 1339 3.79 4.63 3.72 (15.4 ( 0.4) 1.18 90.29 2331 2.07 1085 5.36 6.13 5.27 (8.4 ( 0.2) 1.53 65.53 2335 2.22 1240 4.27 5.46 4.19 (14.0 ( 0.2) 1.42 99.56 2337 2.04 1058 5.81 7.58 5.70 (8.1 ( 0.2) 1.87 129.10 2335 1.93 964 7.00 8.16 6.88 (5.1 ( 0.1) 2.13 99.60 2335 2.03 1056 5.80 8.45 5.69 (8.4 ( 0.2) 1.83 aKey: P
1, pressure of reactant gas mixture; P4, pressure of driver gas; Ms, Mach number; T5, temperature of reaction. Concentrations are in units
of molecule cm-3, k1in cm3molecule-1s-1are fitted with kinetic modeling, and k′1are derived from pseudo-first-order decays; see text.
C2H5OH (+M) f C2H4+ H2O (+M) (11a) f CH2OH + CH3(+M) (11b) O + CH2OH f CH2O + OH (12) O + CH3f CH2O + H (13) O + CH3CHOH f CH3CHO + OH (14) O + SO f S + O2 (15a) O + SO (+M) f SO2(+M) (15b) O + SO2(+M) f SO3(+M) (16a) O + SO2f SO + O2 (16b)
becomes more important, we found that rate coefficient k1would increase by as much as 15% if k11 were neglected in the model.
Some representative decay curves covering the whole tem-perature range of study were also modeled with a complete model consisting of 372 reactions employed by Marinov,12with updated rate coefficients for reactions 2, 3, 18, 19, 21, 25, 29, 34, 46, 47, 60, 107, 108, 110, 130, 132, 135, 137, 138, 139, 143, 144, 145, 159, 191, 193, 204, and 205 in their model, reactions involving sulfur compounds listed as reactions 15 and 16 in this paper, and two additional reactions (reactions 15 and 57 in Table 3) not included in the model of Marinov.12Derived rate coefficients are similar to those listed in Table 3 using our model, with deviations less than 3%.
We tested the effect of branching ratio k1a/(k1a+ k1b+ k1c) on derived total rate coefficient k1by using the ratios proposed by Marinov12and found that derived rate coefficient k1varied
by at most 5%. We also tested the effect of thermal decomposi-tion of C2H5OH before its reacdecomposi-tion with O atoms. Thermal decomposition of C2H5OH has two effects: the decrease in [C2H5OH] and effects due to secondary reactions involving pyrolysis products CH3and CH2OH. When we took out the simulation of the first period (i.e., to assume that thermal decomposition of C2H5OH was negligible before the photolysis laser arrived), we found that fitted rate coefficients k1increased by <1% for reaction temperatures below 1200 K, indicating that the small decrease in [C2H5OH] during the first period was negligible. In contrast, we found that k1increased by <5% for temperatures at 1410 K because 6% C2H5OH dissociated in the first period.
Calculated branching ratios of thermal decomposition of ethanol, R19 and R20 in Table 2, in the literature have large discrepancies. The rate coefficients of R19 predicted by Li et al.34are smaller by factors of 0.75 and 0.40 at 1400 and 1000
TABLE 4: Sensitivity Factors Dln [O]/Dln k of Important Reactions in the Mechanism for the System O + C2H5OH under Various Experimental Conditions
top 10 important reacns
expt conditns at∼1300 K R11 R19 R23 R30 R31 R33 R35 R37 R49 R54
C2H5OH (40 ppm) + SO2(652 ppm) -0.24 -0.14 -0.03 0.04 -0.04 -0.03 0.03 -0.07 -0.07 -0.14
C2H5OH (165 ppm) + SO2(504 ppm) -0.87 -0.28 -0.19 0.28 -0.17 -0.24 0.25 -0.17 -0.16 -0.38
C2H5OH (164 ppm) + SO2(206 ppm) -0.49 -0.19 -0.09 0.11 -0.11 -0.14 0.13 -0.07 -0.09 -0.14
top 10 important reacns
expt conditns at∼1200 K R8 R11 R23 R25 R30 R31 R33 R35 R37 R54
C2H5OH (442 ppm) + SO2(192 ppm) 0.02 -0.26 -0.07 -0.17 0.08 -0.07 -0.41 0.30 -0.04 -0.04
C2H5OH (165 ppm) + SO2(504 ppm) 0.12 -0.56 -0.20 -0.08 0.20 -0.19 -0.34 0.35 -0.13 -0.45
C2H5OH (164 ppm) + SO2(206 ppm) 0.05 -0.31 -0.08 -0.10 0.12 -0.09 -0.24 0.22 -0.06 -0.18
top 10 important reacns
expt conditns at∼1100 K R8 R11 R21 R23 R25 R30 R31 R33 R35 R54
C2H5OH (442 ppm) + SO2(192 ppm) 0.01 -0.16 0.04 -0.03 -0.14 0.04 -0.03 -0.49 0.23 -0.08
C2H5OH (165 ppm) + SO2(504 ppm) 0.17 -0.47 0.10 -0.10 -0.36 0.12 -0.10 -0.68 0.63 -0.58
C2H5OH (164 ppm) + SO2(206 ppm) 0.07 -0.28 0.06 -0.06 -0.23 0.07 -0.06 -0.51 0.40 -0.22
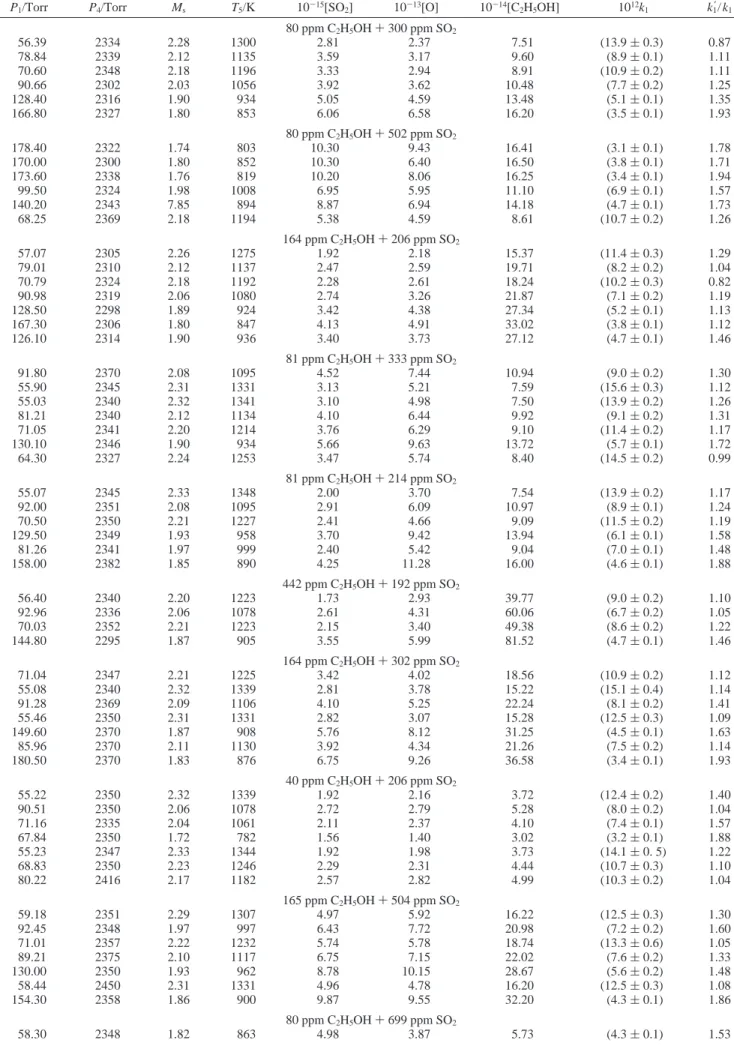
Figure 3. Comparison of experimental total rate coefficient k1with theoretical calculations: medium dash line, CVT with SCT tunneling correction;
dotted line, CVT; O, this work; 2, GNK;9
3, AR;7b, KC;39, Washida.8Inset: Expanded view of data from this work. Key: O, SO2(300 ppm)
+ C2H5OH (80 ppm); 0, SO2(502 ppm) + C2H5OH (80 ppm);×, SO2(206 ppm) + C2H5OH (164 ppm); 1, SO2(333 ppm) + C2H5OH (81 ppm);
9, SO2(214 ppm) + C2H5OH (81 ppm); 0, SO2(192 ppm) + C2H5OH (442 ppm); 0, SO2(302 ppm) + C2H5OH (164 ppm); 0, SO2(206 ppm)
+ C2H5OH (40 ppm); 0, SO2(504 ppm) + C2H5OH (165 ppm); b, SO2(699 ppm) + C2H5OH (80 ppm); 4, SO2(404 ppm) + C2H5OH (40 ppm);
K, respectively, than those predicted by Park et al.28In contrast, rate coefficients of R20 predicted by Li et al.34are greater by factors of 4.8 at 1400 K and 2.9 at 1000 K than those predicted by Park et al.28We employed these rate coefficients reported by Li et al. in our model and derived rate coefficients of the title reaction k1smaller by <6% than those using the smaller values of Park et al.; the largest deviation was observed at higher temperatures and with greater concentration of SO2.
Values of k1are compared with previous reports in Figure 3; an expanded view is shown in the inset. Fitting our results to an Arrhenius equation yields
for 782 < T/K < 1410, in which listed errors represent one standard deviation in fitting, unless otherwise noted. Observed value of Ea/R ) 3040 K is much greater than the value Ea/R ) 758 K reported previously from measurements at temperatures 301-439 K,6,7indicating clearly the non-Arrhenius temperature dependence with an upward curvature. Our rate coefficients are smaller by 23-28% than those reported by Grotheer et al. in the overlapped range of temperature 782-886 K;9the deviations are within experimental error limits. Our work extends the temperature range of study from 886 to 1410 K. Fitting combined data for this work and those of Grotheer et al. yields
B. Potential Energy Surfaces and Reaction Mechanism.
As shown in Figure 4, the C2H5OH has trans (dihedral angle φ(HOCC) ) 180.0°) and gauche (dihedral angle φ(HOCC) ) 60.3°) conformers. They can transform to each other via a transition state t-g-C2H5OH-TS with a small barrier of 0.7 kcal mol-1. The oxygen atom reacts with both conformers. The potential energy diagram obtained by single-point CCSD(T)/ 6-311+G(3df, 2p) calculations on the basis of geometries optimized at the B3LYP/6-311+G(3df) level is presented in Figure 5. Total energies of the reactants and relative energies of the transition states and products are listed in Table 5. Vibrational wavenumbers and moments of inertia of all species are summarized in Table 6.
The oxygen atom may attack C2H5OH at one of the two hydrogen atoms of the CH2group (reaction 1a), one of the three hydrogen atoms of the CH3group (reaction 1b), or the H atom of the hydroxyl group (reaction 1c). As shown in Figure 5, the trans path of reaction 1a proceeds via trans-TS1(dihedral angle φ(HOCC) ) 174.0°) with a barrier of 4.2 kcal mol-1and forms trans-CH3CHOH and OH with energy -6.6 kcal mol-1relative to that of the reactants. The corresponding gauche path has a barrier of 4.3 kcal mol-1 via gauche-TS1 (dihedral angle φ-(HOCC) ) -40.6°) and∆H of -6.3 kcal mol-1for formation of cis-CH3CHOH.
The trans path for reaction 1b proceeds via the trans-TS2a (dihedral angle φ(HOCC) ) -174.0°, φ(OCCO) ) 71.9°) with a barrier of 9.3 kcal mol-1 or the trans-TS2b (dihedral angle φ(HOCC) ) φ(OCCO) ) 180.0°) with a barrier of 9.8 kcal mol-1to form trans-CH2CH2OH and OH with∆H ) 0.7 kcal mol-1. The corresponding gauche path for reaction 1b proceeds via gauche-TS2a(dihedral angle φ(HOCC) ) -58.2°, φ(OCCO) ) 56.0°) with a barrier of 7.7 kcal mol-1 or gauche-TS2b (dihedral angle φ(HOCC) ) 68.6°, φ(OCCO) ) -175.7°) with a barrier of 9.7 kcal mol-1 to form gauche-CH2CH2OH and OH with∆H ) 0.3 kcal mol-1.
The trans path of reaction 1c proceeds via trans-TS3(dihedral angle φ(HOCC) ) 180.0°) with a barrier of 10.7 kcal mol-1 and forms CH3CH2O and OH with∆H ) 2.1 kcal mol-1. The corresponding gauche path proceeds via gauche-TS3(dihedral angle φ(HOCC) ) 64.7°) with a barrier of 10.0 kcal mol-1.
The predicted enthalpies of reaction for the three branching reactions are compared with experimental values in Table 5. The predicted enthalpy changes for reactions 1a-c at 0 K, -6.5 ( 0.2, 0.5 ( 0.2, and 2.1 kcal mol-1, are close to the experimental values -8.7 ( 0.2, 2.3 ( 0.2, and 1.7 ( 1.2 kcal mol-1, respectively, on the basis of∆Hf,0(O) ) 58.99 ( 0.02,35 ∆Hf,0(C2H5OH) ) -51.88 ( 0.12,35 ∆Hf,0(CH3CHOH) ) -10.5,36∆Hf,0(CH3CH2O) ) -0.05 ( 0.96,35and∆Hf,0(OH) ) 8.87 ( 0.07 kcal mol-1;35∆Hf,0(CH2CH2OH) ) 0.57 kcal mol-1 is derived from ∆Hf,298(CH2CH2OH) ) -2.46 kcal mol-1.37
As shown in Figure 4, the reacting atoms O, H, and C in trans-TS1are almost linear, with∠OHC ) 177°; the length of the breaking C-H bond increases by 0.11 Å from that of trans-C2H5OH. The imaginary vibrational wavenumber of trans-TS1 is 355i cm-1. For gauche-TS1,∠OHC ) 176°and the C-H bond length increases by 0.12 Å from that of gauche-C2H5OH. The imaginary vibrational wavenumber of gauche-TS1is 505i cm-1. For trans-TS2aand trans-TS2b,∠OHC ) 177-8° and the C-H bond length increases by 0.22-0.23 Å from that of trans-C2H5OH; the imaginary vibrational wavenumbers are Figure 4. Geometries of reactant C2H5OH, transition states, and
products of the O + C2H5OH system optimized at the
B3LYP/6-311+G(3df) level. Listed bond lengths are in Å, and bond angles are in deg. 2OI indicates that two optical isomers exist for this configuration.
k1) (1.34 ( 0.11) ×
10-10exp[-(3040 ( 80)/T] cm3molecule-1s-1 (17)
k1) (2.89 ( 0.09) ×
1487i and 1504i cm-1, respectively. For gauche-TS2a and gauche-TS2b with ∠OHC ) 168 and 180°, respectively, the C-H bond length increases by 0.21 and 0.20 Å from that of trans-C2H5OH and the imaginary vibrational wavenumbers are 1412i and 1486i cm-1, respectively. For trans-TS3and gauche-TS3,∠OHO ) 161-162°and the C-H bond length increases by 0.17 Å from the corresponding C2H5OH conformer; the imaginary vibrational wavenumbers are 1536i and 1512i cm-1,
respectively. The geometry parameters and imaginary frequen-cies of the trans and gauche transition states are similar. The trans and gauche transition states can be transformed to each other by the internal rotation about the C-O single bond. The trans or gauche TS2aand TS2bcan be transformed to each other by the internal rotation about the C-C bond.
C. Calculations and Comparison of Rate Coefficients. As
shown in Figure 5, the three direct H-abstraction reaction Figure 5. Potential energy diagram for various channels of the reaction O + C2H5OH on the basis of energies calculated with
CCSD(T)/6-311+G-(3df, 2p)//B3LYP/6-311+G(3df). Listed energies are in kcal mol-1.
TABLE 5: Total and Relative Energiesaof Reactants, Transition States, and Products of the Reaction O + C2H5OH
species or reacns ZPE B3LYP/6-311+G(3df) CCSD(T)b/6-311+G(3df, 2p) ∆H
0exptc O(3P) + trans-C 2H5OH 0.079602 -230.105859 -229.681620 O(3P) + gauche-C 2H5OH 0.0 0.1 0.1 O(3P) + t-g-C 2H5OH-TS -0.3 0.8 0.7 trans-TS1 -2.6 -4.8 4.2 gauche-TS1 -2.7 -4.3 4.7 trans-TS2a -4.0 1.9 9.3 gauche-TS2a -3.7 0.7 7.7 trans-TS2b -4.1 2.9 9.8 gauche-TS2b -3.8 2.3 9.7 trans-TS3 -4.7 0.1 10.7 gauche-TS3 -4.6 -0.5 10.0 trans-CH3CHOH + OH -4.4 -11.8 -6.6 -8.7 ( 0.2 trans-CH2CH2OH + OH -4.0 -3.4 0.7 2.3 ( 0.2 cis-CH3CHOH + OH -3.5 -11.5 -6.3 -8.7 ( 0.2 gauche -CH2CH2OH + OH -3.5 -3.9 0.3 2.3 ( 0.2 CH3CH2O + OH -4.6 -5.3 2.1 1.7 ( 1.2
aTotal energies for O(3P) + C
2H5OH are in au, and relative energies for others are in kcal mol-1.bBased on optimized geometries calculated
at B3LYP/6-311+G(3df).cAt 0 K,∆H
f,0are taken from ref 35 unless noted: ∆Hf,0(O) ) 58.99 ( 0.02 kcal mol-1;∆Hf,0(C2H5OH) ) -51.88 (
0.12 kcal mol-1;∆Hf,0(OH) ) 8.87 ( 0.07 kcal mol-1;∆Hf,0(CH2CH2OH) ) 0.57 kcal mol-1using∆Hf,298(CH2CH2OH) ) -2.46 kcal mol-1;37
∆Hf,0(CH3CHOH) ) -10.5 kcal mol-1;36∆Hf,0(CH3CH2O) ) -0.05 ( 0.96 kcal mol-1.
TABLE 6: Vibrational Wavenumbers and Moments of Inertia Iifor the Reactants, Transition States, and Products of the
Reaction O + C2H5OH Calculated with B3LYP/6-311+G(3df)
species Ii(au) vibrational wavenumbers (cm-1)
trans-C2H5OH 51.1, 193.3, 221.8 241, 285, 417, 820, 896, 1030, 1100, 1179, 1268, 1297, 1405, 1448, 1482, 1498, 1522, 2983, 3007, 3034, 3099, 3104, 3827 gauche-C2H5OH 52.2, 197.1, 222.9 259, 276, 420, 804, 883, 1059, 1070, 1134, 1281, 1371, 1403, 1417, 1488, 1491, 1515, 2990, 3018, 3065, 3087, 3100, 3810 t-g-C2H5OH-TS 51.5, 198.1, 222.9 253, 417, 800, 888, 1041, 1097, 1129, 1283, 1350, 1401, 1420, 1483, 1493, 1514, 3010, 3026, 3040, 3089, 3102, 3863, 275i trans-TS1 206.4, 414.5, 571.7 91, 159, 242, 377, 428, 822, 918, 968, 1056, 1152, 1179, 1199, 1278, 1398, 1407, 1438, 1477, 1488, 3010, 3030, 3095, 3120, 3808, 355i guach-TS1 209.8, 409.7, 565.1 127, 163, 253, 386, 448, 767, 878, 924, 1061, 1118, 1171, 1215, 1306, 1366, 1403, 1426, 1483, 1485, 3020, 3073, 3093, 3112, 3789, 505i trans-TS2a 157.2, 490.8, 584.7 80, 148, 244, 398, 486, 534, 819, 897, 1030, 1083, 1109, 1180, 1208, 1267, 1277, 1421, 1454, 1487, 2929, 3007, 3085, 3169, 3831, 1487i gauche-TS2a 182.1, 395.6, 525.2 87, 163, 382, 424, 458, 573, 821, 886, 1021, 1077, 1132, 1177, 1205, 1251, 1381, 1409, 1440, 1484, 2991, 3025, 3076, 3162, 3763, 1412i trans-TS2b 69.8, 678.8, 725.4 67, 117, 179, 360, 430, 569, 817, 891, 1013, 1047, 1155, 1173, 1179, 1247, 1295, 1429, 1472, 1518, 3004, 3034, 3087, 3169, 3812, 1504i gauche-TS2b 69.9, 689.4, 731.7 82, 116, 297, 361, 458, 557, 805, 900, 1030, 1041, 1096, 1173, 1184, 1281, 1363, 1400, 1464, 1505, 3029, 3072, 3104, 3157, 3805, 1486i trans-TS3 59.9, 611.7, 648.8 62, 145, 242, 253, 342, 641, 820, 889, 997, 1092, 1156, 1170, 1281, 1356, 1396, 1475, 1496, 1523, 3006, 3040, 3048, 3111, 3124, 1536i gauche-TS3 158.6, 414.3, 520.2 76, 160, 259, 366, 430, 615, 788, 884, 1052, 1096, 1124, 1209, 1269, 1373, 1405, 1431, 1484, 1495, 2965, 3007, 3031, 3096, 3107, 1512i OH 0.0, 3.2, 3.2 3701 trans-CH3CHOH 38.8, 191.3, 217.9 179, 363, 408, 540, 928, 1023, 1059, 1205, 1275, 1398, 1446, 1464, 1489, 2945, 3037, 3097, 3139, 3830 cis-CH3CHOH 40.5, 191.1, 218.8 195, 334, 411, 564, 916, 1021, 1063, 1200, 1309, 1403, 1435, 1468, 1485, 2927, 3003, 3093, 3195, 3800 trans-CH2CH2OH 44.5, 180.1, 212.5 95, 269, 412, 455, 869, 957, 1058, 1108, 1216, 1266, 1420, 1459, 1484, 2891, 2944, 3158, 3267, 3829 gauche-CH2CH2OH 46.8, 182.9, 210.7 177, 329, 423, 537, 827, 950, 1082, 1121, 1184, 1359, 1398, 1452, 1481, 2974, 2992, 3142, 3247, 3800 CH3CH2O 45.1, 188.8, 211.8 46, 256, 435, 860, 886, 1070, 1097, 1239, 1327, 1389, 1408, 1485, 1493, 2882, 2893, 3031, 3096, 3106
channels have barriers of 4.2-10.7 kcal mol-1; hence, tunneling effects for these channels should be considered. Rate coefficients for the three channels in the temperature range 300-3000 K have been computed with the CVT and the CVT/SCT methods on the basis of the geometries, vibrational frequencies, and rotational constants calculated at the B3LYP/6-311+G(3df) level and the energies calculated at the CCSD(T)/6-311+G(3df, 2p)// B3LYP/6-311+G(3df) level. Because there are two optical isomers for trans-TS1, gauche-TS1, trans-TS2a, gauche-TS2a, gauche-TS2b, and gauche-TS3, a statistical factor of 2 is employed in the calculations.
Because of the existence of the trans-C2H5OH and gauche-C2H5OH conformers and their corresponding transition states, the rate coefficient for the reaction of each conformer should be taken into account using their equilibrium concentrations at each temperature. The thermal equilibrium constant, K ) [gauche-C2H5OH]/[trans-C2H5OH] ) 0.87-0.97 in the tem-perature range 300-3000 K, can be calculated with the Chemrate Program.38The predicted rate coefficients for channels (1a), (1b), and (1c) are derived from rate coefficients of the trans-C2H5OH and gauche-C2H5OH reactions:39
In calculations of rate coefficients, the internal rotation about the C-O bond in trans-TS1, TS2a, and TS2bhave been treated as hindered rotors. Such treatments increase the total rate coefficient by 59-17% in the temperature range 300-3000 K. To compare predicted rate coefficients with experimental data quantitatively, we fit rate coefficients predicted with CVT/SCT for channels (1a), (1b), and (1c) in the temperature range 300-3000 K to the three-parameter form to yield
The total rate coefficients calculated with the CVT and CVT/ SCT methods for the temperature range 300-3000 K are represented as
and
respectively. At 300 K, rate coefficients of reactions 1a-c predicted with CVT/SCT are 10, 58, and 1330 times those predicted with CVT, respectively, resulting to a difference in total rate coefficient of about a factor of 10. The total rate coefficients calculated with CVT (dotted line) and CVT/SCT (medium dash line) are plotted in Figure 3 to compare with experimental data of GNK,9AR,7KC,3Washida,8and this work. In general, rates predicted with CVT/SCT are in satisfactory agreement with experimental values, indicating the SCT method treats tunneling effects adequately. At low temperatures, rate coefficients predicted with CVT/SCT are slightly greater than experimental values of GNK9but within uncertainties of other experiments. An expanded plot for high temperatures is shown in the inset of Figure 3. At high temperatures, predicted rate coefficients fit satisfactorily with experimental data of this work but slightly smaller than those of GNK.9The uncertainty of the barriers of transition states has significant effect on the rate coefficient at low temperatures. The uncertainty of the calculated barriers of transition states is estimated to be (0.5 kcal mol-1 due to errors in accuracy of computations and treatment of hindered rotors and tunneling corrections. The predicted total rate coefficients increase to 2.4-1.1 times the original value in the temperature range 300-3000 K when the calculated barriers of transition states decrease by 0.5 kcal mol-1; they decrease to 0.4-0.9 times when the calculated barriers increase by 0.5 kcal mol-1. The uncertainties are shown as shaded region in Figure 3; they cover the uncertainty range of the experimental data in the whole temperature range.
The branching ratios of channels (1a), (1b), and (1c) predicted with the CVT/SCT method for T ) 300-3000 K are plotted in Figure 6. Reaction 1a is the predominant channel for T < 500 K; its branching ratio decreases from 1.00 at 300 K to 0.81 at 1000 K and 0.31 at 3000 K. The branching ratio for reaction 1b is 0.03 at 600 K and increases to 0.15 at 1000 K and 0.49 at 3000 K. The branching ratio for reaction 1c is 0.03 at 1000 K and 0.20 at 3000 K. The branching ratios employed by Marinov are also compared in Figure 6 (dotted lines);12 branching ratios of k1a appear to be underestimated whereas those of k1care greatly overestimated throughout the temperature range 300-3000 K.
It should be noted that Marinov reported that reaction 1c is more important than reaction 1b and becomes the most important channel at T > 1400 K, but we predicted reaction 1b to be the most important. On the basis of the PES of channels (1a), (1b), and (1c), the barriers of trans- and gauche-TS3are greater than those of trans- and gauche-TS2aand TS2b, so it is unlikely that the rate coefficients of reaction 1c are greater than those of reaction 1b, especially at low temperatures. The revised branch-ing ratios for production of CH3CHOH, CH2CH2OH, and CH3-CH2O should have significant impacts on the chemical modeling of combustion systems involving ethanol, particularly at high temperatures.
Conclusion
Total rate coefficients of the reaction O(3P) + C2H5OH in the temperature range 782-1410 K were determined using a Figure 6. Branching ratios for reactions 1a-c: solid line, this work;
dotted line, Marinov.12
k1a(T) ) (ktrans-1a(T) + kgauche-1a(T)K)/(1+ K) (19) k1b(T) ) (ktrans-1b(T) + kgauche-1b(T)K)/(1+ K) (20) k1c(T) ) (ktrans-1b(T) + kgauche-1b(T)K)/(1+ K) (21) k1a(T) ) 2.41× 10-19T2.47exp(-441/T) cm3molecule-1s-1 (22) k1b(T) ) 1.61× 10-21T3.23exp(-2344/T) cm3molecule-1s-1 (23) k1c(T) ) 2.43× 10-27T4.73exp(-869/T) cm3molecule-1s-1 (24) k1(T) ) 3.77× 10-20T2.86exp[-(1265/T)] cm3molecule-1s-1 (25) k1(T) ) 1.60× 10-22T3.50exp(16/T) cm3molecule-1s-1 (26)
diaphragmless shock tube with atomic resonance absorption detection of O atoms. Our results extended the upper limit of the temperature range of study from 886 to 1410 K and clearly indicated a non-Arrhenius behavior of the rate coefficient. Rate coefficients obtained in this work is slightly smaller than those determined previously by Grotheer et al.9 in the overlapped temperature region 782-886 K but within experimental errors. Theoretical calculations at the CCSD(T)/6-311+G(3df, 2p)// B3LYP/6-311+G(3df) level predict transition states and barriers for various channels. Rate coefficients predicted with CVT/SCT show that branching ratios of three accessible reaction channels to form CH3CHOH + OH (1a), CH2CH2OH + OH (1b), and CH3CH2O + OH (1c) varies with temperature. At T < 600 K, reaction 1a dominates by >96%, whereas, above 2300 K, reaction 1b becomes more important with a branching ratio >44%. The branching for the channel (1c) is less than 12% for T < 2000 K. Predicted total rate coefficients are in satisfactory agreement with our experimental data at high temperature (782-1410 K) and those reported previously.
Acknowledgment. Y.-P.L. thanks the National Science Council of Taiwan (Grant No. NSC95-2119-M-009-032) for support. M.C.L. and S.X. thank the support from the Basic Energy Science, Department of Energy, under Contract DE-FG02-97-ER14784, and Cherry L. Emerson Center for Scientific Computation of Emory University for the use of its resources, which are in part supported by a National Science Foundation Grant (CHE-0079627) and an IBM Shared University Research Award. M.C.L. also acknowledges the support from the National Science Council of Taiwan for a Distinguished Visiting Profes-sorship at the National Chiao Tung University in Hsinchu, Taiwan.
References and Notes
(1) Hansen, A. C.; Zhang, Q.; Lyne, P. W. L. Bioresour. Technol. 2005,
96, 277 and references therein.
(2) Deluga, G. A.; Salge, J. R.; Schmidt, L. D.; Verykios, X. E. Science
2004, 303, 993.
(3) Kato, A.; Cvetanovic, R. J. Can. J. Chem. 1967, 45, 1845. (4) Avramenko, L. I.; Kolesnikova, R. V.; Savinova, G. I. Bull. Acad.
Sci. USSR, DiV. Chem. Sci. (Engl. Transl.) 1967, 16, 19.
(5) Avramenko, L. I.; Kolesnikova, R. V. Bull. Acad. Sci. USSR, DiV.
Chem. Sci. (Engl. Transl.) 1971, 20, 2700.
(6) Owens, C. M.; Roscoe, J. M. Can. J. Chem. 1976, 54, 984. (7) Ayub, A. L.; Roscoe, J. M. Can. J. Chem. 1979, 57, 1269. (8) Washida, N. J. Chem. Phys. 1981, 75, 2715.
(9) Grotheer, H. H.; Nesbitt, F. L.; Klemm, R. B. J. Phys. Chem. 1986,
90, 2512.
(10) Herron,. J. T. J. Phys. Chem. Ref. Data 1988, 17, 967. (11) Held, T. J.; Dryer, F. L. Int. J. Chem. Kinet. 1998, 30, 805. (12) Marinov, N. M. Int. J. Chem. Kinet. 1999, 31, 183.
(13) Dutton, N. J.; Fletcher, I. W.; Whitehead, J. C. J. Phys. Chem.
1985, 89, 569.
(14) Lu, C.-W.; Chou, S.-L.; Lee, Y.-P.; Xu, S.; Xu, Z. F.; Lin, M. C.
J. Chem. Phys. 2005, 122, 244314.
(15) Koshi, M.; Yoshimura, M.; Fukuda, K.; Matsui, H.; Saito, K.; Watanabe, M.; Imamura, A.; Chen, C. J. Chem. Phys. 1990, 93, 8703.
(16) Hsiao, C.-C.; Lee, Y.-P.; Wang, N. S.; Wang, J. H.; Lin, M. C. J.
Phys. Chem. A 2002, 106, 10231.
(17) Tsuchiya, K.; Yokoyama, K.; Matsui, H.; Oya, M.; Dupre, G. J.
Phys. Chem. 1994, 98, 8419.
(18) Greene, E. F.; Toennies, J. P. Chemical Reactions in Shock WaVes; Academic Press: New York, 1964.
(19) Michael, J. V. J. Chem. Phys. 1989, 90, 189.
(20) Michael, J. V.; Sutherland, J. W. Int. J. Chem. Kinet. 1986, 18, 409.
(21) Ross, S. K.; Sutherland, J. W.; Kuo, S.-C.; Klemm, R. B. J. Phys.
Chem. A 1997, 101, 1104.
(22) Raghavachari, K.; Trucks, G. J.; Pople, J. A.; Head-Gordon, M.
Chem. Phys. Lett. 1989, 157, 479.
(23) Corchado, J. C.; Chuang, Y.-Y.; Fast, P. L.; Villa`, J.; Hu, W.-P.; Liu, Y.-P.; Lynch, G. C.; Nguyen, K. A.; Jackels, C. F.; Melissas, V. S.; Lynch, B. J.; Rossi, I.; Coitin˜o, E. L.; Fernandez-Ramos, A.; Pu, J.; Albu, T. V.; Steckler, R.; Garrett, B. C.; Isaacson, A. D.; Truhlar, D. G.
POLYRATE v9.3; 2004.
(24) Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery, J. A., Jr.; Stratmann, R. E.; Burant, J. C.; Dapprich, S.; Millam, J. M.; Daniels, A. D.; Kudin, K. N.; Strain, M. C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G. A.; Ayala, P. Y.; Cui, Q.; Morokuma, K.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Cioslowski, J.; Ortiz, J. V.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Gonzalez, C.; Challacombe, M.; Gill, P. M. W.; Johnson, B. G.; Chen, W.; Wong, M. W.; Andres, J. L.; Head-Gordon, M.; Replogle, E. S.; Pople, J. A. Gaussian 03, revision A.7; Gaussian, Inc.: Pittsburgh, PA, 2003.
(25) Li, J.; Kazakov, A.; Dryer, F. Int. J. Chem. Kinet. 2001, 33, 859 and references therein.
(26) Yamabe, T.; Koizumi, M.; Yamashits, K.; Tachibana, A. J. Am.
Chem. Soc. 1984, 106, 2255.
(27) Butkovskaya, N. I.; Zhao, Y.; Setser, S. W. J. Phys. Chem. 1994,
98, 10779.
(28) Park, J.; Zhu, R. S.; Lin, M. C. J. Chem. Phys. 2002, 117, 3224. (29) Park, J.; Zhu, R. S.; Lin, M. C. J. Chem. Phys. 2003, 118, 9990. (30) Xu, Z. F.; Park, J.; Lin, M. C. J. Chem. Phys. 2004, 120, 6593. (31) Feng, R.; Brion, C. E. Chem. Phys. Lett. 2002, 282, 419. (32) Cheng, B. M.; Bahou, M.; Chen, W. C.; Yu, C.-h.; Lee, Y.-P.; Lee, L. C. J. Chem. Phys. 2002, 117, 1633.
(33) FACSIMILE (AEA Technology, Oxfordshire, U.K) is a computer software for modeling process and chemical reaction kinetics.
(34) Li, J.; Kazakov, A.; Dryer, F. L. J. Phys. Chem. A 2004, 108, 7671. (35) Ruscic, B.; Boggs, J. E.; Burcat, A.; Csaszar, A. G.; Demaison, J.; Janoschek, R.; Martin, J. M. L.; Morton, M. L.; Rossi, M. J.; Stanton, J. F.; Szalay, P. G.; Westmoreland, P. R.; Zabel, F.; Berces, T. J. Phys. Chem.
Ref. Data 2005, 34, 573.
(36) Ruscic, B.; Berkowitz, J. J. Chem. Phys. 1994, 101, 10936. (37) Meier, U.; Grotheer, H. H.; Riekert, G.; Just, T. Chem. Phys. Lett.
1985, 115, 221.
(38) Mokrushin, W.; Bedanov, V.; Tsang, W.; Zachariah, M.; Knyazev, V. ChemRate, version 1.20; National Institute of Standards and Technol-ogy: Gaithersburg, MD 20899, 2003.
(39) Tzeng, C. M.; Choi, Y. M.; Huang, C. L.; Ni, C. K.; Lee, Y. T.; Lin, M. C. J. Phys. Chem. A 2004, 108, 7928.
(40) Srinivasan, N. K.; Su, M.-C.; Sutherland, J. W.; Michael, J. V. J.
Phys. Chem. A 2005, 109, 7902.
(41) Wooldridge, M. S.; Hanson, R. K.; Bowman, C. T. Int. J. Chem.
Kinet. 1994, 26, 389.
(42) Baulch, D. L.; Cobos, C. J.; Cox, R. A.; Esser, C.; Frank, P.; Just, Th.; Kerr, J. A.; Pilling, M. J.; Troe, J.; Walker, R. W.; Warnatz, J. J.
Phys. Chem. Ref. Data 1992, 21, 411.
(43) Tsang, W.; Hampson, R. F. J. Phys. Chem. Ref. Data 1986, 15, 1087.
(44) Miller, J. A.; Melius, C. F. Comb. Flame 1992, 91, 21. (45) Miyoshi, A.; Tsuchiya, K.; Yamauchi, N.; Matsui, H. J. Phys. Chem.
1994, 98, 11452.
(46) Baulch, D. L.; Cobos, C. J.; Cox, R. A.; Frank, P.; Hayman, G.; Just, Th.; Kerr, J. A.; Murrells, T.; Pilling, M. J.; Troe, J.; Walker, R. W.; Warnatz, J. J. Phys. Chem. Ref. Data 1994, 23, 847.
(47) Atkinson, R.; Baulch, D. L.; Cox, R. A.; Crowley, J. N.; Hampson, R. F., Jr., Kerr, J. A.; Rossi, M. J.; Troe, J. J. Phys. Chem. Ref. Data 1997,
26, 521.
(48) Edelbuttel-Einhaus, J.; Hoyermann, K.; Rohde, G.; Seeba, J. Symp.
Int. Combust. Proc. 1992, 24, 661.
(49) Dagaut, P.; Cathonnet, M.; Boettner, J. C. Int. J. Chem. Kinet. 1991,
23, 437.
(50) Baulch, D. L.; Cobos, C. J.; Cox, R. A.; Frank, P.; Hayman, G.; Just, Th.; Kerr, J. A.; Murrells, T.; Pilling, M. J.; Troe, J.; Walker, R. W.; Wamatz, J. Comb. Flame 1994, 98, 59.
(51) Lu, C.-W.; Wu, Y.-J.; Lee, Y.-P.; Zhu, R. S.; Lin, M. C. J. Phys.
Chem. A. 2003, 107, 11020.
(52) Grillo, A.; Reed, R.; Slack, M. W. J. Chem. Phys. 1979, 70, 1634. (53) Smith, O. I.; Tseregounis, S.; Wang, S.-N. Int. J. Chem. Kinet.
1982, 14, 679.
![Figure 2. A typical temporal profile of [O] observed after irradiation](https://thumb-ap.123doks.com/thumbv2/9libinfo/7619198.131440/3.918.490.821.62.316/figure-typical-temporal-profile-o-observed-irradiation.webp)