255
*To whom correspondence and reprint requests should be addressed. Tel and Fax: 886-2-23639570.
E-mail: wnt@ccms.ntu.edu.tw
T
he Japanese eel, Anguilla japonica Temminck and Schlegel, is a catadromous fish (Ege 1939), spawning in the waters west of the Mariana Is. (Tsukamoto 1992) and growing in the estuaries and rivers of East Asia (Tesch 1977). After hatching, the eel larvae (leptocephali) drift with the North Equatorial Current and Kuroshio Current from the spawning ground to the continen-tal shelves of East Asian countries and then meta-morphose into glass eels which leave the strong oceanic currents and migrate into coastal waters (Cheng and Tzeng 1996, Tzeng 2003). Glass eels become elvers in the estuaries then feed and grow as yellow eels in rivers for 5-8 yrs untilmetamor-phosis from yellow to silver eels (Tzeng et al. 2000). After maturation, eels migrate back to their birthplace to spawn and die (Tesch 1977). The Japanese eel is a commercially important cultured species. For cultivation purposes, elvers in the estuary have been overexploited for many years, which has severely influenced the recruitment of the eel and diminished eel populations in the rivers of Taiwan (Tzeng 1984, 1985 Tzeng et al. 1995, Liao 2001, Tzeng 2004).
European (A. anguilla) and American (A. ros-trata) eels have been reported to have ZW sex chromosomes (Passakas and Klekowski 1972, Park and Grimm 1981). However, further
kary-Use of the Sex Ratio as a Means of Resource Assessment for the
Japanese Eel Anguilla japonica: A Case Study in the Kaoping River,
Taiwan
Yu-San Han1and Wann-Nian Tzeng2,*
1Division of Biotechnology and Pharmaceutical Research, National Health Research Institutes, Taipei, Taiwan 115, R.O.C. 2Institute of Fisheries Science, College of Life Science, National Taiwan University, Taipei, Taiwan 106, R.O.C.
(Accepted December 1, 2005)
Yu-San Han and Wann-Nian Tzeng (2006) Use of the sex ratio as a means of resource assessment for the Japanese eel Anguilla japonica: a case study in the Kaoping River, Taiwan. Zoological Studies 45(2): 255-263. A negative relationship between the proportion of females and the population density was found when compil-ing historical data of both wild and cultured Japanese, European, and American eels. Based on the relation-ship, the population status of the Japanese eel Anguilla japonica was assessed. Japanese eel samples were collected from the lower reaches of the Kaoping River in southwestern Taiwan from 1998 to 2002, and the pop-ulation density was estimated by mark-recapture experiments in 2001 and 2002. The sex ratio of the eel was skewed towards females, accounting for 81.3%-88.3% in the total samples of yellow and silver eels and for 63.6%-81% in the silver stage eels. The significant female-skewness of the sex ratio validated that the popula-tion of Japanese eels in the Kaoping River has declined to a very low level in recent years. The populapopula-tion density of the Japanese eel in the lower reaches of the Kaoping River was approximately 0.01 eels/m2in 2001
and 2002. The decline in the Japanese eel population in the Kaoping River has probably resulted from both overfishing of glass eels in the estuary for aquaculture needs which severely influences recruitment, and degra-dation of the growth habitat of the yellow eel along the river. The sex ratio therefore is a fast and reliable indi-cator for eel resource assessment. http://zoolstud.sinica.edu.tw/Journals/45.2/255.pdf
Key words: Japanese eel (Anguilla japonica), Resource assessment, Sex ratio, Mark-recapture, Population density.
otype studies failed to identify heteromorphic sex chromosomes (Sola et al. 1980, Wiberg 1983). The eel before the elver stage is considered to be intersexual, and sexual differentiation occurs in the yellow eel stage. Ovaries are found in European eels of 22-30 cm in total length, possibly derived from undifferentiated gonads or from the Syrski organ, a gonad containing both early testes and oogonia. Spermatogonium B-type testes were observed in eels longer than 30 cm (Colombo and Grandi 1996). Sexually undifferentiated elvers can be feminized by treatment with estradiol-17β (Degani and Kushnirov 1992, Satoh et al. 1992, Chiba et al. 1993), indicating that the sex of the eel is labile. However, the mechanisms of sexual labi-lization and delayed sexual development in eels are poorly known.
In the wild, eel sex ratios can vary widely, ranging from almost all males to predominantly females (Matsui 1972, Parsons et al. 1977, Tesch 1977, Jessop 1987, Tzeng et al. 1995 2002, Oliveira 1997 1999, Oliveira and McCleave 2000, Oliveira et al. 2001). If the sex determination of the eel is genotype-dependent, then the uneven distributions of sexes in rivers might result from habitat choice, with females preferring to migrate upstream and males preferring to stay in estuaries (Bertin 1956, Tesch 1977), or perhaps females prefer a habitat suitable for faster growth, while males prefer a slow-growth habitat (Helfman et al. 1987). If eel sex determination is phenotype-dependent, then the habitat in which they grow will affect their sex differentiation. Parsons et al. (1977) found that each period of elver restocking into Lough Neagh of the Bann River was followed by a marked increase in the proportion of male sil-ver eels migrating from the lough. Degani and Kushnirov (1992) indicated that 77% of European eels maintained in groups became males, whereas 60% of those maintained in isolation became females. Oliveira et al. (2001) found that the lake area of a river system probably influences the sex ratio of the eel such that eels migrating from lacus-trine habitats were mostly females, and eels migrating from fluvial habitats were mostly males. These studies inferred the possible effects of pop-ulation density on the eel sex ratio. In pond cul-tures, captivity limits eel migration thus preventing differences in migratory behavior between the sexes. The sex ratio of cultured eels changes with stocking density, and a negative relationship between the proportion of females and stocking density was noted, similar to that found in the wild (Roncarati et al. 1997, Tzeng et al. 2002). Recent
studies favor the environmental sex-determination hypothesis, i.e., at high population densities, males dominate and at low population densities females dominate (Krueger and Oliveira 1999, Tzeng et al. 2002).
Usually, fish stocks are assessed by either laborious mark-recapture experiments or by the time-consuming and fishery-biased data collection system of catch and fishing effort. Finding a sim-ple and reliable way to monitor the resource status would be of great use. Given the hypothesis that the proportion of female eels is negatively related to its population density, 3 major temperate eels Anguilla anguilla, A. japonica, and A. rostrata from both wild and cultured systems were analyzed. The population status of the Japanese eel in a river in Taiwan was assessed by examining the sex ratio. The sex ratio of the Japanese eel col-lected in the Kaoping River from 1998 to 2002 was analyzed, and the population density was estimat-ed using the mark-recapture method. This adap-tive strategy of a labile sex ratio of anguillid eels is also discussed.
MATERIALS AND METHODS Analysis of the historic sex ratio and popula-tion density data
We searched MEDLINE and EMBASE from 1970 to 2004 to acquire sex ratio and population density data of anguillid eels. The search used combined key words like eel with sex ratio, popula-tion density, and sex determinapopula-tion. We also searched specialist books and conference pro-ceedings we had on hand regardless of the lan-guage. Data of the eel included 4 studies on the Japanese eel, 5 studies on the American eel, and 3 studies on the European eel (Appendix I). Data on the population density of the eel with continu-ous variables were defined as quantitative and were used for analysis of relationship between the proportion of females and population density. For Japanese eels, all data were included for analysis. For American eels, only the studies of (Oliveira 1997) and (Oliveira and McCleave 2000) were used. For European eels, data from (Roncarati et al. 1997) were used.
Field investigations of the sex ratio
The sex ratio of the Japanese eels caught with eel traps in the lower reaches of Kaoping
River in southwestern Taiwan (120
°
50'E, 22°
40'N) were recorded approximately every 2 mo during the period from Nov. 1998 to Nov. 2002. Total length (TL, ± 1 mm) and body weight (BW, ± 0.1 g)of the eels were measured. The sex and develop-mental stage of each eel was determined by body coloration and gonadal histology when possible (Han et al. 2002).
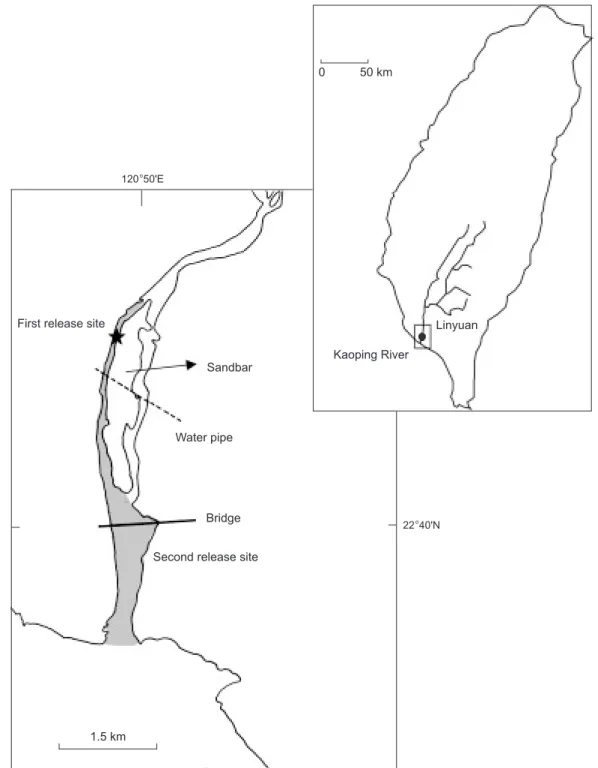
Fig. 1. Sampling site for wild Japanese eels in the lower reaches of the Kaoping River. The asterisk and shaded region indicate the 1st and 2nd release sites, respectively. The area of the shaded region was approximately 1.2 km2as estimated by a 1: 10000 scale map
copied onto paper with a square grid. Multiplying the squared unit area by the estimated number of squares covering the shaded region gave the area.
Second release site
1.5 km Bridge Water pipe Sandbar Kaoping River Linyuan 0 50 km
First release site
22°40'N 120°50'E
The mark-recapture experiment in 2001
In total, 277 eels collected from a culture pond at Lukang in Aug. 2001 were used for a mark-recapture experiment. After anesthetization with 2-phenoxyethanol, microchips were implanted intramuscularly behind the anus of the eels so that they could be detected using an external scanner. The tagged eels were released into the estuary of the Kaoping River on 29 Aug. 2001 (Fig. 1). Captured eels caught by traps within the shaded region of the estuary shown in figure 1 were recorded from 11 Sept. - 29 Nov. 2001. In total, 360 eels were caught and scanned to identify tagged eels.
The mark-recapture experiment in 2002
To eliminate a possible bias in the distribution-al behavior of cultured eels, 180 eels caught within the shaded region of the Kaoping River estuary shown in figure 1 in June 2002 were used for a tagging experiment. After anesthetization with 2-phenoxyethanol, visible plastic streamer tags were attached to the dorsal fin of the eels, and the eels were then released back into the estuary of the Kaoping River on 11 June 2002 (Fig. 1). In total, 150 eels were caught in the same area from 11 to 25 July 2002, and the tagged eels were identified.
Estimation of population density
The population size of wild Japanese eels in the Kaoping River was estimated by the mark-recapture method of Chapman (Seber 1982) which uses the following equations:
N=(M+1)(C+1)/(R+1)-1, (1)
V(N)=(M+1)(C+1)(M-R)(C-R)/(R+1)2(R+2), and (2)
95%CI=N ± Zαx; (3)
where N is the population size, M is the num-ber of the marked eels, C is the numnum-ber of eels caught, R is the number recaptured, V(N) is vari-ance of N, CI is the confidence interval, and Zαis 1.96 with α = 0.05. Because of the small size of the recapture sample, Poisson confidence inter-vals were also introduced (Clopper and Pearson 1934). The exact Poisson confidence intervals for samples were computed using a website tool (available at http://members.aol.com/johnp71/con-fint.html#Poisson). The population density (d) of
eels in the river was estimated by the formula:
d = N/A; (4)
where N is the population size and A is the area investigated.
Data analysis
Statistical differences in the sex ratios of wild eels among years were examined using the Chi-square test of homogeneity, and differences in the mean TL (± SD) and BW (± SD) between sexes were examined using Student,s t-test. A regres-sion between the proportion of females and densi-ty was calculated using the program SigmaPlot 2001.
RESULTS
Relationship between the eel sex ratio and population density
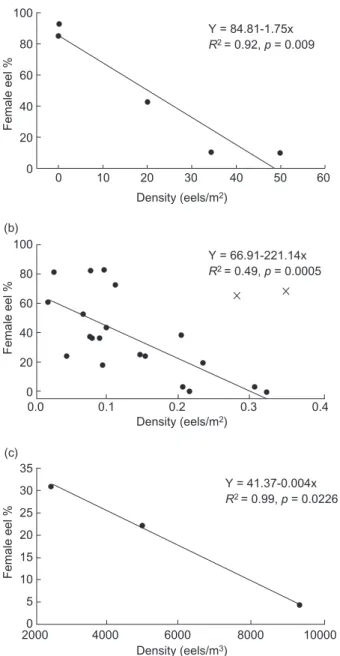
Historic quantitative data on the sex ratio and population density of the Japanese, American, and European eels all showed a similar trend of the proportion of females being significantly nega-tively correlated to the total population density (Fig. 2). The relationship in the American eel showed no significance when all data were included (R2 =
0.14, p = 0.09), however, the relationship turned out to be strongly significant when 2 extreme data points (may be due to some measurement bias) were excluded from the analysis (R2 = 0.49, p =
0.0005, Fig 2b). In other words, the eel population was dominated by females when the population density was lower and dominated by males when the population density was higher irrespective of eel species.
Comparison of the length (TL) and weight (BW) between sexes
There were no significant differences in either mean TL (p = 0.61) or BW (p = 0.96) between females (499.0 ± 4.8 mm, 198.2 ± 7.5 g) and males (505.3 ± 10.4 mm, 197.3 ± 12.9 g) regard-less of the developmental stage. The ranges of the TL and BW were wider for females (TL: 271-794 mm; BW: 25.8-851.6 g) than for males (TL: 315-675 mm; BW: 37.7-468.7 g) (Fig. 3a). How-ever, mean values of TL and BW of silver eels were significantly larger (p = 0.0002 for both) for females (642.9 ± 10.3 mm, 457.3 ± 25.8 g) than
males (563.9 ± 17.9 mm, 275.5 ± 26.6 g). Silver eels larger than 70 cm TL were all females, while silver ones less than 45 cm TL were all males (Fig. 3b)
Sex ratio
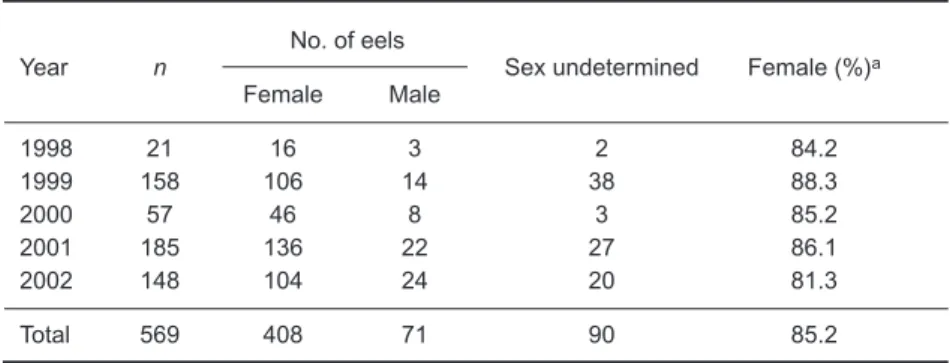
From 1998 through 2002, the sex of 569 Japanese eels collected in the Kaoping River was
determined (Table 1). The proportions of female eels in the river ranged from 81.3% to 88.3% with a mean of 85.2% and did not differ significantly among years (χ2 = 9.764 < χ2(4, 0.025) = 11.143). Silver eels were also predominantly females, rang-ing from 63.6% to 81.0% with a mean of 73.1% (data not shown), and the proportions did not sig-nificantly differ among years (χ2 = 1.298 <
χ2
(3, 0.025)= 9.348). Population density
In 2001, totally 360 eels with a mean total length of 527.1 ± 39.8 mm and a mean body weight of 222.7 ± 58.8 g were captured after the
Y = 84.81-1.75x R2 = 0.92, p = 0.009 (a) (b) (c) Y = 66.91-221.14x R2 = 0.49, p = 0.0005 Density (eels/m2) Female eel % Female eel % Female eel % Density (eels/m2) 0.0 0.1 60 50 40 30 20 10 0 0.2 0.3 0.4 Density (eels/m3) Y = 41.37-0.004x R2 = 0.99, p = 0.0226 100 80 60 40 20 0 100 80 60 40 20 0 35 30 25 20 15 10 5 0 2000 4000 6000 8000 10000
Fig. 2. Relationship between the eel sex ratio and population density in Japanese (a), American (b), and European (c) eels. The density units for Japanese and American eels are eels/m2
and for European eel are eels/m3, respectively. The regression
equation in (b) was determined by omitting 2 extreme data points (cross). Studies used for analysis of the relationship are shown in the Appendix I.
Fig. 3. (a) Frequency distribution of the total lengths of females, males, and gender-undetermined Japanese eels in the Kaoping River. (b) Frequency distribution of the total lengths of silver female and male Japanese eels in the Kaoping River. 30 25 20 15 10 5 0 14 12 10 8 6 4 2 0 Frequency (%) Frequency (%) Female (n = 408) Male (n = 71) Unknown (n = 90) Total length (mm) Total length (mm) 440-449 350-389 <349 390-429 430-469 470-509 510-549 550-589 590-529 630-669 >670 450-499 500-549 550-599 600-649 650-699 >700 Female (n = 49) Male (n = 18) (a) (b)
release. Among them, 6 tagged eels were recap-tured. The recapture rate was 1.7% (Table 2). In 2002, totally 150 eels with a mean total length of 447.2 ± 52.7 mm and a mean body weight of 126.9 ± 60.0 g were captured after the release. Among them, 2 tagged eels were recaptured. The recap-ture rate was 1.3% (Table 2). The population size of wild Japanese eels in the estuary of the Kaoping River was estimated to be approximately 14,335 ± 9713 (95% CI) in 2001 and 9109 ± 8765 (95% CI) in 2002 using Chapman,s method (Table 2). Since the population size estimation may have a large bias when fewer than 7 tagged eels are recaptured and the recapture rate is < 10%, Poisson confi-dence limits were introduced for comparison. The population size ranged 7137-31,360 in 2001 and 3324-22,040 in 2002 using the Poisson confidence intervals with 95% CI (Table 2). As indicated, the ranges of eel population sizes estimated by both Chapman,s method and the Poisson confidence intervals were similar. The population density was estimated to be approximately 0.01 eels/m2in both
years.
DISCUSSION
The mean TL and BW of both sexes showed
no significant differences (p > 0.05) when all eel phases were included, but were significantly larger for silver females than for silver males (p < 0.05). This is due to the wider TL and BW ranges of females. Since development of the male gonads occurs later than that of females (Colombo and Grandi 1996), yellow males were sometimes diffi-cult to identify. Thus, some of them may have been grouped with eels of undetermined sex, thus increasing the mean TL and BW of the males but reducing their proportion. This may explain the higher mean proportion of females when all phas-es were included (85.2%) (Table 1) compared with only silver phase eels (73.1%). Although the ratio of total males may have been underestimated, the sex ratio should be close to the true value when only silver phase eels, which all have developed gonads, were considered.
In today,s world of pollution and the preva-lence of endocrine-disrupting chemicals, environ-mentally induced changes in sex ratios are possi-ble. Thus, we analyzed the sex ratio of a goby fish (Glossogobius aureus) in the estuary of the Kaoping River for comparison. Out of 136 fish examined, there were 78 males and 58 females. Somewhat more males were found in this case. Therefore, the possible effects of environmental estrogen-like hormones on eel sex determination could be excluded.
Table 1. Sex ratios by year of wild Japanese eels in the Kaoping River
Year n
No. of eels
Sex undetermined Female (%)a
Female Male 1998 21 16 3 2 84.2 1999 158 106 14 38 88.3 2000 57 46 8 3 85.2 2001 185 136 22 27 86.1 2002 148 104 24 20 81.3 Total 569 408 71 90 85.2
aSex-undetermined eels were omitted from the calculation of percent of females.
Table 2. Population size of the Japanese eel in the Kaoping River estimated
by Chapman's mark-recapture method
No. of eels
Sampling date Marked Catch Recaptured Population Chapman ,
s Poisson
CI (95%) CI (95%)
Sept.-Nov. 2001 277 360 6 14,335 4622-24,048 7137-31,360
In this study, the number of recaptured eels with tags relative to the total number recaptured was low (Table 2). This may have led to a large bias in the population density estimation. Despite this, the estimated population density still remains quite low even if the abundance estimates are off by an order of magnitude. According to conversa-tions with local fishermen who have been catching eels for more than 30 yrs in the Kaoping River, the eel resources were once abundant (pers. comm.). They said that the eel harvest was several dozen-fold greater 20 yrs ago than it is now with a similar fishing effort, and most eels were males when the resource was abundant. Since more than 80% of Japanese eels are concentrated in the lower reaches (unpubl. data), we can speculate that a sex ratio reversal seems to have occurred for the Japanese eel, and the eel resources in the Kaoping River are endangered. A previous inves-tigation of Japanese eel resources in the Tanshui River of northern Taiwan, which has experienced similar fishery and habitat pressures (Tzeng et al. 1995), also showed a very low population density, and thus may also be experiencing similar short-ages in eel resources.
The negative relationship between the female sex ratio and population density seems to be a common phenomenon among eel species (Fig. 2). The population density was proposed as being a key factor in determining the sex of eels, with habi-tats having high densities of eels dominated by males and habitats with low densities dominated by females (Colombo and Rossi 1978). Sex differ-entiation of the eel is probably an adaptive strategy to achieve maximum fitness in which male eels exhibit a time-minimizing growth strategy by matur-ing as soon as possible, while females postpone maturation with a size-maximizing growth strategy to attain higher fecundity (Vøllestad and Johnson 1986, Helfman et al. 1987, Larsson et al. 1990, Vøllestad 1992). In high-density habitats, food resources are limited, and the earlier maturation of males may promote an earlier spawning migration. This possibly reduces intraspecific competition and the mortality rate of the eel. In contrast, in low-density stocks, food resources are relatively plenti-ful and eels differentiating into females would have enough time to fully utilize the resources and grow larger to achieve higher fecundity and increased reproductive success (Tzeng et al. 2000 2002). Furthermore, although the sex ratios of the eels in different habitats may be highly variable, the final sex ratio of the entire spawning population would be suitable for maximum population growth when
they migrate back to the spawning ground.
In Taiwan, Japanese eel elvers in the estuary have been overexploited for aquaculture, and the adult eels in the rivers have also been over-fished due to their high price (Tzeng 1984 1997). These factors have probably led to both population declines and the skewed sex ratios. In Japan, the catches of both glass eels and adult eels have also shown decreasing tendencies (Tatsukawa 2003). If Japanese eel resources severely decline throughout East Asia, it might result in a wide-spread sex ratio bias and catastrophic population collapse. Thus, to sustain natural eel resources, habitat protection and prevention of over-fishing are urgently needed.
Giving the unique character of the eel sex ratio in relationship to population density, long-term changes in the eel sex ratio may reflect long-term changes in the eel resource. In conclusion, the use of the sex ratio as an indicator of the eel resource status may be helpful for eel conserva-tion. An analysis of the latest 5 yrs of eel sampling found that the sex ratio was strongly skewed towards females, which contrasts with historic information, suggesting imminent danger to the eel resource in the Kaoping River. The hypothesis that the variation in sex ratio in conjunction with different growth strategies for each sex serves to maximize the adaptive fitness of the eel might turn out to be hazardous when eel sex ratios in all habi-tats significantly shift toward females.
Acknowledgments: This study was financially
supported by the Council of Agriculture, Executive Yuan, Taiwan, R.O.C. (90AS-1.4.5-FA-F1-36 and 91AS-2.5.1-FA-F1-8). The authors are grateful to Mr. G. H. Cheng for sample collection and data processing and Mr. B. M. Jessop of Canada for reviewing an early draft of the manuscript.
REFERENCES
Bertin L. 1956. Eels: a biological study. London: Cleaver-Hume Press, 192 pp.
Chiba H, K Iwatsuk, K Hayami, K Yamauchi. 1993. Effect of dietary estradiol-17βon feminization, growth and body composition in the Japanese eel (Anguilla japonica). Comp. Biochem. Physiol. 106A: 367-372.
Cheng PW, WN Tzeng. 1996. Timing of metamorphosis and estuatine arrival across the dispersal range of the Japanese eel Anguilla japonica. Mar.Ecol.Prog.Ser.113: 89-96.
Clopper CJ, ES Pearson. 1934. The use of confidence or fidu-cial limits illustrated in the case of the binomial. Biometrika 26: 404-413.
Colombo G, G Grandi. 1996. Histological study of the devel-opment and sex differentiation of the gonad in the European eel. J. Fish Biol. 48: 493-512.
Colombo G, R Rossi. 1978. Environmental influences on growth and sex ratio in different eels population (Anguilla
anguilla L.) of Adriatic coasts. In Ds McLusky, AJ Berry,
eds. Physiology and behaviour of marine organisms. Proceedings of the 12th European Symposium on Marine Biology. Stirling, Scotland, Sept. 1977. Oxford and New York: Pergamon Press, pp. 313-320.
Degani G, D Kushnirov. 1992. Effects of 17β-estradiol and grouping on sex determination of European eels. Prog. Fish-Culturist 54: 88-91.
Ege V. 1939. A revision of the genus Anguilla Shaw, a system-atic, phylogenetic and geographical study. Dana Rep. 16: 1-256.
Han YS, IC Liao, YS Huang, JT He, CW Chang, WN Tzeng. 2002. Synchronous changes of morphology and gonadal development of silvering Japanese eel Anguilla japonica. Aquaculture 219: 783-796.
Helfman GS, DE Facey, LS Hales, EL Bozeman Jr. 1987. Reproductive ecology of the American eel. In MJ Dadswell, RJ Klauda, CM Moffit, RA Saunders, RL Rulifson, JE Cooper, eds. Common strategies of anadro-mous and catadroanadro-mous fishes. Bethesda, MD: American Fisheries Society Symposium 1, pp. 42-56.
Jessop BM. 1987. Migrating American eels in Nova Scotia. Trans. Am. Fish. Soc. 116: 161-170.
Krueger W, K Oliveira. 1999. Evidence for environmental sex determination in the American eel, Anguilla rostrata. Environ. Biol. Fish. 55: 381-389.
Larsson P, S Hamrin, L Okla. 1990. Fat content as a factor inducing migratory behavior in the eel (Anguilla anguilla L.) to the Sargasso Sea. Naturwissenschaften 77: 488-490.
Liao IC. 2001. A general review on aquaculture in Asia: a focus on anguillid eel. In 5th and 6th Asian Fisheries Forums. Chiang Mai, Thailand: AFS Special Publication no. 11, pp. 39-54.
Matsui I. 1972. Unagigaku: eel biology. Tokyo: Kosei-sha Kosei-Kaku Press.
Oliveira K. 1997. Movements and growth rates of yellow phase American eels, Anguilla rostrata, in the Annaquatucket River, Rhode Island. Trans. Am. Fish. Soc. 126: 638-646.
Oliveira K. 1999. Life history characteristics and strategies of the American eel, Anguilla rostrata. Can. J. Fish. Aquat. Sci. 56: 795-802.
Oliveira K, JD McCleave. 2000. Variation in population and life history traits of the American eel, Anguilla rostrata, in four rivers in Maine. Environ. Biol. Fish. 59: 141-151.
Oliveira K, JD McCleave, GS Wippelhauser. 2001. Regional variation and the effect of lake: river area on sex distribu-tion of American eels. J. Fish Biol. 58: 943-952.
Park EH, H Grimm. 1981. Distribution of C-band heterochro-matin in the ZW sex chromosomes of European and American eels (Anguillidae, Teleostomi). Cytogenet. Cell Genet. 31: 167-174.
Parsons J, KU Vickers, Y Warden. 1977. Relationship between elver recruitment and changes in the sex ratio of silver eels Anguilla anguilla L. migrating from Lough Neagh, Northern Ireland. J. Fish Biol. 10: 211-229.
Passakas T, RZ Klekowski. 1972. Chromosomes of European eel (Anguilla anguilla) as related to in vivo sex chromo-some. Pol. Arch. Hydrobiol. 20: 517-519.
Roncarati A, P Melotti, O Mordenti, L Gennari. 1997. Influence of stocking density of European eel (Anguilla anguilla, L.) elvers on sex differentiation and zootechnical perfor-mances. J. Appl. Ichthyol. 13: 131-136.
Satoh H, Y Nimura. 1991. Growth promotion in Japanese eel by oral administration of an estrogen (diethylstilbostrol). Nippon Suisan Gakkaishi 57: 21-27.
Satoh H, Y Nimura, T Hibiya. 1992. Sex control of the Japanese eel by an estrogen (DES-Na) in feed. Nippon Suisan Gakkaishi 58: 1211-1218.
Seber GAF. 1982. The estimation of animal abundance and related parameters. 2nd ed. London: Griffin Press. Sola L, G Gentili, S Cataudella. 1980. Eel chromosomes:
cyto-taxonomical interrelationships and sex chromosomes. Copeia 1980: 911-913.
Tatsukawa K. 2003. Eel resources in East Asia. Eel biology. Tokyo: Springer Press, pp. 293-298.
Tesch FW. 1977. The eel. Biology and Management of Anguillid Eels. London: Chapman and Hall Press. Tsukamoto K. 1992. Discovery of the spawning area for
Japanese eel. Nature 356: 789-791.
Tzeng WN. 1984. An estimate of the exploitation rate of
Anguilla japonica elvers immigrating into the coastal
waters of Shuang-Chi River, Taiwan. Bull. Inst. Zool. Acad. Sinica 23: 173-180.
Tzeng WN. 1985. Immigration timing and activity rhythms of the eel Anguilla japonica elvers in the estuary of northern Taiwan with emphasis on environmental influences. Bull. Jap. Soc. Fish. Oceanog. 47/48: 11-28.
Tzeng WN. 1997. Short- and long-term fluctuations in catches of elvers of the Japanese eel, Anguilla japonica. In Developing and sustaining world fisheries resources: the state of science and management. Victoria, Australia: 2nd World Fisheries Compress Proceedings, pp. 85-89. Tzeng WN. 2003. The processes of onshore migration of the
Japanese eel Anguilla japonica as revealed by otolith microstructure. In K Aida, K Tsukamoto, K Yamauchi, eds. Eel biology. Tokyo: Springer-Verlag, pp. 181-190. Tzeng WN. 2004. Modern research on the Natural life history
of the Japanese eel Anguilla japonica. J. Fish. Soc. Taiwan 31: 73-84.
Tzeng WN, PW Cheng, FY Lin. 1995. Relative abundance, sex ratio and population structure of the Japanese eel
Anguilla japonica in the Tanshui River system of northern
Taiwan. J. Fish Biol. 46: 183-201.
Tzeng WN, YS Han, JT He. 2002. The sex ratios and growth strategies of wild and captive Japanese eels Anguilla
japonica. In B Small, D MacKinlay, eds. Developments in
understanding fish growth. International Congress on the Biology of Fish. Vancouver, Canada: Univ. of British Columbia, pp. 25-42.
Tzeng WN, HR Lin, CH Wang, SN Xu. 2000. Differences in size and growth rates of male and female migrating Japanese eels in Pearl River, China. J. Fish Biol. 57: 1245-1253.
Vollestad LA. 1992. Geographic variation in age and length at metamorphosis of maturing European eel: environmental effects and phenotypic plasticity. J. Anim. Ecol. 61: 41-48.
Vøllestad LA, B Jonsson. 1986. Life-history characteristics of the European eel Anguilla anguilla in the Imsa River, Norway. Trans. Am. Fish. Soc. 115: 864-871.
Wiberg UH. 1983. Sex determination in the European eel (Anguilla anguilla L). Cytogenet. Cell Genet. 36: 589-598.
APPENDIX I:: Studies used for analysis of the relationship between the eel sex ratio and population density
Study Speciesa n Habitat Density character
This study AJ 479 Wild Eels/m2
Tzeng et al. 1995 AJ 97 Wild Eels/m2
Satoh and Nimura 1991 1992 AJ 84 Cultured Eels/m2
Tzeng et al. 2002 AJ 49 Cultured Eels/m2
Tzeng et al. 2002 AJ 40 Cultured Eels/m2
Jessop 1987 AR 635 Wild Lake area/Drainage area
Oliveira 1997 AR 245 Wild Eels/m2
Oliveira 1999 AR 1718 Wild Lake area/Drainage area
Oliveira and McCleave 2000 AR 1098 Wild Eels/m2
Oliveira et al. 2001 AR 3273 Wild Lake area/Drainage area
Parsons et al. 1977 AA 10346 Wild Elver restocking
Degani and Kushnirov 1992 AA 18 Cultured Isolated/Grouped Roncarati et al. 1997 AA 212622 Cultured Eels/m3