Clinical Pharmacology
Cardiology 2001;95:146–150
Efficacy and Safety of Imidapril in Patients
with Essential Hypertension:
A Double-Blind Comparison with Captopril
Por-Jau Huang Kou-Liong Chien
Ming-Fong Chen Ling-Ping Lai
Fu-Tien Chiang
Department of Internal Medicine (Cardiology Division), National Taiwan University Hospital, Taipei, Taiwan
Received: September 26, 2000 Accepted after revision: March 13, 2001
Por-Jau Huang, MD
Department of Internal Medicine (Cardiology Division)
ABC
© 2001 S. Karger AG, Basel0008–6312/01/0953–0146$17.50/0
Key Words
ImidaprilW HypertensionW CaptoprilW
Angiotensin-converting enzyme inhibitor
Abstract
In this 12-week, double-blind, parallel-group, compara-tive trial, 57 adult patients with mild-to-moderate hyper-tension were randomly allocated to receive imidapril or captopril, initially at a dose of 5 mg once a day and 25 mg twice daily, respectively. After 4 weeks of therapy, the dose of each drug was increased twice if diastolic blood pressure (DBP) remained 690 mm Hg. Both treatments effectively lowered DBP in a comparable manner. Mean changes from baseline in DBP at 12 weeks were –9.9 mm Hg for imidapril and –8.8 mm Hg for captopril (p = 0.488). Responder rates in patients receiving active treatment for at least 6 weeks were 53.9% for imidapril and 48% for captopril (p = 0.676). Both treatments were well tolerat-ed. Adverse drug reactions were observed in 20.7% (6/29) of the imidapril group and 46.4% (13/28) of the cap-topril group (p ! 0.05). A cough was the most frequent side effect, reported in 13.8% of the imidapril group and 35.7% of the captopril group. The results indicate that imidapril is as effective as captopril in the treatment of hypertension. Imidapril produces less adverse effects compared with captopril.
Copyright © 2001 S. Karger AG, Basel
Introduction
Angiotensin-converting enzyme (ACE) inhibitors have been established as one of the first choice of the antihyper-tensive drugs [1, 2]. They are also effective in retarding the progression of diabetic nephropathy [3] and in reduc-ing mortality in heart failure [4]. Although ACE inhibitors are usually well tolerated, a dry persistent cough with the incidence ranging from 1 to 33% [5, 6] is an annoying problem that occasionally necessitates a withdrawal of the drug.
Imidapril is a novel ACE inhibitor without the sulfhy-dryl group, currently under investigation for treatment of hypertension [7–9] and heart failure [10, 11]. It is a pro-drug that is converted by the liver to its active metabolite, imidaprilat. It has been reported that 5–10 mg of imida-pril once a day effectively decreases blood pressure in essential hypertensives [7–9]. Interestingly, the drug may have a relatively low incidence of cough [8, 9, 12]. Saruta et al. [8] observed that imidapril had a lower incidence (0.9%) of cough than that of enalapril (7%). Recently, in a multicenter, double-blind, cross-over study, Saruta et al. [9] also found that 52.8% of the patients with an enalapril-associated cough did not develop a cough after switching to imidapril, while all of the patients with an imidapril-associated cough developed a cough after switching to enalapril. In contrast, Shionoiri et al. [13] observed that 98.3% of the patients with an ACE inhibitor-induced
cough developed cough-related symptoms after receiving imidapril. They stressed that imidapril is not different from other ACE inhibitors in inducing a cough.
In Chinese patients receiving ACE inhibitors, an even higher incidence of cough has been observed than in Cau-casians [14]. The aim of the present study was to perform a randomized, double-blind study to evaluate the efficacy and cough incidence of imidapril, compared with capto-pril, in the treatment of mild to moderate hypertension in Chinese patients.
Patients and Methods
Patients
Male and female outpatients with mild to moderate essential hypertension ranging in age from 30 to 70 years were eligible to par-ticipate in this study. Mild to moderate hypertension was defined as sitting diastolic blood pressure (DBP) ranging from 95 to 115 mm Hg on two occasions (1 week apart) during a placebo run-in period of 2 or 3 weeks. Important exclusion criteria included the presence of heart failure, a history of myocardial infarction in the preceding 3 months, pregnancy, poorly controlled diabetes, angina pectoris, sec-ond- or third-degree heart block, significant hepatic or renal disease and secondary hypertension. All patients gave written consent to par-ticipate in the study. The study protocol was approved by the Institu-tional Review Board of the NaInstitu-tional Taiwan University Hospital and by the Taiwan Government (the Department of Health, Taiwan). The trial was conducted according to Good Clinical Practice require-ments.
Study Design
This study was a randomized, double-blind, parallel-group and dose titration trial. The design included a placebo run-in period of 2 or 3 weeks and an active treatment period of 12 weeks. After a single-blind, double-dummy, placebo run-in period, patients who met the inclusion criteria were randomly assigned to receive either 5 mg imi-dapril daily or 25 mg captopril twice daily. The blind, double-dummy treatment period consisted of a 4-week titration phase fol-lowed by an 8-week maintenance phase. Imidapril, its placebo and captopril were put into completely identical capsules. Patients on the imidapril regimen received a capsule (containing 5 mg imidapril) in the morning and a placebo capsule in the evening. Patients on the captopril regimen received a capsule (containing 25 mg captopril) each morning and evening. After 4 weeks of therapy, the dose of each drug was increased twice if sitting DBP was still 690 mm Hg. Con-comitant medications with antihypertensive properties were prohib-ited throughout the duration of the trial.
Patients were assessed at baseline and every 2 weeks during the treatment period. At each visit, pulse rate, sitting systolic blood pres-sure (SBP) and DBP and adverse events were recorded. For each patient, blood pressure was measured on the same arm and by the same physician. Blood pressure was measured twice at 5-min inter-vals after the patient had rested in a sitting position for 10 min, using a mercury sphygmomanometer. The average of the 2 measurements was taken for evaluation. Measurements were carried out at each vis-it at the same time of the morning before daily dosing. Therefore, for
imidapril, blood pressure was measured 22–26 h after the intake of the prior morning dose. Korotkoff phase V was used for the measure-ment of DBP. The 12-lead electrocardiogram and body weight were recorded at the beginning and at the end of the trial. Patient com-pliance was estimated by a pill count at each visit to the clinic.
Efficacy Assessment
The primary efficacy variable was the change from baseline in the mean sitting DBP at the endpoint of therapy. Secondary efficacy variables included the change in mean sitting SBP from baseline and responder rates (defined as the percentage of patients at the endpoint with sitting DBP !90 mm Hg or decrease in DBP 610 mm Hg com-pared to baseline).
Safety Assessment
Routine hematological, biochemical and urinary parameters were measured during the run-in period, at 4 weeks and at 12 weeks. The hematology measurements included hemoglobin, hematocrit, white blood count, differential cell count and platelet count. The blood chemistry examinations included total bilirubin, alkaline phospha-tase, blood urea nitrogen, serum transferase, creatinine, serum sodi-um, potassisodi-um, chloride, total cholesterol and glucose. The urinalysis included sediment and dipstick urinalysis for protein, blood and glu-cose.
All adverse events reported by the patient or observed by the investigator were recorded according to their duration, severity and relation to study treatment. The latter two were graded on a three-point scale. Any adverse event was reported in detail on the Case Report Form. A dry cough without identifiable cause lasting for more than 1 week and of sufficient severity was regarded as test drug-induced, whereas a cough which accompanied sneezing and/or a sore throat and ceased after treatment with medication was not consid-ered as drug-induced.
Statistical Analysis
Two data sets were used for data analysis. The ‘intent-to-treat’ data set included all patients who took at least one treatment dose and had baseline data. The ‘per-protocol’ population included all patients who received active treatment for at least 6 weeks.
For comparison of differences between the two treatment groups, an unpaired t test was utilized. ¯2 test or Fisher’s exact test was applied for the categorical variables. The changes in blood pressure and heart rate from the baseline were compared between the treat-ment groups by using the analysis of covariance with each baseline value as a covariate. The changes from baseline of blood pressure, heart rate and laboratory parameters in each medication group were assessed by paired t test. Differences were considered as statistically significant if p ! 0.05.
Results
Patient Disposition and Baseline Demographics A total of 57 patients were allocated randomly to imi-dapril (n = 29) and captopril (n = 28). All of these patients were qualified as the intent-to-treat population. There were no significant differences between the two treatment groups with respect to baseline demographic data
(ta-Males
Baseline
Coughing
Table 1. Baseline demographic characteristics
Imidapril (n = 29) Captopril (n = 28) 14 (48) 17 (61) Females 15 (52) 11 (39) Age, years 52.4B6.9 52.5B7.1 Weight, kg 65.4B10.6 67.9B10.5 Hypertension duration Newly diagnosed 2 (7) 2 (7) ! 5 years 16 (55) 19 (68) 5–10 years 8 (28) 3 (11) 1 10 years 3 (10) 4 (14) SBP, mm Hg 152.2B15.5 149.1B14.0 DBP, mm Hg 99.2B4.4 99.0B3.4
Pulse rate, beats/min 76.9B9.3 75.3B7.0
Values are expressed as means B SD. Figures in parentheses represent percentage. No significant differences were found between groups.
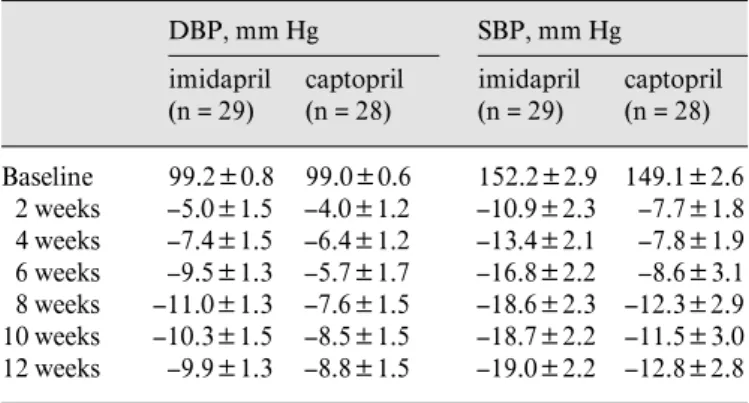
Table 2. Sitting DBP and SBP (intent-to-treat data set) DBP, mm Hg imidapril (n = 29) captopril (n = 28) SBP, mm Hg imidapril (n = 29) captopril (n = 28) 99.2B0.8 99.0B0.6 152.2B2.9 149.1B2.6 2 weeks –5.0B1.5 –4.0B1.2 –10.9B2.3 –7.7B1.8 4 weeks –7.4B1.5 –6.4B1.2 –13.4B2.1 –7.8B1.9 6 weeks –9.5B1.3 –5.7B1.7 –16.8B2.2 –8.6B3.1 8 weeks –11.0B1.3 –7.6B1.5 –18.6B2.3 –12.3B2.9 10 weeks –10.3B1.5 –8.5B1.5 –18.7B2.2 –11.5B3.0 12 weeks –9.9B1.3 –8.8B1.5 –19.0B2.2 –12.8B2.8
Values are means B SEM.
Table 3. Adverse drug reactions
Imidapril (n = 29) Captopril (n = 28) 4 (13.8) 10 (35.7) Impotence 1 (3.4) 0 Dysgeusia 1 (3.4) 2 (7.1) Pruritus 0 1 (3.6) Diarrhea 0 1 (3.6) Proteinuria 1 (3.4) 0
Abnormal liver function 0 1 (3.6)
Total number of patients with ADR 6 (20.7) 13 (46.4)* Figures in parentheses represent percentage. One of 6 patients taking imidapril and 2 of 13 patients taking captopril had more than one adverse drug reaction (ADR). * p ! 0.05 vs. imidapril.
ble 1). Fifty-one of the 57 patients receiving medications 66 weeks were classified as the per-protocol population. The remaining 6 patients, 3 in each group, received treat-ment for less than 6 weeks. Of the 6 patients, 2 in each group discontinued the treatment because of side effects. The other 2 patients (one in each group) withdrew be-cause there was no therapeutic effect.
Antihypertensive Efficacy
Table 2 shows serial changes in the blood pressure measured at 2-week intervals. Both the imidapril and
cap-topril treatments were effective at lowering sitting DBP and SBP. The reduction from baseline for either group was statistically significant after 2 weeks of treatment (p ! 0.05), and the effect persisted throughout the treatment period. Before week 8, there was a tendency toward a greater reduction in DBP and SBP as the treatment period lengthened. There was no statistically significant differ-ence between the two groups at any measurement point. After 12 weeks of monotherapy, mean changes from base-line in DBP were –9.9 mm Hg for imidapril and –8.8 mm Hg for captopril (p = 0.488). Mean changes from baseline in SBP were –19.0 and –12.8 mm Hg for imidapril and captopril, respectively (p = 0.065). There were no signifi-cant changes of the mean heart rate from baseline at each visit in either treatment group.
In the intent-to-treat population, the responder rate was 48.3% in the imidapril group and 46.4% in the capto-pril group (p = 0.889). In the per-protocol population, the responder rate was 53.9% in patients taking imidapril and 48% in those taking captopril (p = 0.676). The normalized rate (defined as the percentage of patients with DBP ! 90 mm Hg) was 46.2% in the imidapril group and 40% in the captopril group (p = 0.657).
Safety
Twenty-two (75.9%) patients in the imidapril group and 24 (85.7%) patients in the captopril group reported one or more adverse events at some point during the 12-week double-blind, comparative treatment period. Of these, only 6 (20.7%) patients in the imidapril group and 13 (46.4%) patients in the captopril group (p ! 0.05 vs. imidapril) suffered adverse events that were considered
by the investigator to be related to the trial medication (table 3). In both treatment regimens, the most frequently reported side effect was a dry cough. The incidence of drug-related coughing was more than twice as high with captopril (35.7%) than with imidapril (13.8%). Statistical-ly the difference was of borderline significance (p = 0.055).
One patient receiving imidapril had mild proteinuria. Another patient taking captopril had mild elevation of levels of aminotransferases. The remaining patients in either group did not have relevant changes in laboratory parameters during the study. There were no serious ad-verse experiences reported in this trial.
Discussion
Imidapril is a long-acting ACE inhibitor. The elimina-tion half-life was 2 h for imidapril and 8 h for imidaprilat [15]. ACE activity was still suppressed to under 40% of the baseline value 24 h after 5- and 10-mg doses [7]. In patients with mild to moderate hypertension, 5–10 mg once daily of imidapril was judged as effective in 71% of treated patients [8]. High doses did not significantly increase the response rate [7]. Captopril, on the other hand, is the first ACE inhibitor. In the treatment of mild to moderate hypertension, 50–100 mg per day was found effective [2, 16]. A starting dose of 25 mg twice a day seems appropriate for most patients [16]. Therefore, the doses and the frequency of dosing selected in the present double-blind study were 5–10 mg once daily for imidapril and 25–50 mg twice per day for captopril.
Results of this study show that imidapril 5–10 mg once a day is at least as effective as captopril 25–50 mg twice daily in reducing blood pressure in patients with mild to moderate hypertension. There are no significant differ-ences in antihypertensive efficacy between the two agents. The reductions in DBP and SBP by imidapril and capto-pril are of a similar magnitude. In the present study, the normalized rate (DBP !90 mm Hg) in the per-protocol population was 46.2% for imidapril and 40% for capto-pril. This is consistent with the study by Saruta et al. [8] who reported that the normalized rate was 40.7% for imi-dapril and 40% for enalapril.
The present study also assessed the safety of the two drugs. We found that both trial medications were well tol-erated during the treatment period. However, adverse drug reactions occurred less commonly in patients taking imidapril than in those taking captopril (20.7 vs. 46.4%, p ! 0.05).
The fact that there are fewer side effects, especially coughing, in the imidapril group compared with captopril is of interest. Woo et al. [14] reported that Chinese patients had a very high incidence of cough associated with ACE inhibitors. Using a quality-of-life question-naire, they observed that the prevalence of cough was 54% in Chinese patients receiving captopril for 16 B 5 months compared with 19% in Caucasians receiving cap-topril for a similar period of time. In this study coughing occurred in 35.7% of patients taking captopril for 12 weeks. The high incidence of a captopril-induced cough in Chinese in the present study is comparable with the results of Woo et al. However, a cough occurred only in 13.8% of the cohort taking imidapril. The relatively low incidence of cough in patients with imidapril has also been observed among Japanese patients [8, 9, 12]. Saruta et al. [8] reported that a cough occurred in 0.9% (1/108) of patients treated with imidapril for 12 weeks compared with 7% (8/115) with enalapril. In a multicenter study [9], the incidence of cough was reported to be significantly lower in the imidapril group (15.2%) than in the enalapril group (38.6%).
The exact mechanism of an ACE inhibitor-induced cough remains unclear. Several mechanisms have been proposed [17–23]. Bradykinin, which is normally inacti-vated in part by ACE, accumulates in the lung as a result of inhibition of ACE. Accumulation of bradykinin stimu-lates afferent vagal C fibers [18] and enhances broncho-constriction [19], thus promoting a cough. Substance P, a potent bronchoconstrictor, is also degraded by ACE. Therapy with ACE inhibitors could result in local accu-mulation of this substance, leading to a cough in suscepti-ble patients [20]. Both bradykinin and substance P aug-ment formation of prostaglandins, which may be involved in the pathogenesis of a cough [21, 22].
Sasaguri et al. [24] in an in vitro study using purified canine lung ACE disclosed that different ACE inhibitors had different potencies in the hydrolysis of bradykinin and angiotensin I. Their data showed that the accumula-tion of bradykinin relative to the inhibiaccumula-tion of angiotensin II formation was significantly reduced with imidaprilat than with enalaprilat, ramiprilat or captopril. This may partly explain the relatively low incidence of cough for imidapril, as noted in the present study as well as other clinical trials.
Conclusions
This study shows that imidapril 5–10 mg once a day is at least as effective as captopril 25–50 mg twice daily in reducing blood pressure. Both drugs were generally well tolerated. However, imidapril produced fewer side effects compared with captopril. Thus, imidapril can be pre-scribed effectively and safely in patients with mild to moderate hypertension.
Acknowledgment
This study was partly supported by Taiwan Tanabe Seiyaku Co., Ltd., Taipei.
References
1 Guidelines Subcommittee: 1999 World Health Organization–International Society of Hyper-tension guidelines for the management of hy-pertension. J Hypertens 1999;17:151–183. 2 Joint National Committee: The sixth report of
the Joint National Committee on prevention, detection, evaluation and treatment of high blood pressure. Arch Intern Med 1997;157: 2413–2446.
3 Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, for the Collaborative Study Group: The effect of angiotensin-converting enzyme inhibition on diabetic nephropathy: The Collaborative Study Group. N Engl J Med 1993;329:1456– 1462.
4 Garg R, Yusuf S, for the Collaborative Group on ACE Inhibitor Trials: Overview of random-ized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in pa-tients with heart failure. JAMA 1995;273: 1450–1456.
5 Israili ZH, Hall WD: Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy: A review of the liter-ature and pathophysiology. Ann Intern Med 1992;117:234–242.
6 Karlberg BE: Cough and inhibition of the re-nin-angiotensin system. J Hypertens 1993; 11(suppl 3):S49–S52.
7 Vandenburg MJ, Mackay EM, Dews I, Pullan T, Brugier S: Dose-finding studies with imida-pril: A new ACE inhibitor. Br J Clin Pharmacol 1994;37:265–272.
8 Saruta T, Omae T, Kuramochi M, Iimura O, Yoshinaga K, Abe K, Ishii M, Watanabe T, Takeda T, Ito K, Kokubu T, Fujishima M, Ara-kawa K, Nakajima M: Imidapril hydrochloride in essential hypertension: A double-blind com-parative study using enalapril maleate as a con-trol. J Hypertens 1995;13(suppl 3):S23–S30.
9 Saruta T, Arakawa K, Iimura O, Abe K, Mat-suoka H, Nakano T, Nakagawa M, Ogihara T, Kajiyama G, Hiwada K, Fujishima M, Naka-shima M: A multicenter comparative study of imidapril and enalapril on usefulness and inci-dence of cough. J New Remedies Clin 1998;47: 249–282.
10 Pinto YM, van Veldhuisen DJ, Tjon-Ka-Jie RT, Rooks G, Netzer T, Lie KI: Dose-finding study of imidapril, a novel angiotensin convert-ing enzyme inhibitor, in patients with stable chronic heart failure. Eur J Clin Pharmacol 1996;50:265–268.
11 Van Veldhuisen DJ, Genth-Zotz S, Brouwer J, Boomsma F, Netzer T, Man in’t Veld AJ, Pinto YM, Lie KI, Crijns HJGM: High-versus low-dose ACE inhibition in chronic heart failure: A double-bind, placebo-controlled study of imi-dapril. J Am Coll Cardiol 1998;32:1811–1818. 12 Nishikawa Y, Ogawa S: Incidence of cough induced by imidapril in patients with hyperten-sion with enalapril-associated cough. Curr Ther Res 1997;58:601–608.
13 Shionoiri H, Takasaki I, Minainisawa K, Ueda S, Kihara M, Shindo K, Hiroto S, Sugimoto K, Himeno H, Naruse M, Nagamochi I, Yasuda G: Cough-challenge trial with a new angioten-sin-converting enzyme inhibitor, imidapril. J Clin Pharmacol 1998;38:442–446.
14 Woo KS, Norris RM, Nicholls G: Racial differ-ence in inciddiffer-ence of cough with angiotensin-converting enzyme inhibitors (a tale of two cities). Am J Cardiol 1995;75:967–968. 15 Hirota Y, Kawamura K, Ooyagi A, Hayashi S,
Morioka A, Terasaki Y, Tagawa K, Mizobe M: Phase I study of TA-6366 (I): Single oral ad-ministration. Rinshou Iyaku 1992;8:507–522.
16 Kaplan NM: Angiotensin-converting enzyme inhibitors; in Kaplan NM (ed): Clinical Hyper-tension, ed 6. Baltimore, Williams & Wilkins, 1994, pp 240–246.
17 Bucknall CE, Neilly JB, Carter R, Stevenson RD, Semple PF: Bronchial hyperreactivity in patients who cough after receiving angiotensin converting enyzme inhibitors. Br Med J 1988; 296:86–88.
18 Kaufman MP, Coleridge HM, Coleridge JCG, Baker DG: Baker DG: Bradykinin stimulates afferent vagal C-fibers in intrapulmonary air-ways of dogs. J Appl Physiol 1980;48:511– 517.
19 Fuller RW, Dixon CM, Cuss FM, Barnes PJ: Bradykinin-induced bronchoconstriction in humans: Mode of action. Am Rev Respir Dis 1987;135:176–180.
20 Shore SA, Stimler-Gerard NP, Coats SR, Draz-en JM: Substance P-induced bronchoconstric-tion in the guinea pig: Enhancement by inhibi-tors of neutral metalloendopeptidase and an-giotensin-converting enzyme. Am Rev Respir Dis 1988;137:331–336.
21 Hartung HP, Heininger K, Schafer B, Toyka KV: Substance P and astrocytes: Stimulation of the cyclooxygenase pathway of arachidonic acid metabolism. FASEB J 1988;2:48–51. 22 Coleridge HM, Coleridge JC, Ginzel KH,
Bak-er GD, Banzett RB, Morrison MA: Stimulation of ‘irritant’ receptors and afferent C-fibres in the lungs by prostaglandins. Nature 1976;264: 451–453.
23 Yeo WW, Ramsay LE, Morice AH: ACE inhib-itor cough: A genetic link? Lancet 1991;337: 187.
24 Sasaguri M, Ideishi M, Kinoshita A, Arakawa K: Differential inhibition of bradykinin hydro-lysis by four ACE inhibitors: A possible expla-nation for differences in induced coughing. Hy-pertens Res 1994;17:253–258.