行政院國家科學委員會專題研究計畫 期中進度報告
研究腫瘤浸潤性淋巴球所分泌的 IL-6 調控腫瘤細胞 MHC 表
現的機制(2/3)
期中進度報告(精簡版)
計 畫 類 別 : 個別型 計 畫 編 號 : NSC 95-2313-B-002-025- 執 行 期 間 : 95 年 08 月 01 日至 96 年 07 月 31 日 執 行 單 位 : 國立臺灣大學獸醫學系暨研究所 計 畫 主 持 人 : 朱瑞民 處 理 方 式 : 期中報告不提供公開查詢中 華 民 國 96 年 05 月 31 日
行政院國家科學委員會專題研究計畫 期中進度報告
研究腫瘤浸潤性淋巴球所分泌的IL-6 調控腫瘤細胞MHC表
現的機制(2/3)
計畫類別: 個別型計畫
計畫編號: 95-2313-B-002-025
執行期間: 95 年08 月01 日至96 年07 月31 日
執行單位: 國立臺灣大學獸醫學系暨研究所
計畫主持人: 朱瑞民
報告類型: 精簡報告
處理方式: 本計畫可公開查詢
中 華 民 國 96 年 5 月 15 日
摘要
腫瘤細胞具有逃避免疫系統監測之機制,因而在體內不停增生,使宿主本身 生理功用失常,加上惡性腫瘤細胞的轉移最終造成嚴重後果。腫瘤細胞逃避免疫 系統監測機制之的主要幾種包括(1)使免疫系統失去辨識能力。最常見的是主要 組織相容複合物 (Major histocopatibility complex, MHC)不表現或表現量極低,(2)
免疫系統毒殺腫瘤細胞能力下降。抑制免疫細胞之毒殺作用包括T 細胞及 NK 細
胞,及 (3)分泌抑制性分子,抑制免疫細胞的作用。
因為犬傳染性花柳性腫瘤(canine transmissible venereal tumor, CTVT)生長期
時,TGF-β 除了抑制 IFN-γ 誘導 MHC 作用,並抑制(tumor infiltrating lymphocyte,
TIL)的毒殺能力,造成 CTVT 在宿主體內快速生長。但實驗接種 CTVT 於犬隻 背部皮下後會自然地進入消退期(R 期)。造成消退的主因來自 R 期時 TIL 分泌大
量的IL-6,拮抗 TGF-β 抑制 IFN-γ 之作用,使 IFN-γ 能誘導 CTVT 的 MHC 表現,
使T 細胞活化。另外 IL-6 也拮抗 TGF-β 抑制 NK 細胞的毒殺能力,腫瘤因此進 入R 期。 本實驗乃針對CTVT 之特性,嘗試以 IL-6 導入基因解除 TGF-β 對免疫系統 抑制能力,使CTVT 細胞之 MHC 分子表現量上升,更進一步導入 IL-15 基因活 化免疫系統,達到成功治療腫瘤之目的。因此構築IL-6 及 IL-15 基因的質體,用 腫瘤內電衝方式治療CTVT,並研究此基因治療的效果與抗腫瘤機制。實驗結果 證明合併使用IL-6 與 IL-15 不但能顯著的延緩 CTVT 的生長更可以成功使腫瘤 消退。在複合式療法下,腫瘤中IL-6 與 IL-15 的濃度增加、腫瘤細胞的 MHC 表 現量上昇。同時發現腫瘤內TIL 浸潤程度增加,並有效提升 NK 細胞與 CTL 的 毒殺能力。以上這些結果顯示使用腫瘤內電衝,進行IL-6 與 IL-15 基因治療,在 實驗犬模式,可成功破壞腫瘤逃避免疫系統的作用並提昇免疫反應,達到成功治 療腫瘤之目的。本研究結果所使用之策略:一方面提高MHC 的表現,一方面強 化NK 細胞的作用,可做為未來對腫瘤治療上的一個新選擇。
關鍵字:CTVT、IL-6、IL-15、gene therapy、intratumoral electroporation
Abstract
Malignant tumor cells that can escape the surveillance of immune system and proliferate unrestrictedly. This mechanism make host physiological function be abnormal. Furthermore, malignant tumor cells can metastasize and influence many tissues and organs. There are three mechanisms of tumor cell escape: (1)Lack of recongizion; (i.e., tumor cells do not express MHCⅠmolecule to escape T lymphocytes recongizionm.)(2) Decrease of cytotoxicity; (i.e., escape from the cytotoxic mechanisms of T cells and NK cells) (3)Inhibition of immune system: Tumor cells or stromal cells can express some inhibiting factor to suppress the function of immune stystem.
In P phase, Canine transmissible venereal tumor (CTVT) can secrete TGF-β to inhibit the inducing MHC molecule activity of IFN-γ and the cytotoxicity of tumor infiltrating lymphocytes (TIL). CTVT can proliferate in canine, but experimentally transplanted CTVT exhibits a spontaneous regression (R phase). This is because that high concentration of IL-6 from TIL in R phase antagonizes the activity of tumor-derived TGF-β. So, IFN-γ can induce MHC expression and make T cells repair its activity. IL-6 can restore the cytotoxicity of NK cells. The effects make CTVT regress.
Based on these finding of CTVT, our laboratory tested that transfer IL-6 gene to antagonize TGF-β activity and induce MHC expression of CTVT. Transferring IL-15 gene activate immune system. We combined two strategies to perform successful tumor therapy. So we constructed IL-6 and IL-15 plasmid and used intratumoral electroporation to treat CTVT. Besides, we studied this gene therapy and anti-tumor mechanism. In the results of my experiment, combined treatment of IL-6 and IL-15 can inhibit and suppress the growth of CTVT. This treatment elevated IL-6 and IL-15 concentration in tumors. The surface expression of MHC molecules on CTVT cells was significantly increased after gene therapy. In addition, the IL-6 and L-15 gene therapy induced more infiltrating lymphocytes in the tumors and influence cytokine profile of CTVT. NK cells and CTL cytotoxicity also can be increased. Our results demonstrate that intratumoral electroporation-mediated IL-6 and IL-15 gene therapy for canine transmissible venereal tumor in the canine model can destroy tumor escape successfully and increase immune response. The strategy of the study can be a new choice to treat tumor.
前言
Tumor escapes are important mechanisms of tumor proliferation. There are many strategies that include : (1) Evasion of recognition:Tumor cell can express low MHC class I or alter MHC class I (1), (2) Insensitive to the effect of cytotoxicity : The effect of NK and CTL are Lack of susceptibility. Immune cell can’t express of Fas/CD95(2), (3) Expression of specific cytokines and chemokines(3, 4) There are inhibiting effects of TGF-β (5)and IL-10 (6)on immune system. The chronic inflammation associated with cancer may induce local and systemic immune suppression. If want to perform the therapy well, we need to understand the character of tumor cells.
Canine transmissible venereal tumor (CTVT) is an excellent animal model with which to study tumor-host interactions. Experimentally transplanted CTVT exhibits a predictable growth pattern that includes a progressive growth (P phase) and a
spontaneous regression (R phase)(7). There are many effects of tumor escape on CTVT proliferation. (1)CTVT expresses extremely low numbers of MHC, much like malignant tumors in humans (8). (2) The activity of IFN-γ to induce MHC molecules expression and the cytotoxicity of tumor infiltrating lymphocytes (TIL) are seriously suppressed by TGF-β secreting from CTVT cells.
Interleukin-6 (IL-6) is a pleiotropic cytokine that can influence a wide range of biological activities(9). It is produced by both lymphoid and nonlymphoid cells and helps regulate immune reactivity(10), the acute phase response(11), inflammation(12), oncogenesis(13), and hematopoiesis(14). IL-6-mediated anti-tumor effects have been shown in several tumor type, such as some breast(MCF-7, ZR-75-1)(15), colon(16), pancreas(17), prostate(18) cancer cells or no effects on adenocarcinoma(19),
malignant glioma cells(20), T47D, MDA-MB-231, and ZR-75-1 human breast carcinoma cells line.(21)
Interleukin (IL-15) is a pleiotropic cytokine that plays an important role in both the innate and adaptive immune system. I IL-15 promotes activation of NK cell(22), T cells(23, 24), neutrophils(25, 26) and macrophages(27), and is critical to dendritic cell (28)function in several model systems. In addition, IL-15 promotes the development, activation, homing and survival of immune effector cells, particularly NK cells(29, 30), natural killer T (NKT) cells(29) and CD8+T cells(31, 32). So, IL-15 is a common candidate to treat tumors.
Gene therapy is a powerful strategy to treat tumor. In this study, we constructed IL-6 and IL-15 plasmids and performed intratumoral electroporation with these pasmids. IL-6 and IL-15 can activates CTL and NK cells cytotoxicity and suppresses CTVT growth.
結果 Fig1A
1 2 3 4 5 6 7 8 9 10 11
E. coli clones 1-10
100 bp DNA Ladder marker 11
Lane Parameter
Fig1B
1 2 3 4 5 6 7 8 9 10 11
Lane Parameter
11 100 bp DNA Ladder marker
1~10 E. coli clones
Fig1C
IL-6 500 bp
Fig1D.
IL-15 500 bp
Fig1A IL-6 rapid screening. Using T7 and hIL-6 AS primer can detect IL-6 expression in agarose gel. IL-6 sequence is about 720bp.
Fig1B. IL-15 rapid screening. Using T7 and hIL-15 AS primer can detect IL-15 expression in agarose gel. IL-6 sequence is about 490bp
Fig1C Restriction enzyme digestion of IL-6 plasmid. IL-6 plasmid was digested with HindⅢ and XhoⅠ and IL-6 nucleotide sequence is about 720bp.
Fig1D Restriction enzyme digestion of IL-15 plasmid. IL-15 plasmid was digested with HindⅢ and XhoⅠ and IL-15 nucleotide sequence is about 720bp.
L-6 and IL-15 gene of plasmids were amplified by the polymerase chain reaction with T7, IL-6AS or IL-15AS primers. Agarose gel electrophoresis of the IL-6 and IL-15 PCR reaction products revealed a single band of about 727bp(Fig.1A) and 402 bp(Fig.1B). IL-6 and IL-15 plasmids were digested by restriction enzymes (HindⅢ and XhoⅠ). The results of the restriction enzyme digestion also revealed a single band as expected(Fig.1C, D). BALB/3T3 cells were transfected by IL-6 or IL-15 plasmids. After 48 h of incubation at 37°C, supernatants were collected and the concentration were measured with ELISA(Fig.2A, 2B) IL-6 dependent cell line “TF-1” and IL-15 dependent cell line “HT-2” were performed IL-6 and IL-15 functional assay(Fig.2C, 2D). The proliferation assay performed with CellTiter 96® AQueous One Solution.
Fig2A Fig2B
IL-6 expression in Balb/3T3 sup
Vector IL-6 0 250 500 750 1000 1250 IL-6 expression Balb/3T3 supernatant IL-6 conc ent rai on ( pg/ m l)
IL-15 expression in Balb/3T3 supernatant
300 400 500 600 700 800 900 pcDNA3.1 IL-15 0 100 200 Balb/3T3 supernatant IL -15 con cent rat ion ( p g/ m l) 6 IL-15 expression
Fig2C
Fig2D
Fig2A. IL-6 concentration can be detected in BALB/3T3 supernatant. “Vector” is an empty plasmid that do not insert any sequence. “IL-6” is an IL-6 plasmid. IL-6 concentration is about 1043.6pg/ml in BALB/3T3 supernatant.
Fig2B. IL-15 concentration can be detected in BALB/3T3 supernatant. “IL-15” is an IL-15 plasmid. IL-15 concentration is about 694pg/ml in BALB/3T3
supernatant.
Fig2C. BALB/3T3 supernatant of IL-6 promoted TF-1 proliferation.TF-1 cells are IL-6 dependent cell line. “PC” is a positive control using TF-1 growth medium. “PC-hIL-6” is a positive control using 5ng/ml IL-6 protein. “IL-6” is the
BALB/3T3 supernatant of IL-6. “Vector” is the BALB/3T3 supernatant of empty vectors. “IL-6” promoted TF-1 proliferation significantly. (*, p<0.05) Fig2D. BALB/3T3 supernatant of IL-15 promoted HT-2 proliferation. “IL-15” is the
BALB/3T3~IL-15 supernatant. “Vector” is the BALB/3T3 supernatant of empty vectors. “IL-15” promoted HT-2 proliferation significantly. (*, p<0.05)
Fig3A. Fig3B.
IL-6 concentration in tumor mass IL-6 concentration in serum
Fig3C. Fig3D.
When tumoral diameter were about 2cm, we performed intratrumoal
electroporation-mediated IL-6 and IL-15 genes. 1mg IL-6 and 1mg IL-15 plasmids were mixed in 1ml isotonic sodium chloride solution. 1mg empty vector
(pcDNA3.1/V5-His-TOPO) was performed in control. We determined the secretion of IL-6/IL-15 from IL-6/IL-15 gene transfected, CTVT-IL-6/15 cells. The solution of CTVT-IL-6/IL-15 tumor lysis can detected highest IL-6/IL-15 expression after gene therapy 1 week (Fig3A,3B). In the solution of tumor lysis, the level of IL-6/IL-15
IL-15 concentration in tumor mass
0 1 2 4 0 250 500 750 1000 1250 Vector GT
W eeks after IL-6/IL-15 GT
IL-1 5c once nt ra ti on ( pg/ m l)
IL-15 concentration in serum
0 1 2 4 0 250 500 750 1000 1250 Vector GT
Weeks after IL-6/IL-15 GT
IL -15 co n c en tr a ti o n ( p g /m l) 0 1 2 4 0 250 500 750 1000 1250 Vector GT
W eeks after IL-6/IL-15 GT
IL -6 con c ent rat io n ( p g/ m l) 200 Vector 0 1 2 4 0 50 100 150 GT
W eeks after IL-6/IL-15 GT
IL -6 con c ent rat io n ( p g/ m l) 8
increased much higher in CTVT-IL-6/IL-15 tumor mass than in CTVT-vector. The highest IL-6 expression was the case of gene therapy 1 week(Fig3C, Fig3D). Therefore, IL-15 can not be detected in serum.
Fig4A
CTVT growth curve
0 1 2 3 4 5 6 7 8 9 10 0 25 50 75 100 pcDNA3.1 GT-1 GT-2 weeks Tum o r s iz e ( c m 3 ) Fig4B. VectorFig4C GT-1 Fig4D GT-2 Fig4C MHC class I BG Vector GT 0 5 10 15 20 25 30 35 E x pr essi o n ( % ) * * MHC class II BG Vector GT 0 5 10 15 20 25 30 35 E x pr essi o n ( % ) * *
Fig4A. Tumor growth can be inhibited after transfected by IL-6 and IL-15 plsamid. After gene therapy 6 weeks, Tumor mean volume in the “vector” was
92.76cm3. Tumor mean volume in the “vector” was 20.77 and 2.2 cm3. IL-6/IL-15 gene therapy can inhibit CTVT growth.
Fig4B. There are the pictures of the experimental canine. These picture were taken after gene therapy 6 weeks. “Vector” was treated with an empty plasmid. “GT-1” was one treated dog with IL-6/IL-15 plasmid and “GT-1” was another one.
Fig4C.FACS analysis of MHC expression from CTVT treated with IL-6/IL-15 cells. MHC expression of CTVT were elevated continuedly from gene therapy. In gene therapy 4 week, the percentage of CTVT MHCⅠexpression and CTVT MHCⅡexpression were 34% and 25%.
We measured with tumor dimensions once a week and estimated the tumor volume. After IL-6 and IL-15 transfection, the CTVT growth were inhibited.(Fig4A) and elevated MHC molecules expression on CTVT cells(Fig4C). MHC expression were defined by canine MHC class I, canine MHC class II and mouse IgG2a. The MHC
expression of CTVT cells were about 30% after gene therapy. There are the picture of CTVT growth. (Fig4B) Fig5A CTVT cells TIL Vector GT Fig5B. CD3 CD4 CD8 CD21 Non-CD4,8,21 0 10 20 30 40 50 60 BG Vector GT E xp ressi o n ( % )
*
Fig5A. The level of TIL infiltration in tumor mass was elevated. Tumor sections
fixed with formalde were stained by hematoxylin and eosin. This is the sections from gene therapy 1 weeks.
Fig5B. CD8+ T cells were up-regulated by IL-6/IL-15.
CD8+ T cells can up-regulate and the percentage of TIL population were about 21.63% from the results of FACS Calibur flow cytometer(Becton Dickinson, NJ, USA)at 4 weeks after gene therapy. There were no significant effects of
the other cell surface marker. (*, p<0.05)
In order to understand the level of TIL infiltration influenced by IL-6/IL-15, tumor sections fixed with formalde were stained by hematoxylin and eosin. On the sections, we can detect higher TIL infiltration of IL-6/IL-15 plasmids after gene therapy 1 week than vector(Fig5A).
TIL population were stained by mouse anti-canine CD3、mouse anti-canine CD4、mouse anti dog CD8 mAb、mouse anti-canine B cells、IgG1. CD8+ T cells were up-regulated by IL-6/IL-15(Fig5B).
Fig 6.
Fig 6. CTVT cytokine profile
We performed Real-time RT-PCR to compare the difference of cytokine secretion form CTVT cells. IL-2、IL-4、IL-6、IL-12、IFN-γ can not be determined. After gene therapy, IL-8 up-regulation can be detected. This results was the same as R phase. The mRNA level of TNF-α increased, too. (p<0.05)
Real time RT-PCR is the powerful methods to detect the level of mRNA expression. We focused on IL-1β、IL-2、IL-4、IL-6、IL-8、IL-10、IL-12、IL-13、IL-15、IL-18、 TNF-α、TGF-β、IFN-γ and wanted to compare the difference between control and IL-6/IL-15 group. The results showed that mRNA level (relative to β-actin) were in P, R, before treatment, control(Vector) and IL-6/IL-15 groups. IL-8 in R phase and IL-6/IL-15 group were significantly higher than P phase and Vector (p<0.05). TNF-α was significantly higher than P phase and Vector, too (p<0.05). IL-13 in R phase was significantly higher than P phase (p<0.05). The level of TGF-β mRNA can not be influenced after IL-6 and IL-15 transfection. IL-2、IL-4、IL-6、IL-12、IFN-γ can not be determined. The difference of cytokine expression may correlate with tumor
regression.
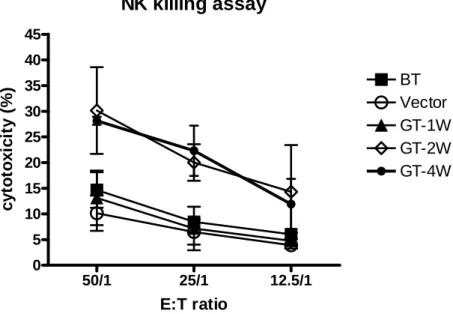
Fig7 NK killing assay 50/1 25/1 12.5/1 0 5 10 15 20 25 30 35 40 45 BT Vector GT-1W GT-2W GT-4W E:T ratio c yt o to xi ci ty ( % )
Fig7. Transfecting IL-6 and IL-15 genes activates NK cells cytotoxicity.
After gene therapy 2weeks, the cytotoxicity of NK was up-regulated significantly. After gene therapy 4 weeks, the cytotoxicity of NK was similar with 2 weeks. IL-6 (33)and IL-15(30, 34) can activate NK cytotxic activity. In this study, we used CTAC to play a role of target cells in NK killing assay. Cytotoxicity of PBL against the CTAC cell line was measured between “Vector” and “GT” group. The cytotoxic percentage of CTAC was found to be significantly increased (P<0.05) after gene therapy 2 weeks
Fig8 13 0 5 10 15 20 25 30 BT Vector GT-1W GT-2W GT-4W cyt o to xi ci ty ( % )
Fig8. CTVT specific killing activity of CTL was up-regulated after gene therapy
After gene therapy 2weeks, the cytotoxicity of CTL was up-regulated
significantly. (p<0.05) After gene therapy 4 weeks, the cytotoxicity of CTL was similar with 2 weeks.
To investigate the effect of tumor antigen specific CTL on CTVT of intratumoral electroporation-mediated IL-6 and IL-15. PBMC of canine cultured with CTVT. After 6 days, tumor antigen specific CTL were induced. CTL activity increased after gene therapy 1 week. The cytotoxicity of CTL was significantly increased after gene therapy 2 weeks and 4 weeks. (p<0.05)
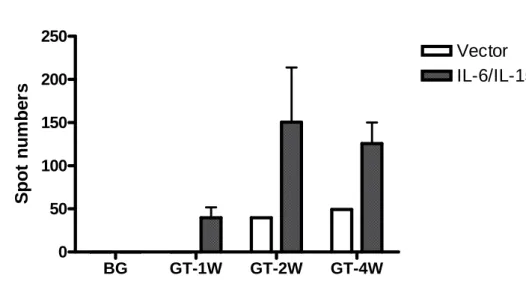
Fig9 BG GT-1W GT-2W GT-4W 0 50 100 150 200 250 Vector IL-6/IL-15 Spot num be rs
Fig9 IFN-γELISPOT results. Forming spots were counted. After gene therapy, IL-6
and IL-15 can increase CTVT-specific CTL cytotoxicity. If tumor antigens combined with T cell receptors, T cells activate and secrete IFN-γ. Using tumor lysis to activate CTL, we counted spots and determined CTVT-specific CTL activity. CTL killing activity increased since gene therapy 1 weeks.
討論
The results of the present study strongly suggest that cotransfection of IL-6 and IL-15 genes elicits powerful antitumor immune responses against CTVT in canine model. Canine transmissible venereal tumor (CTVT) is an excellent animal model to study tumor-host interactions. Experimentally transplanted CTVT exhibits a
predictable growth pattern that includes a progressive growth (P phase), stasis (S phase) and a spontaneous regression (R phase). CTVT expresses extremely low numbers of MHC during P phase, much like malignant tumors in humans, but MHC expression in CTVT increases to 30–40% during R phase. So, studying tumor-host interactions of CTVT and perform good therapy are important for cancer reaserches.
The major suppressive effect of CTVT comes from TGF-β. TGF-β can suppress immune system, consisting of inhibiting DCs antigen presentation, T cells secreting IFN-γ activity, decrease T cells proliferating activity, inactivate NK cytotoxicity and activate T regulatory cells proliferation. CTVT produces functional TGF-β during P and R phase. The activity of IFN-γ to induce MHC molecules expression and the cytotoxicity of TIL are seriously suppressed by TGF-β secreting from CTVT in P phase(35, 36). TIL isolated from regressing CTVT secrete high concentrations of IL-6. Host high concentrations of IL-6 antagonize the anti-IFN-γ activity of tumor TGF-β to restore MHC expression and anti-TIL activity of tumor TGF-β to recover the cytotoxicity of TIL (35, 36). The mechanism is the reason of tumor regression. Based on these results, IL-6 is the most important factor to make CTVT regress and we wanted that using this cytokine to antagonize TGF-β activity.
Interleukin-6 (IL-6) is a pleiotropic cytokine that can influence a wide range of biological activities(9).Several kinds of tumor cells transfected with IL-6 gene had been studied in vivo. IL-6 gene -transfected B16 melanoma cells showed stronger adhesion to the lymphokine-activated killer (LAK) cells or cytotoxic T lymphocytes (CTL), and higher sensitivity to cytotoxicity of LAK cells or CTL, because MHC class I and ICAM-1 expression were increased after IL-6 gene transfection(37). IL-6 inhibit of growth of the human ductal breast carcinoma cell line T-47(38). IL-6 also has the ability to enhance natural killer (NK) cells activity(38). In previous studies, IL-6 can antagonize TGF-β inhibiting activity to restores LAK (lymphokine-activated killer) killing activity, but IL-6 does not promote LAK killing activity(35). We can not sure that increasing NK killing activity is influenced by IL-6. The percentage of MHC expression on CTVT after intracellular electroporated with IL-6 and IL-15 is significantly higher than control. The results can confirm the previous data that IL-6 can induce MHC expression of CTVT. Increasing MHC classⅠ expression can promotes to recognize tumor antigens by the presentation of small antigenic peptides
to the receptor of CTL and reject the tumor. In CTL and ELISpot experiments, CTL can recognize small antigenic peptides and CTL are activated by clonal selection.
In the aspect that IL-6 influence tumor cell growth, although IL-6 can activate anti-apoptosis protein, such as Bcl-2 and Bcl-XL, and induce some tumor cells proliferation, IL-6 activates Bax protein that can induce apoptosis in Lewis lung carcinoma (LLC) cells(39). Their results suggest that LLC transfected IL-6 gene results from the high ratio of Bax to Bcl-2 and cytotoxicity in the IL-6-dependent apoptotic pathway(39). In order to study the effect on influence tumor cells growth, . In this study, we did not confirm the relationship between IL-6-transfected and apoptosis.
IL-15 is a key regulator of NK development, homeostasis and activity. Resting NK cells always express IL-15 receptor complex (consisting of IL-15Rα and
IL-15Rβγ) that can respond to IL-15 at picomolar concentrations(40). LL-15 increases the number and percentage of NK cells in the spleen, the proliferation and survival of NK cells, and their cytolytic activity and cytokine secretion. Therefore, we used IL-15 in order to activate NK cells activity. Although we did not find IL-15 concentration in serum, IL-15 can activate NK cells function in this therapy. (In NK killing assay, the percentage of CTAC killed by NK cells from GT cases is significantly higher than from control. IL-15 protein is hardly found in serum or body fluids and often referred to as a "ghost cytokine "(41). It may be why we did not find IL-15 concentration in serum.
IL-15 is important for T cells, too. IL-15 has a role in the survival and
antigen-independent expansion of naive and memory CD8+ T cells. Because IL-15 can activate Bcl-2 and Bcl-XL(42), it performs the survival effects on CD8+ T cells(42). In IL-15 and IL-15R-deficient mice, CD8+ T cells shows a significant decrease(7).
CD8+T cells are cytotoxic T lymphocytes. IL-15 also activate CTL cytotoxic activity. In our study, CTL cytotoxic activity of GT cases are higher than control. There are two reasons of activating cytotoxic function of CTL . (a) IL-6 antagonized TGF-β and activate CTVT with IFN-γ. The level of MHC molecules expression increased. MHC class Ⅰ molecules are necessary for the presentation of peptide antigens to CTL. The interaction triggers a casade of T-signaling events that lead to cell proliferation, cytokine producton, and target cell lysis(43).
Because IL-15 has been demonstrated to have a pivotal role in the function of memory CD8t T cells and NK cells, intratumoral IL-15 gene therapy had been performed in several studies. The results of these studies were also successful.
C57BL/6 mice were injected with B16.F10 melanoma cells and treated intratumorally with 50 mg of IL-15 plasmid on days 0, 4 and 7 and tumor volume/size, tumor
regression and long-term survival were measured. These results demonstrate the ability of pIL-15 to mediate B16 melanoma regression, with the effect being significantly enhanced by electroporative delivery(44).
Combined different cytokine gene to treat with tumor had also been performed. Mice had been intravenously inoculated with the RLmale1 lymphoma cells. 40% of mice that received a single injection of a mixture IL-15 and IL-21 genes successfully rejected the preestablished metastatic lymphoma and showed tumor-free survival for more than 300 days(45).
In vivo electroporation has been used to efficiently deliver drugs and
‘therapeutic’ genes to tumors. We combined immune character of IL-6/IL-15 and advantages of electroporation. CTVT growth can be inhibited and regressed. This strategy may be used in other tumor therapies.
參考資料
1. Garcia-Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol 2003;195(3):346-55.
2. Maecker HL, Yun Z, Maecker HT, Giaccia AJ. Epigenetic changes in tumor Fas levels determine immune escape and response to therapy. Cancer Cell
2002;2(2):139-48.
3. Murgia C, Pritchard JK, Kim SY, Fassati A, Weiss RA. Clonal origin and evolution of a transmissible cancer. Cell 2006;126(3):477-87.
4. Mozos E, Mendez A, Gomez-Villamandos JC, Martin De Las Mulas J, Perez J. Immunohistochemical characterization of canine transmissible venereal tumor. Vet Pathol 1996;33(3):257-63.
5. Bierie B, Moses HL. TGF-beta and cancer. Cytokine Growth Factor Rev 2006;17(1-2):29-40.
6. Kovalchuk AL, Kim JS, Park SS, et al. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc Natl Acad Sci U S A 2002;99(3):1509-14.
7. Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med
2000;191(5):771-80.
8. Hsiao YW, Liao KW, Hung SW, Chu RM. Effect of tumor infiltrating
lymphocytes on the expression of MHC molecules in canine transmissible venereal tumor cells. Vet Immunol Immunopathol 2002;87(1-2):19-27.
9. Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther 2006;8 Suppl 2:S2.
10. Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol 2006;80(2):227-36.
11. Nishimoto N. Interleukin-6 in rheumatoid arthritis. Curr Opin Rheumatol 2006;18(3):277-81.
12. Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol 2006;63(5):321-9.
13. Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res Treat 2006.
14. Arnoult D, Petit F, Lelievre JD, et al. Caspase-dependent and -independent T-cell death pathways in pathogenic simian immunodeficiency virus infection: relationship to disease progression. Cell Death Differ 2003;10(11):1240-52.
15. Chiu JJ, Sgagias MK, Cowan KH. Interleukin 6 acts as a paracrine growth factor in human mammary carcinoma cell lines. Clin Cancer Res 1996;2(1):215-21.
16. Inoue K, Okabe S, Sueoka E, Sueoka N, Tabei T, Suganuma M. The role of interleukin-6 in inhibition of lung metastasis in subcutaneous tumor-bearing mice. Oncol Rep 2000;7(1):69-73.
17. Saito K, Ishikura H, Kishimoto T, et al. Interleukin-6 produced by pancreatic carcinoma cells enhances humoral immune responses against tumor cells: a possible event in tumor regression. Int J Cancer 1998;75(2):284-9.
18. Wang Q, Horiatis D, Pinski J. Interleukin-6 inhibits the growth of prostate cancer xenografts in mice by the process of neuroendocrine differentiation. Int J Cancer 2004;111(4):508-13.
19. Kobel M, Budianto D, Schmitt WD, Borsi L, Siri A, Hauptmann S. Influence of various cytokines on adhesion and migration of the colorectal adenocarcinoma cell line HRT-18. Oncology 2005;68(1):33-9.
20. Poppenborg H, Knupfer MM, Galla HJ, Ernst J, Wolff A. In vitro modulation of cisplatin resistance by cytokines. Cytokine 1999;11(9):689-95.
21. Asgeirsson KS, Olafsdottir K, Jonasson JG, Ogmundsdottir HM. The effects of IL-6 on cell adhesion and e-cadherin expression in breast cancer. Cytokine
1998;10(9):720-8.
22. Gosselin J, TomoIu A, Gallo RC, Flamand L. Interleukin-15 as an activator of natural killer cell-mediated antiviral response. Blood 1999;94(12):4210-9.
23. Sato N, Patel HJ, Waldmann TA, Tagaya Y. The IL-15/IL-15R{alpha} on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc Natl Acad Sci U S A 2007;104(2):588-93.
24. White L, Krishnan S, Strbo N, et al. Differential effects of IL-21 and IL-15 on perforin expression, lysosomal degranulation and proliferation in CD8 T cells of patients infected with Human Immunodeficiency Virus-1 (HIV). Blood 2006. 25. Bouchard A, Ratthe C, Girard D. Interleukin-15 delays human neutrophil apoptosis by intracellular events and not via extracellular factors: role of Mcl-1 and decreased activity of caspase-3 and caspase-8. J Leukoc Biol 2004;75(5):893-900. 26. Ratthe C, Girard D. Interleukin-15 enhances human neutrophil phagocytosis by a Syk-dependent mechanism: importance of the IL-15Ralpha chain. J Leukoc Biol 2004;76(1):162-8.
27. Alleva DG, Kaser SB, Monroy MA, Fenton MJ, Beller DI. IL-15 functions as a potent autocrine regulator of macrophage proinflammatory cytokine production: evidence for differential receptor subunit utilization associated with stimulation or inhibition. J Immunol 1997;159(6):2941-51.
28. Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev 2002;13(2):169-83.
natural killer cells. Adv Immunol 2005;86:209-39.
30. Sutherland CL, Rabinovich B, Chalupny NJ, Brawand P, Miller R, Cosman D. ULBPs, human ligands of the NKG2D receptor, stimulate tumor immunity with enhancement by IL-15. Blood 2006;108(4):1313-9.
31. Diab A, Cohen AD, Alpdogan O, Perales MA. IL-15: targeting CD8+ T cells for immunotherapy. Cytotherapy 2005;7(1):23-35.
32. Teague RM, Sather BD, Sacks JA, et al. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med
2006;12(3):335-41.
33. Cao X, Wang Q, Ju DW, Tao Q, Wang J. Efficient inducation of local and systemic antitumor immune response by liposome-mediated intratumoral co-transfer of interleukin-2 gene and interleukin-6 gene. J Exp Clin Cancer Res
1999;18(2):191-200.
34. Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol 2003;3(5):413-25.
35. Hsiao YW, Liao KW, Hung SW, Chu RM. Tumor-infiltrating lymphocyte secretion of IL-6 antagonizes tumor-derived TGF-beta 1 and restores the lymphokine-activated killing activity. J Immunol 2004;172(3):1508-14. 36. Hsiao YW, Liao KW, Hung SW, Chu RM. Mechanism of Major
Histocompatibility Complex Expression in Canine Transmissible Venereal Tumor Cells during Tumor Regression. (In preperation).
37. Cao X, Chen G, He L, Zhang W, Yu Y, Wang J. Involvement of MHC class I molecule and ICAM-1 in the enhancement of adhesion and cytotoxic susceptibility to immune effector cells of tumor cells transfected with the interleukin (IL)-2, IL-4 or IL-6 gene. J Cancer Res Clin Oncol 1997;123(11-12):602-8.
38. Reiter Z, Chen L, Revel M, Rubinstein M. Interleukin-6 protects ductal breast carcinoma cells from MHC-unrestricted cell-mediated cytotoxicity. Lymphokine Cytokine Res 1992;11(3):175-81.
39. Usuda J, Okunaka T, Furukawa K, et al. Increased cytotoxic effects of photodynamic therapy in IL-6 gene transfected cells via enhanced apoptosis. Int J Cancer 2001;93(4):475-80.
40. Carson WE, Fehniger TA, Haldar S, et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest 1997;99(5):937-43. 41. Van Belle T, Grooten J. IL-15 and IL-15Ralpha in CD4+T cell immunity. Arch Immunol Ther Exp (Warsz) 2005;53(2):115-26.
42. Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 1998;8(5):591-9. 43. Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown
and tumor growth. Immunol Today 2000;21(9):455-64.
44. Ugen KE, Kutzler MA, Marrero B, et al. Regression of subcutaneous B16 melanoma tumors after intratumoral delivery of an IL-15-expressing plasmid followed by in vivo electroporation. Cancer Gene Ther 2006;13(10):969-74. 45. Kishida T, Asada H, Itokawa Y, et al. Interleukin (IL)-21 and IL-15 genetic transfer synergistically augments therapeutic antitumor immunity and promotes regression of metastatic lymphoma. Mol Ther 2003;8(4):552-8.