Notes
Structural Transformations of a Series of
Tellurium-Iron Carbonylates and the Isolation of the
Cubic Cluster [Te
4Fe
4(CO)
10(dppm)]
Kuo-Chih Huang,
†Mei-Huey Shieh,
†Ren-Jay Jang,
†Shie-Ming Peng,
‡Gene-Hsiang Lee,
§and Minghuey Shieh*
,†Department of Chemistry, National Taiwan Normal University, Taipei 11718, Taiwan, Republic of China, and Department of Chemistry and Instrumentation Center,
National Taiwan University, Taipei 10764, Taiwan, Republic of China Received June 22, 1998
Summary: The cluster growth from the small anionic cluster [TeFe3(CO)9]2-to the double-cubic cluster [Te10Fe8 -(CO)20]2-was systematically established. Further treat-ment of [Te10Fe8(CO)20]2- with [Cu2(dppm)2(MeCN)4 ]-[BF4]2 led to the formation of the new dppm-bridged cubic cluster [Te4Fe4(CO)10(dppm)].
Introduction
Tellurium-rich metal compounds have attracted ex-tensive attention, since tellurides exhibit unusual struc-tural and reactivity patterns and are potentially pre-cursors for new solid-state materials.1,2 Recently, a
series of novel tellurium-iron carbonylates have been synthesized and structurally characterized by several different methodologies. These interesting anionic clus-ters include [TeFe3(CO)12]2-,3a[TeFe3(CO)9]2-,3,4[Te4Fe5
-(CO)14]2-,5,6[Te6Fe8(CO)24]2-,5 and [Te10Fe8(CO)20]2-.6
Nevertheless, their structural relationships and trans-formations have never been systematically studied. The reaction sequences in which the small molecules trans-form to larger clusters are of great importance to the extended inorganic solids.7 As a consequence,
develop-ing and understanddevelop-ing the conversion of small clusters to larger ones is of great interest. In this paper, we demonstrate a facile stepwise cluster growth from [TeFe3(CO)9]2- to [Te10Fe8(CO)20]2-. The reactions of
[Te10Fe8(CO)20]2- with electrophiles are described as
well, from which the cubic cluster [Te4Fe4(CO)10(dppm)]
is obtained and compared with the double-cubic cluster [Te10Fe8(CO)20]2-.
Experimental Section
All reactions were performed under an atmosphere of pure nitrogen by using standard Schlenk line techniques.8 Solvents were purified, dried, and distilled under nitrogen prior to use. K2TeO3‚H2O (Alfa), TeO2(Strem), Fe(CO)5(Aldrich), and bis-(diphenylphosphino)methane (dppm; Acros) were used as received. Infrared spectra were recorded on a Jasco 700 IR or a Perkin-Elmer Paragon 500 spectrometer using CaF2liquid cells. Mass spectra were obtained on a Finnigan TSQ700 mass spectrometer. Elemental analyses were performed on a Per-kin-Elmer 2400 analyzer at the NSC Regional Instrumental Center at National Taiwan University. 1H NMR spectra were taken on a Varian 200 (200 MHz) instrument. [TeFe3(CO)9]2-,3,4 Te2Fe3(CO)9,9and [Cu2(dppm)2(MeCN)4][BF4]210were prepared by the published methods.
Formation of [Et4N]2[Te4Fe5(CO)14]. To a mixture of 0.239 g (0.296 mmol) of [Et4N]2[TeFe3(CO)9] and 0.100 g (0.148 mmol) of Te2Fe3(CO)9was added 20 mL of THF. After being stirred for 3 days at room temperature, the solution was filtered and solvent was removed under vacuum. The residue was washed with hexanes and ether and then extracted with CH2Cl2to give 0.110 g (0.076 mmol) of [Et4N]2[Te4Fe5(CO)14] (26% based on [Et4N]2[TeFe3(CO)9]). IR (νCO, MeCN) for [Et4N]2[Te4Fe5(CO)14]: 2025 w, 2008 s, 1975 vs, 1940 m cm-1.
Formation of [Et4N]2[Te6Fe8(CO)24]. To a mixture of 0.100 g (0.069 mmol) of [Et4N]2[Te4Fe5(CO)14] and 0.086 g (0.127 mmol) of Te2Fe3(CO)9was added 40 mL of CH2Cl2. The solution turned reddish brown immediately and was filtered, and solvent was removed under vacuum. The residue was washed with hexanes and ether and then extracted with CH2Cl2to give 0.110 g (0.051 mmol) of [Et4N]2[Te6Fe8(CO)24] * To whom correspondence should be addressed.
†Department of Chemistry, National Taiwan Normal University. ‡Department of Chemistry, National Taiwan University. §Instrumentation Center, National Taiwan University.
(1) (a) Ansari, M. A.; Ibers, J. A. Coord. Chem. Rev. 1990, 100, 223. (b) Roof, L. C.; Kolis, J. W. Chem. Rev. 1993, 93, 1037. (c) Kanatzidis, M. G.; Huang, S.-P. Coord. Chem. Rev. 1994, 130, 509.
(2) (a) Bogan, L. E., Jr.; Lesch, D. A.; Rauchfuss, T. B. J. Organomet. Chem. 1983, 250, 429. (b) Mathur, P.; Thimmappa, B. H. S.; Rheingold, A. L. Inorg. Chem. 1990, 29, 4658 and references therein. (c) Mathur, P.; Reddy, V. D.; Das, K.; Sinha, U. C. J. Organomet. Chem. 1991, 409, 255 and references therein. (d) Roof, L. C.; Pennington, W. T.; Kolis, J. W. J. Am. Chem. Soc. 1990, 112, 8172.
(3) (a) Bachman, R. E.; Whitmire, K. H. Inorg. Chem. 1994, 33, 2527. (b) Roof, L. C.; Smith, D. M.; Drake, G. W.; Pennington, W. T.; Kolis, J. W. Inorg. Chem. 1995, 34, 337.
(4) Shieh, M.; Chen, P.-F.; Tsai, Y.-C.; Shieh, M.-H.; Peng, S.-M.; Lee, G.-H. Inorg. Chem. 1995, 34, 2251.
(5) Shieh, M.; Chen, P.-F.; Peng, S.-M.; Lee, G.-H. Inorg. Chem. 1993, 32, 3389.
(6) Roof, L. C.; Pennington, W. T.; Kolis, J. W. Angew. Chem., Int. Ed. Engl. 1992, 31, 913.
(7) (a) Steigerwald, M. L.; Siegrist, T.; Stuczynski, S. M. Inorg. Chem. 1991, 30, 4940. (b) Steigerwald, M. L.; Siegrist, T.; Gyorgy, E. M.; Hessen, B.; Kwon, Y.-U.; Tanzler, S. M. Inorg. Chem. 1994, 33, 3389.
(8) Shriver, D. F.; Drezdzon, M. A. The Manipulation of Air Sensitive Compounds; Wiley: New York, 1986.
(9) Lesch, D. A.; Rauchfuss, T. B. Inorg. Chem. 1981, 20, 3583. (10) Dı´ez, J.; Gamasa, P.; Gimeno, J.; Tiripicchio, A.; Camellini, M. T. J. Chem. Soc., Dalton Trans. 1987, 1275.
5202 Organometallics 1998, 17, 5202-5205
10.1021/om980516f CCC: $15.00 © 1998 American Chemical Society Publication on Web 10/16/1998
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009
(74% based on [Et4N]2[Te4Fe5(CO)14]). IR (νCO, CH2Cl2) for [Et4N]2[Te6Fe8(CO)24]: 2026 s, 2004 vs, 1956 m (br) cm-1.
Formation of [TMBA]2[Te10Fe8(CO)20]. To a mixture of 3.20 g (1.46 mmol) of [TMBA]2[Te6Fe8(CO)24] and 0.967 g (7.58 mmol) of Te powder was added 40 mL of THF. After being stirred and heated at 50 °C for 3 days, the solution was filtered and solvent was removed under vacuum. The residue was washed with hexanes and ether and then extracted with CH2Cl2to give 2.24 g (0.867 mmol) of [TMBA]2[Te10Fe8(CO)20] (60% based on [TMBA]2[Te6Fe8(CO)24]). IR (νCO, CH2Cl2): 2026 s, 2015 sh, 1979 s, 1924 w cm-1. Anal. Calcd (found) for [TMBA]2[Te10Fe8(CO)20]: C, 18.57 (18.59); H, 1.24 (1.44); N, 1.08 (0.95).
Reaction of [TMBA]2[Te10Fe8(CO)20] with
Na/Naphtha-lene. To a sample of 0.297 g (0.115 mmol) of [TMBA] 2[Te10-Fe8(CO)20] in 15 mL of THF was added 1.2 mL of a solution which was prepared from 0.349 g (0.015 mol) of Na and 1.94 g (0.015 mol) of naphthalene in 40 mL of THF. The mixed solution was stirred for 10 h and filtered, and solvent was removed under vacuum. The residue was washed with hex-anes and then extracted with CH2Cl2 to give 0.11 g (0.050 mmol) of [TMBA]2[Te6Fe8(CO)24] (43% based on [TMBA]2[Te10-Fe8(CO)20]). IR (νCO, CH2Cl2) for [TMBA]2[Te6Fe8(CO)24]: 2027 m, 2006 vs, 1959 m, br cm-1.
Reaction of [TMBA]2[Te10Fe8(CO)20] with MeSO3CF3. To a sample of 0.203 g (0.079 mmol) of [TMBA]2[Te10Fe8(CO)20] in 10 mL of THF was added 0.04 mL (0.35 mmol) of MeSO3-CF3. The mixed solution was stirred for 3 h and filtered, and solvent was removed under vacuum. The residue was ex-tracted with hexanes to give the known butterfly complex 0.02 g (0.034 mmol) (Fe2(CO)6(µ-TeMe)2,11which was identified by IR and mass spectroscopy.
Formation of [Te4Fe4(CO)10(dppm)]. To a mixture of 0.774 g (0.300 mmol) of [TMBA]2[Te10Fe8(CO)20] and 0.369 g (0.300 mmol) of [Cu2(dppm)2(MeCN)4][BF4]2was added 30 mL of THF. After being stirred and heated at 50 °C for 40 h, the solution was filtered and solvent was removed under vacuum. The residue was washed with MeOH and ether and then extracted with THF to give 0.25 g (0.169 mmol) of [Te4-Fe4(CO)10(dppm)] (28%). IR (νCO, CH2Cl2) for [Te4Fe4(CO)10-(dppm)]: 2053 s, 2032 vs, 1994 vs, 1940 w cm-1. Anal. Calcd (found) for [Te4Fe4(CO)10(dppm)]: C, 29.19 (29.10); H, 1.63 (1.81). 1H NMR (CDCl3, 298 K): δ 7.55-7.22 (m, 20H), δ 4.04 (t, 2H, JP-H) 10 Hz). Mp: 319-320 °C dec. This complex is soluble in ether, CH2Cl2, and THF.
X-ray Structural Characterization of [Te4Fe4(CO)10
-(dppm)]‚CH2Cl2(1). A summary of selected crystallographic data for [Te4Fe4(CO)10(dppm)]‚CH2Cl2(1) is given in Table 1. Data collection was carried out on a Nonius CAD-4 dif-fractometer using graphite-monochromated Mo KR radiation at 25 °C employing theθ/2θ scan mode. A ψ-scan absorption correction was made.12 The crystal was mounted on glass fibers with epoxy cement. Data reduction and structural refinement were performed using the NRCC-SDP-VAX pack-ages,13and atomic scattering factors were taken from ref 14. Reddish black crystals of 1 suitable for X-ray analysis were grown from hexane/CH2Cl2solutions. A total of 7842 unique reflections were collected and corrected for absorption and decay. The structure was solved by the heavy-atom method and refined by least-squares cycles. All non-hydrogen atoms were refined with anisotropic thermal parameters. Full-matrix least-squares refinement of 1 led to convergence with
R ) 3.4% and Rw) 3.2% for those reflections with I > 2.0
σ(I).
Selected bond distances and angles of 1 are listed in Table 2. Additional crystallographic data are available as Support-ing Information.
Results and Discussion
The previous study3,4 showed that the reaction of
K2TeO3with 3 equiv of Fe(CO)5/KOH in MeOH led to
the formation of the open cluster [TeFe3(CO)12]2-, which
then decarbonylated to generate the tetrahedral com-plex [TeFe3(CO)9]2-. However, when K2TeO3 reacted
with 1 equiv of Fe(CO)5/KOH in MeOH, the large cluster
anion [Te6Fe8(CO)24]2- was formed.4 These results
indicated that the Te-Fe-CO system is very versatile, and different stoichiometries and subtle changes of the reaction conditions could give rise to different outcomes. These prompted us to explore the cluster transforma-tions and to attempt to elucidate the cluster growth processes in this system.
Cluster Growth Processes. The cluster growth processes are summarized in Scheme 1. When the tetrahedral cluster [TeFe3(CO)9]2-was treated with Te2
-Fe3(CO)9, the mediun-sized cluster [Te4Fe5(CO)14]2-was
obtained. One can envision [Te4Fe5(CO)14]2- to be
composed of the anion [Te2Fe2(CO)6]2-and one Te2Fe3
-(CO)9with the loss of one CO, which seems to have no
direct relevance to [TeFe3(CO)9]2- and Te2Fe3(CO)9.
Therefore, the formation of [Te4Fe5(CO)14]2- can be
regarded as resulting from complicated bond breakage and rearrangement processes. It was more interesting (11) Bachman, R. E.; Whitmire, K. H. Organometallics 1993, 12,
1988.
(12) North, A. C. T.; Philips, D. C.; Mathews, F. S. Acta Crystallogr. 1968, A24, 351.
(13) Gabe, E. J.; Le Page, Y.; Charland, J. P.; Lee, F. L.; White, P. S. J. Appl. Crystallogr. 1989, 22, 384.
(14) International Tables for X-ray Crystallography; Kynoch Press: Birmingham, England, 1974; Vol. IV.
Table 1. Selected Crystallographic Dataafor [Te4Fe4(CO)10(dppm)]‚CH2Cl2(1) empirical formula C36H24Cl2Fe4O10P2Te4
fw 1483.21
cryst syst triclinic
space group P1h a, Å 11.724(5) b, Å 13.836(5) c, Å 15.116(5) R, deg 84.97(3) β, deg 86.70(3) γ, deg 66.24(3) V, Å3 2235(2) D(calcd), Mg m-3 2.204 abs coeff, cm-1 40.837 Tmin/Tmax 0.90/1.00 residuals: R; Rwb 0.034; 0.032
aAll data were obtained from a Nonius (CAD-4) diffractometer
with Mo KR radiation (λ ) 0.7107 Å) at 25 °C.bThe functions
minimized during least-squares cycles were R )∑|Fo- Fc|/∑Fo and Rw) [∑w(Fo- Fc)2/∑w(Fo)2]1/2.
Table 2. Selected Bond Distances (Å) and Bond Angles (deg) for [Te4Fe4(CO)10(dppm)]‚CH2Cl2(1)
(A) Distances Te(1)-Fe(1) 2.615(2) Te(1)-Fe(2) 2.607(2) Te(1)-Fe(3) 2.619(2) Te(2)-Fe(1) 2.620(2) Te(2)-Fe(2) 2.623(2) Te(2)-Fe(4) 2.608(2) Te(3)-Fe(1) 2.605(2) Te(3)-Fe(3) 2.619(2) Te(4)-Fe(2) 2.621(2) Te(4)-Fe(3) 2.621(2) Te(4)-Fe(4) 2.613(2) Te(3)-Fe(4) 2.625(2) (B) Bond Angles Fe(1)-Te(1)-Fe(2) 92.95(6) Fe(1)-Te(1)-Fe(3) 97.88(6) Fe(2)-Te(1)-Fe(3) 97.40(5) Fe(1)-Te(2)-Fe(2) 92.47(6) Fe(1)-Te(2)-Fe(4) 96.66(5) Fe(2)-Te(2)-Fe(4) 98.64(6) Fe(1)-Te(3)-Fe(3) 98.14(6) Fe(1)-Te(3)-Fe(4) 96.60(5) Fe(3)-Te(3)-Fe(4) 95.51(6) Fe(2)-Te(4)-Fe(3) 96.99(5) Fe(2)-Te(4)-Fe(4) 98.55(6) Fe(3)-Te(4)-Fe(4) 95.71(6) Te(1)-Fe(1)-Te(2) 86.23(5) Te(1)-Fe(1)-Te(3) 82.04(5)
Notes Organometallics, Vol. 17, No. 23, 1998 5203
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009
to note that when [Te4Fe5(CO)14]2-further reacted with
Te2Fe3(CO)9, the large cluster [Te6Fe8(CO)24]2- was
cleanly yielded. This cluster growth process is quite reasonable, because [Te6Fe8(CO)24]2- can be
approxi-mately viewed as one [Te4Fe5(CO)14]2- anion and one
Te2Fe3(CO)9.
Utilizing this methodology, we wondered whether the larger cluster can be obtained when [Te6Fe8(CO)24]
2-was treated with Te2Fe3(CO)9or Te or other Fe sources.
This is indeed the case. When [Te6Fe8(CO)24]2- was
treated with 4 equiv of Te, the double-cubic cluster [Te10Fe8(CO)20]2- was obtained in good yield. The
double-cubic cluster was previously reported to be generated from the reaction of Fe(CO)5with “Zintl” ion
Te42-and excess Te. The route we describe here from
[Te6Fe8(CO)24]2-to [Te10Fe8(CO)20]2-is convenient and
high yielding. From a structural viewpoint, there is no obvious relationship between these two clusters; how-ever, the structural transformation is possible by ad-justment of the appropriate stoichiometry.
In this study, the facile processes of the cluster growth from the small [TeFe3(CO)9]2- to the large [Te10Fe8
-(CO)20]2-are established. Other types of cluster
expan-sion processes have been seen in our previous studies of Se-Fe and E-Mn (E ) S, Se) systems.15,16 In this
case, larger cluster skeletons are observed probably due to the greater size of the Te atom. The results all
indicate that the cluster formation is gradual and may be predictable and also provide some useful information on further cluster expansion reactions.
Reactivity of [Te10Fe8(CO)20]2-. Since the
double-cubic cluster [Te10Fe8(CO)20]2-is structurally interesting
and can be obtained easily from [Te6Fe8(CO)24]2-,
further reactivity studies of [Te10Fe8(CO)20]2- were
therefore carried out. It was found that [Te10Fe8(CO)20]
2-can be reconverted to [Te6Fe8(CO)24]2-upon reduction
with Na/naphthalene (eq 1). To test the basic sites of
[Te6Fe8(CO)24]2-, we treated [Te10Fe8(CO)20]2-with the
methylation agent MeSO3CF3. However, the reaction
proceeded with severe bond breakage and formation to give the known butterfly complex11Fe
2(CO)6(µ-TeMe)2.
When [Te10Fe8(CO)20]2-was treated with [Cu2(dppm)2
-(MeCN)4][BF4]2, the dppm-bridged cubic cluster [Te-4Fe4(CO)10(dppm)] (1) was obtained (eq 2). Cluster 1
can be considered to result from the oxidative fragmen-tation of [Te10Fe8(CO)20]2-by the Cu(I) complex followed
by the chelation of the dppm ligand. Direct reaction of [Te10Fe8(CO)20]2- with dppm failed to produce cluster
1.
The reactivity investigation showed that the double-cubic cluster tended to undergo fragmentation to give the cubic or even smaller fragments depending upon the incoming reagents. However, the potential of [Te10Fe8
-(CO)20]2-to serve as a cubic precursor is promising, and
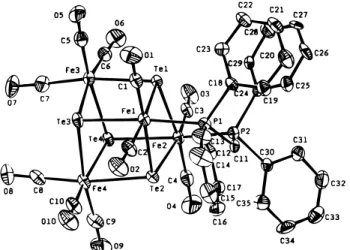
further study of its cluster expansion is now in progress. Structure of [Te4Fe4(CO)10(dppm)]‚CH2Cl2 (1).
[Te4Fe4(CO)10(dppm)] was structurally characterized by
single-crystal X-ray diffraction. As shown in Figure 1, (15) Shieh, M.; Shieh, M.-H.; Tsai, Y.-C.; Ueng, C.-H. Inorg. Chem. 1995, 34, 5088.
(16) Huang, K.-C.; Tsai, Y.-C.; Lee, G.-H.; Peng, S.-M.; Shieh, M. Inorg. Chem. 1997, 36, 4421.
Scheme 1
Figure 1. ORTEP diagram showing the structure and
atom labeling for 1.
[Te6Fe8(CO)24]
2-{\}4Te
Na/naphthalene [Te10Fe8(CO)20]
2-(1)
[Te10Fe8(CO)20]2-+ [Cu2(dppm)2(MeCN)4][BF4]2f [Te4Fe4(CO)10(dppm)] (2)
5204 Organometallics, Vol. 17, No. 23, 1998 Notes
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009
the Te4Fe4core exhibits a cubic geometry with the dppm
ligand bridging across two Fe atoms. Each iron atom in 1 is octahedrally coordinated and obeys the 18-electron count. The Te-Fe distance averages 2.616 Å and is close to those of the structurally related double-cubic cluster [Te10Fe8(CO)20]2- (2.62 Å) and the cubic
clusters [Te4Fe4(SPh)4]3-(2.63 Å),17[Te4Fe4(TePh)4]
3-(2.622 Å),18and [Te
4Fe4(SiPr)4]3-(2.627 Å).19 In 1, the
internal angles in the Te4Fe4 cube are between 82.04
and 98.64°, close to 90°, indicative of a slight distortion
from the ideal cube. This is different from the previ-ously characterized cubes [Te4Fe4(EPh)4]3- (E ) S,
Te),17,18which contain angles deviating greatly from 90°,
probably due to the bridging effect of the dppm ligand on the cubic structure.
Acknowledgment. We thank the National Science Council of the Republic of China for financial support (Grant No. NSC-87-2113-M-003-001).
Supporting Information Available: Complete listings
of crystallographic data, atomic positional parameters, bond distances and angles, and anisotropic thermal parameters of cluster 1 (7 pages). Ordering information is given on any current masthead page.
OM980516F (17) Barbaro, P.; Bencini, A.; Bertini, I.; Briganti, F.; Midollini, S.
J. Am. Chem. Soc. 1990, 112, 7238.
(18) Simon, W.; Wilk, A.; Krebs, B.; Henkel, G. Angew. Chem., Int. Ed. Engl. 1987, 26, 1009.
(19) Stephan, H.-O.; Chen, C.; Henkel, G.; Griesar, K.; Haase, W. J. Chem. Soc., Chem. Commun. 1993, 886.
Notes Organometallics, Vol. 17, No. 23, 1998 5205
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009
![Table 1. Selected Crystallographic Data a for [Te 4 Fe 4 (CO) 10 (dppm)]‚CH 2 Cl 2 (1) empirical formula C 36 H 24 Cl 2 Fe 4 O 10 P 2 Te 4](https://thumb-ap.123doks.com/thumbv2/9libinfo/8665604.195437/2.918.500.827.99.310/table-selected-crystallographic-data-fe-dppm-empirical-formula.webp)